Abstract
Prevention of secondary infection is currently the main goal of treatment for acute necrotizing pancreatitis. Colon was considered as the main origin of secondary infection. Our aim was to investigate whether prophylactic total colectomy would reduce the rate of bacterial translocation and infection of pancreatic necrosis. Forty-two Sprague–Dawley rats were used. Pancreatitis was created by ductal infusion of sodium taurocholate. Rats were divided into four groups: group-1, laparotomy + pancreatic ductal infusion of saline; group-2, laparotomy + pancreatic ductal infusion of sodium taurocholate; group-3, total colectomy + pancreatic ductal infusion of saline; and group-4, total colectomy + pancreatic ductal infusion of sodium taurocholate. Forty-eight hours later, tissue and blood samples were collected for microbiological and histopathological analysis. Total colectomy caused small bowel bacterial overgrowth with gram-negative and gram-positive microorganisms. Bacterial count of gram-negative rods in the small intestine and pancreatic tissue in rats with colectomy and acute pancreatitis were significantly higher than in rats with acute pancreatitis only (group-2 versus group-4; small bowel, p = <0.001; pancreas, p = 0.002). Significant correlation was found between proximal small bowel bacterial overgrowth and pancreatic infection (r = 0,836, p = 0.001). In acute pancreatitis, prophylactic total colectomy (which can mimic colonic cleansing and reduction of colonic flora) induces small bowel bacterial overgrowth, which is associated with increased bacterial translocation to the pancreas.
Keywords: Bacterial translocation, Acute pancreatitis, Colonic cleansing, Intestinal flora
Introduction
Despite recent improvements in the new diagnostic and therapeutic tools, the severe form of acute pancreatitis (AP) remains a critical condition with a high rate of morbidity and mortality [1–3]. Secondary infections generally resulting from translocation of enteric bacteria constitute the leading cause of deaths in AP [4–6]. The isolated predominant pathogens in most cases of pancreatic infection are gastrointestinal gram-negative bacteria, thus supporting this hypothesis [7]. Therefore, prevention of secondary infection has become a principal focus in the management of this disease so far. Bacterial translocation and reduction of colonic flora have been the main focus of experimental trials investigating the course of AP [8–10]. A number of studies have been conducted [11–13] to evaluate the role of different therapeutic modalities in preventing bacterial translocation, but there is lack of studies investigating the impact of elimination of the colonic flora, by means of total colectomy.
The main goal of this study was to evaluate the role of total colectomy on bacterial translocation to pancreas and on pancreatic infection rate during experimental AP.
Methods
This study was approved by the Institutional Animal Use and Care Committee of the Gulhane Medical Academy. A total of 42 male Sprague–Dawley rats, weighing 300–420 g were used. The animals were fed standard rat chow. They were given free access to water and were housed in cages under standard conditions (room temperature with a 12-h light–dark cycle). Rats were allowed to adjust to these conditions for at least 1 week before study to stabilize their intestinal flora.
The rats were randomly divided into four groups. Surgical anesthesia was induced in rats by 50 mg/kg intramuscular ketamine (Ketalar flacon, Parke-Davis, Eczacibasi, Istanbul, Turkey) and 10 mg/kg xylazine hydrochloride (Rompun flakon, Bayer, Leverkusen, Germany). Rats in group-1 (control, n = 10) underwent laparotomy with manipulation of the pancreas and received saline injection into the biliopancreatic duct (BPD). Group-2 (AP, n = 12) rats underwent laparotomy with induction of pancreatitis via intraductal infusion of sodium taurocholate into the BPD. Group-3 (colectomy, n = 10) rats underwent total colectomy and received saline injection into BPD. Group-4 (colectomy + AP, n = 10) rats underwent total colectomy, and AP was also inducted by intraductal sodium taurocholate infusion. During surgical procedures, the intestine was kept moist with sterile saline solution. All surgical procedures were performed under strict aseptic conditions. Animals died before the end of the study, developing ductal perforation or hemorrhage during cannulation of the duct which was excluded from the study.
