Abstract
Porcine acellular dermal collagen (PDC), which is a biological material derived from processing porcine dermis, has already been used for urologic, gynecologic, plastic, and general surgery procedures up to now. The aim of this study is to investigate the effectiveness of PDC on wound healing as a dermal substitute in the rat model. Twenty Wistar albino rats were divided into two groups. Standard full-thickness skin defects were created on the back of the rats. In the control group (Group 1), the dressings moisturized with saline were changed daily. In the study group (Group 2), porcine dermal collagen was implanted onto each wound and fixed with 4–0 polypropylene sutures. Contraction percentages of wound areas were calculated on the third, seventh, tenth, and fourteenth days by using the planimetric program. On fourteenth day, the wound areas were excised for histopathological examination, inflammatory scoring, and evaluation of collagen deposition. The study group was superior to the control group in terms of inflammatory scoring, type I/type III collagen ratio, and wound contraction rates. Porcine dermal collagen may be used effectively and safely on full-thickness wounds as a current dermal substitute.
Keywords: Porcine dermal collagen, Natural biological materials, Dermal substitutes, Wound healing
Introduction
The process of wound healing is a progressive and dynamic event with predictable stages that occur with varying intensities [1]. Most skin wounds can heal naturally. The quality of skin wound healing can be improved by the application of scaffolds as skin replacement materials [2]. Many are based on artificial materials such as polylactic or polyglycolic acid. Alternatively, biologically derived materials such as deepithelialized dermis, collagen-based, or fibrin-based materials have been developed as wound repair biomaterials [3].
Porcine acellular dermal collagen (PDC) implant is a biologic material derived from processing porcine dermis. Up to the present day, it has been used for various urologic, gynecologic, plastic and reconstructive surgery, and general surgery procedures, especially for different hernia repairs.
This study aimed to investigate the effectiveness of PDC on dermal wound healing in the rat model.
Materials and Methods
Animals
Twenty healthy adult male Wistar albino rats weighing 200–250 g were used. All the rats were obtained from our animal research center. The rats were housed in stainless-steel cages in an animal room maintained at 22 °C with 12-h dark–light periods. All were fed with the same amount of a laboratory pellet diet and water ad libitum and fasted for 12 h before the procedures. The procedures in this experimental study were performed in accordance with the National Guidelines for the Use and Care of Laboratory Animals and approved by the Animal Ethics Committee of Ankara Research and Training Hospital.
Surgical Technique
Two groups were randomly constituted with ten rats for each. The rats were anesthetized with intraperitoneal injections of 25 mg/kg ketamine hydrochloride (Ketalar, Parke-Davis, Eczacibasi, Istanbul, Turkey). The operation sites were shaved and disinfected with povidone-iodine. A 2 × 1-cm rectangular-shaped incision was performed on the back of the rats centered on the midline, and then standard full-thickness skin defect, including panniculus carnosus, was created on this site. In the control group (group 1), no intervention was made on dermal wounds. The wounds were cleaned daily with saline and covered with moisturized dressings. In the study group (group 2), PDC was implanted on each wound. Each side of the scaffold overlapped the edge of the defect, and it was fixed with 4–0 polypropylene interrupted sutures. PDC implant was removed to limit the contraction on the third postoperative day. The wound was daily irrigated with saline and covered with moisturized dressings. All wounds were followed for 14 days, and no complication developed