Induction of Acute Pancreatitis
After induction of anesthesia, laparotomy was performed through a 4-cm length midline incision. BPD was cannulated with a 28-gauge, 0.5-in microfine catheter, and a microaneurysm clip was placed on the bile duct below the liver. Another clip was placed around the common BPD at its entry into the duodenum to avoid reflux of enteric contents into the duct. When the catheter was placed in the pancreatic duct, 1 mL/kg of 3 % sodium taurocholate (Sigma, St. Louis, MO, USA) was infused into the BPD (1 mL/min) as described by Liu et al. [14]. Once the injection was finished, the two microclips were removed; the catheter was taken out, and duodenal defect was immediately closed using 8–0 Prolene sutures. Finally, the abdominal wall was sutured with interrupted 2–0 silk.
Surgical Procedures
Total colectomy was performed through a 4-cm length midline abdominal incision. After colonic vasculature was identified and ligated, great volume of colon was excised till 3 cm to the anus, and end-to-end ileorectal anastomosis was handled using a running 8–0 Prolene suture. During all these operations, great care was taken for minimizing surgical trauma. As for sham laparotomy, rats underwent the same surgical processes, including mobilization of the colon and the small intestine. However, instead of sodium taurocholate, the same volume of physiologic solution was infused into BPD in the same manner.
Collection of Samples and Scarification
Forty-eight hours after induction of AP, a relaparotomy was performed under aseptic conditions to allow sterile collection of organ and fluid samples. Samples were collected in the following order: peritoneal fluid, blood (by cardiac puncture), mesenteric lymph nodes (MLN), liver segment, spleen, pancreas, and ileum. After collection of samples, rats were sacrificed by blood loss. Blood samples were divided into two groups: one for biochemical analyses and the other for bacteriologic analyses. All tissue samples were evaluated for microbiological and histopathological analyses.
Microbiological Analysis
The samples were weighed and processed immediately for quantitative culture of aerobic and anaerobic organisms using standard microbiologic methods. Gram-negative bacteria were identified using MacConkey agar supplemented with 10 % lactose (Scott Co. West Warwick, Rhode Island, USA). Gram-positive bacteria were identified using the Schaedler agar (Difco, Detroit, Michigan, USA). Bacterial counts were expressed as colony-forming units per gram of tissue (CFU/g). To represent a positive culture, 1,000 CFU/g and higher bacterial count in MLNs, liver, spleen, and pancreas samples were accepted.
Histologic Examination
Pancreatic tissue samples from the same anatomical location in each rat, including the main pancreatic duct, were fixed with formalin, and thick sections were prepared. After being stained with hematoxylin and eosin (H&E), two pathologists, who were kept unaware of the source of specimens, scored the tissues regarding edema, acinar necrosis, inflammatory infiltrate, hemorrhage, fat necrosis, and perivascular inflammation in 20 fields. The scores for each histological examination were summed, yielding a maximum score of 24, as defined by Schmidt et al. [14]. H&E-stained small bowel sections were examined with standard light microscopy to evaluate morphologic alterations following AP.
Statistical Analysis
Serum amylase concentrations and the severity of inflammatory changes (histopathologic scores) were compared using one-way analysis of variance. A post hoc Tukey’s test was used for paired comparison of the groups. The Kruskal–Wallis test was used to determine whether the frequency of bacterial translocation significantly differed among the groups. Dunn’s multiple-comparison test was used to compare each possible pair of groups. Spearman rank correlation coefficients were computed for linear correlation analyses. A p < 0.05 was considered statistically significant. All statistical measurements were done using SPSS PC 11.0 (SPSS Inc. Chicago, IL, USA).
Results
As shown in Table 1, increased serum amylase levels and histopathological scores of pancreatic tissue confirmed AP in groups 2 and 4, whereas they confirmed the absence of AP in groups 1 and 3. Serum amylase levels in group-1 were significantly lower than those in groups 2 and 4 (p = 0.003 and p = 0.005, respectively). Serum amylase levels in group-3 were significantly lower than those in group-4 (p = 0.04). On the other hand, there was no significant difference between groups 2 and 4 in terms of serum amylase levels (p > 0.05).
Table 1.
Serum amylase levels (mean value ± standard deviation)
| Groups | Amylase (U/lt) (mean ± standard deviation) | P value | |
|---|---|---|---|
| 1 | 2,269 ± 593 | Control vs AP | p = 0.003a |
| Control vs colectomy | p = 0.317 | ||
| Control vs Colectomy + AP | p = 0.005a | ||
| 2 | 7,648 ± 5,589 | AP vs colectomy | p = 0.047a |
| 3 | 4,097 ± 2,346 | Colectomy vs Colectomy + AP | p = 0.04a |
| 4 | 7,649 ± 4,965 | AP vs colectomy + AP | p > 0.05 |
aStatistically significant
Bacterial Overgrowth in the Small Intestine
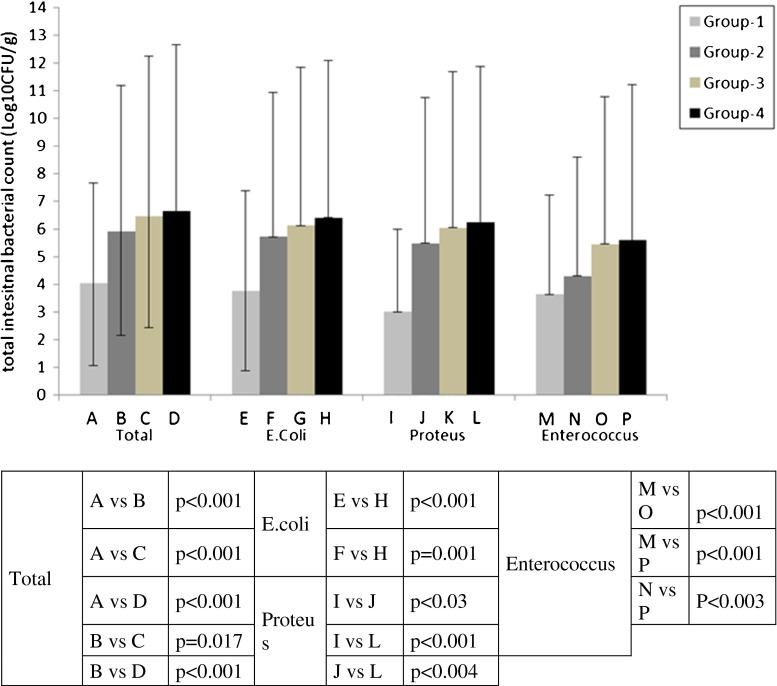
Data related to total number of bacteria in the small intestine is shown in Fig. 1. In group-1, the count of bacteria in the small intestine was found lower than that in group-2, and the difference between these groups was statistically significant (p < 0.001). AP caused significant bacterial overgrowth in the proximal jejunum when group-2 was compared with group-1. This overgrowth was mainly prominent for gram-negative bacteria such as Proteus mirabilis (p < 0.03).
Fig. 1.
The comparison between the groups, in terms of bacterial overgrowth in the small intestine
The number of bacteria was found higher in group-3 than in group-1 (p < 0.001). Significant increase was detected in all types of bacteria. Although the total number of gram-negative and gram-positive bacteria (Escherichia coli, P. mirabilis, Enterococcus) was higher in group-3 than in group-1, there was no superiority for both gram-negative and gram-positive bacteria. Like AP, colectomy caused significant bacterial overgrowth in the proximal jejunum.
The increase in the number of small intestine bacteria was higher in group-4 than in group-2 (p < 0.001). The number, especially, of gram-negative bacteria such as E. coli, P. mirabilis, and gram-positive Enterococcus was higher in group-4 than in group-2 (respectively p = 0.001, p < 0.004, p < 0.003).
Bacterial Translocation (The Amount of Bacteria in the Other Abdominal Organs)
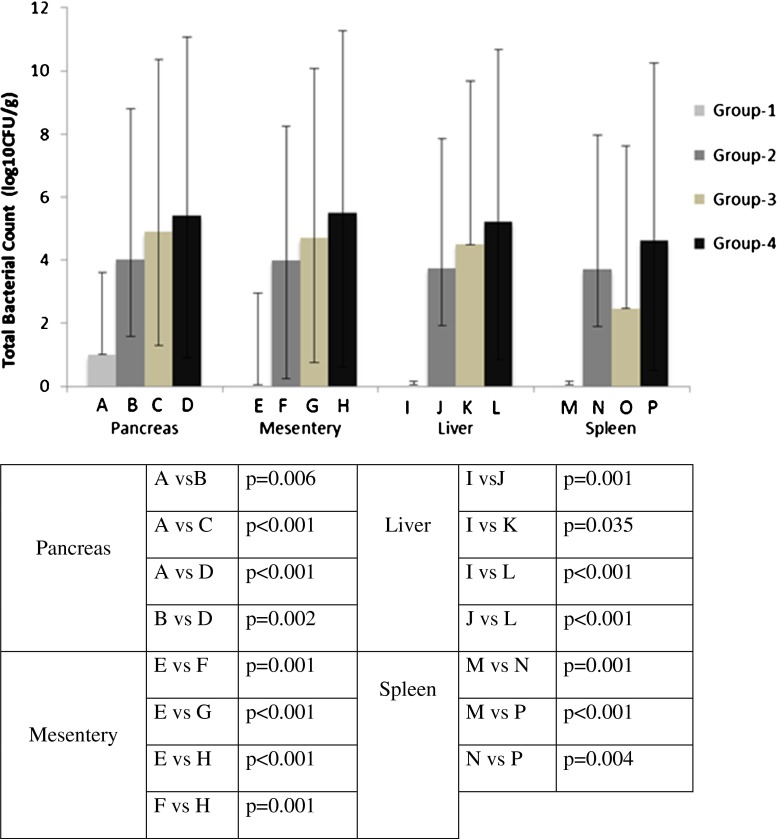
Total number of bacteria isolated from pancreas, MLNs, liver, and spleen is summarized in Fig. 2. While bacterial translocation to pancreas, MLN, liver, and spleen was not detected in group-1, it was detected in group-2. The difference between the two groups was statistically significant (p = 0.006, p = 0.001, p = 0.001, p = 0.001, respectively). In group2, gram-negative microorganisms especially (P. mirabilis was the leading microorganism) were the most commonly isolated bacteria. When compared with group-2, group-4 had a higher rate of bacterial translocation to the pancreas, MLNs, liver, and spleen (p = 0.002, p = 0.001, p = 0.001, p = 0.004, respectively). In group-4, when compared with group-2, the count especially of P. mirabilis increased significantly in the MLNs. As for liver and spleen cultures, E. coli and P. mirabilis were the prominent bacteria in group-4. On the other hand, there was no significant increase for the gram-positive Enterococcus in all culture materials in group-4.
Fig. 2.
The comparison of bacterial translocation to abdominal organs
Although median values were higher in group-4 than in group-3 in terms of bacterial translocations to pancreas, MLN, liver- and spleen, the differences between the two groups were not statistically significant. When compared with group-3, the counts of gram-negative microorganisms such as P. mirabilis on abdominal organs were higher, and the difference was statistically significant in group-4 (p = 0.043, p = 0.009, p = 0.019, p = 0.002, respectively).
There was no significant difference between groups 2 and 3 in terms of bacterial translocations to abdominal organs. Colectomy quantitatively caused greater translocation than pancreatitis.
Morphological Changes in the Small Intestine
Morphological study of the distal small bowel showed significant changes in the animals with AP (groups 2 and 4) when compared with controls. AP was associated with damage to the apical portion of the villi and alteration of the mucosal microvasculature.
The Correlation between Bacterial Translocation and Bacterial Overgrowth
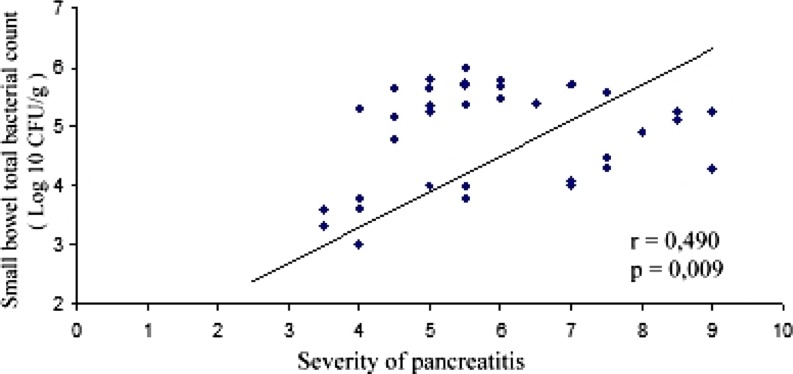
Close correlation between the intestinal bacterial overgrowth and severity of pancreatitis was observed with the help of Spearman’s correlation test (r = 0.490, p = 0.009; Fig. 3). In addition, a significant correlation between the severity of AP and total number of bacteria in pancreas was present (r = 0.399, p = 0.008).
Fig. 3.
The correlation between the scores of acute pancreatitis (AP) and the count of bacteria in the small intestine
Bacterial Translocation Occurring within the Blood and Peritoneum
Numerical results were shown in Table 2. In groups 2–4, abundant positive cultures were detected. While significant differences were observed (p < 0.05) when group-1 was compared with other groups (groups 2–4), there was no such significant difference between other groups. Bacteria cultured in group-2 were predominantly gram-negative P. mirabilis, and also, they were the same ones cultured in the pancreas and small bowel.
Table 2.
Bacterial translocation occurring within the blood and peritoneum
| Group-1 (n = 10) | Group-2 (n = 12) | Group-3 (n = 10) | Group-4 (n = 10) | |
|---|---|---|---|---|
| Blood | 3 | 7 | 6 | 9 |
| Peritoneum | 1 | 6 | 3 | 9 |
Discussion
The infectious complications associated with severe pancreatitis constitute the major source of morbidity and mortality in this patient population, and the prevention of these complications has become a targeted therapy in the current management of the disease. Although the routes of bacterial translocation in the course of AP have not yet been clarified, several different routes have been described. They include three main pathways: lymphatic or hematogenous spread, and direct transmural migration to the peritoneal cavity or retroperitoneum. Webster et al. showed bacteremia occurring early after induction of AP in CDD-induced AP, suggesting a hematogenous route [15]. On the other hand, Runkel et al. found bacteria migrating to regional lymph nodes before translocation to distant sites in a duct ligation model, suggesting a lymphogenous route [16]. Widdison et al. suggested transperitoneal translocation of bacteria originating from the colon in a feline model of severe necrotizing pancreatitis [17]. These findings have directed efforts for many different prophylactic and therapeutic strategies. Our findings were supported by mainly lymphogenous spreading theory. In groups 2 and 4, bacterial translocation was observed in the majority of MLNs, pancreas, liver, and spleen samples at the end of 48 h. Culture of enteric bacteria in the pancreatic tissue 48 h later from the development of pancreatitis supported the idea that pancreatic tissue was being infected in the early stages of AP in animal models. Whereas intensive bacterial invasion was noted in tissue samples, bacterial translocation to blood and peritoneal fluid were not found to be less. Comparison of tissue and blood-peritoneal samples suggested that the hematogenous and transperitoneal spread was present, but it played a lesser and delayed role in development of pancreatic tissue infection.
In the course of AP, because of reduced peristalsis and ischemic changes, bacterial overgrowth occurs in the proximal small intestine, and gram-negative microorganisms translocate into MLNs and pancreas [18, 19]. In our study, MLNs cultures were positive in almost all cases. These findings support the idea that MLNs are the primer stations for bacterial translocation to the pancreas. Additionally, infection of blood and peritoneum shows us that hematogenous spread is another important secondary route. On the contrary, Cicalese et al. reported that bacterial translocation to MLNs did not correlate with the severity of pancreas infection at the end of 24 h [18]. Data obtained from their study revealed that MLNs were non-specific stations during AP. Similarly, some investigators have previously shown that positive MLN cultures have also occurred in physiologic conditions and may not necessarily represent a pathogenic condition [20]. Data collected from our study showed that MNLs were important in the development of pancreatic infection in the course of AP.
Most investigators have accepted colon as the primary source of bacteria translocating to pancreas. Therefore, many studies have been conducted to evaluate the impact of reduction of colonic flora [21–24]. Specific colonic cleansing by large bowel enemas or colonic irrigation after induction of AP has been shown to reduce bacterial translocation to MLNs and liver, but it had no effect on pancreatic infection rates and mortality [9, 10]. Selective decontamination of gastrointestinal system, by using a combination of oral and intravenous antibiotics, has been reported to decrease the incidence of sepsis and the related mortality [23–26]. Widdison et al. created a model of AP in cats by giving a certain amount of E. coli into the pancreas, colon, and bile duct [27]. At the same time, to prevent bacterial translocation, transverse colon was isolated in an impermeable plastic sac during experimental AP. They observed that isolation of transverse colon with an impermeable plastic sac prevented transmural spread of bacteria to the pancreas. This study confirmed the hypothesis that the colon was the main source of translocating bacteria.
In humans, bacterial translocation during AP is a local phenomenon rather than systemic. Hence, the use of intraluminal non-absorbable antibiotics targeting intestinal bacteria seems more reasonable than systemic therapy [28]. Enteral nutrition that increases the motility may have inhibitory effects on intestinal bacterial overgrowth [29–33]. Our study showed that total colectomy, in the setting of experimental pancreatitis, did not prevent bacterial translocation and infection of pancreatic necrosis. On the contrary, total colectomy caused bacterial colonization of the ileum as well as the duodenum with colonic-type bacteria, including gram-negative rods (E. coli, Proteus spp.) and anaerobes. Despite the studies pointing out colonic microflora as the main source of pancreatic infection during AP, our study showed that elimination of colonic flora itself did not prevent the bacterial translocation to the MLN; it also caused bacterial overgrowth in the small intestine, and this overgrowth indeed also had an important role on pancreatic infection.
As a conclusion, in AP, total colectomy induced small bowel bacterial overgrowth, which was associated with increased bacterial translocation to the pancreas.
Acknowledgments
Conflict of Interest Statement
None of the authors have a conflict of interest to disclose.
References
- 1.Sekimoto M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, et al. JPN Guidelines for the management of acute pancreatitis: epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:10–24. doi: 10.1007/s00534-005-1047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller BJ, Henderson A, Strong RW, Fielding GA, DiMarco AM, O’Loughlin BS. Necrotizing pancreatitis: operating for life. World J Surg. 1994;18:906–911. doi: 10.1007/BF00299103. [DOI] [PubMed] [Google Scholar]
- 3.Bradley EL. A fifteen year experience with open drainage for infected pancreatic necrosis. Surg Gynecol Obstet. 1993;177:215–222. [PubMed] [Google Scholar]
- 4.Banks PA, Freeman ML. Practice parameters committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 5.Gloor B, Muller CA, Worni M, et al. Late mortality in patients with severe acute pancreatitis. Br J Surg. 2001;88:975–979. doi: 10.1046/j.0007-1323.2001.01813.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmid SW, Uhl W, Friess H, et al. The role of infection in acute pancreatitis. Gut. 1999;45:311–316. doi: 10.1136/gut.45.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dervenis C, Smailis D, Hatzitheoklitos E. Bacterial translocation and its prevention in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2003;10:415–418. doi: 10.1007/s00534-002-0727-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Valente JF, Alexander JW. The effect of sennosides on bacterial translocation and survival in a model of acute hemorrhagic pancreatitis. Pancreas. 1999;18:39–46. doi: 10.1097/00006676-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Sahin M, Yol S, Ciftci E, et al. Does large-bowel enema reduce septic complications in acute pancreatitis? Am J Surg. 1998;176:331–334. doi: 10.1016/S0002-9610(98)00199-8. [DOI] [PubMed] [Google Scholar]
- 10.Sulkowski U, Boin C, Brockmann J, et al. The influence of caecostomy and colonic irrigation on pathophysiology and prognosis in acute experimental pancreatitis. Eur J Surg. 1993;159:287–291. [PubMed] [Google Scholar]
- 11.Yamanel L, Mas MR, Comert B, Isik AT, Aydin S, Mas N, Deveci S, Ozyurt M, Tasci I, Unal T. The effect of activated protein C on experimental acute necrotizing pancreatitis. Crit Care. 2005;9:184–190. doi: 10.1186/cc3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Minnen LP, Timmerman HM, Lutgendorff F, et al. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery. 2007;141:470–480. doi: 10.1016/j.surg.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Simsek I, Mas MR, Yasar M, Ozyurt M, Saglamkaya U, Deveci S, Comert B, Basustaoglu A, Kocabalkan F, Refik M. Inhibition of inducible nitric oxide synthase reduces bacterial translocation in a rat model of acute pancreatitis. Pancreas. 2001;23:296–301. doi: 10.1097/00006676-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:1. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster MW, Pasculle AW, Myerowitz RL, Rao KN, Lombardi B. Postinduction bacteremia in experimental acute pancreatitis. Am J Surg. 1979;138:418–420. doi: 10.1016/0002-9610(79)90276-9. [DOI] [PubMed] [Google Scholar]
- 16.Runkel NS, Rodriguez LF, Moody FG. Mechanisms of sepsis in acute pancreatitis in opossums. Am J Surg. 1995;169:227–232. doi: 10.1016/S0002-9610(99)80142-1. [DOI] [PubMed] [Google Scholar]
- 17.Widdison AL, Karanjia ND, Reber HA. Routes of spread of pathogens into the pancreas in a feline model of acute pancreatitis. Gut. 1994;35:1306–1310. doi: 10.1136/gut.35.9.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cicalese L, Sahai A, Sileri P. Acute pancreatitis and bacterial translocation. Dig Dis Sci. 2001;46:1127–1132. doi: 10.1023/A:1010786701289. [DOI] [PubMed] [Google Scholar]
- 19.Van Felius D, Akkermans LMA, Bosscha K, et al. Interdigestive small bowel motility and duedonal bacterial overgrowth in experimental acute pancreatitis. Neurogastroenterol Motil. 2003;15:267–276. doi: 10.1046/j.1365-2982.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 20.Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol. 1999;473:11–30. doi: 10.1007/978-1-4615-4143-1_2. [DOI] [PubMed] [Google Scholar]
- 21.Medich DS, Lee TK, Melhem MF, et al. Pathogenesis of pancreatic sepsis. Am J Surg. 1993;165:46–50. doi: 10.1016/S0002-9610(05)80403-9. [DOI] [PubMed] [Google Scholar]
- 22.Runkel NS, Moody FG, Smith GS, et al. The role of the gut in the development of sepsis in acute pancreatitis. J Surg Res. 1991;51:18–23. doi: 10.1016/0022-4804(91)90064-S. [DOI] [PubMed] [Google Scholar]
- 23.Takeda K, Matsuno S, Sunamura M, Kakugawa Y. Continuous regional arterial infusion of protease inhibitor and antibiotics in acute necrotizing pancreatitis. Am J Surg. 1996;171:394–398. doi: 10.1016/S0002-9610(97)89617-1. [DOI] [PubMed] [Google Scholar]
- 24.Isaji S, Suzuki M, Frey CF, et al. Role of bacterial infection in diet-induced acute pancreatitis in mice. Int J Pancreatol. 1992;11:49–57. doi: 10.1007/BF02925994. [DOI] [PubMed] [Google Scholar]
- 25.Araida J, Erey CF, Ruebner B, et al. Therapeutic regimens in acute experimental pancreatitis in rats: effects of a protease inhibitor, a beta-against and antibiotics. Pancreas. 1995;11:132–140. doi: 10.1097/00006676-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Mithofer K, Fernandez del Castillo C, Ferraro MJ, et al (1996) Antibiotic treatment improves survival in experimental acute necrotizing pancreatitis. Gastroenterology 110:232–40 [DOI] [PubMed]
- 27.Widdison AL, Alvarez C, Chang YB, Karanjia ND, Reber HA. Sources of pancreatic pathogens in acute pancreatitis in cats. Pancreas. 1994;9:536–541. doi: 10.1097/00006676-199407000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Marotta F, Geng TC, Wu CC. Bacterial translocation in the course of acute pancreatitis: beneficial role of nonabsorbable antibiotics and lactitol enemas. Digest. 1996;57:446–452. doi: 10.1159/000201373. [DOI] [PubMed] [Google Scholar]
- 29.Meier RF, Beglinger C. Nutrition in pancreatic diseases. Best Pract Res Clin Gastroenterol. 2006;20:507–529. doi: 10.1016/j.bpg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Imrie CW, Carter CR, McKay CJ. Enteral and parenteral nutrition in acute pancreatitis. Best Pract Res Clin Gastroenterol. 2002;16:391–397. doi: 10.1053/bega.2002.0314. [DOI] [PubMed] [Google Scholar]
- 31.Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ. 2004;328:1407. doi: 10.1136/bmj.38118.593900.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakorafas GH, Lappas C, Mastoraki A, Delis SG, Safioleas M. Current trends in the management of infected necrotizing pancreatitis. Infect Disord Drug Targets. 2010;10:9–14. doi: 10.2174/187152610790410936. [DOI] [PubMed] [Google Scholar]
- 33.Hidehiro S, Takashi U, Yoshifumi T, Takeo Y, Makoto S, Naoki M, et al. Treatment outcome of selective digestive decontamination and enteral nutrition in patients with severe acute pancreatitis. J Hepatobiliary Pancreat Surg. 2007;14:503–508. doi: 10.1007/s00534-007-1216-7. [DOI] [PubMed] [Google Scholar]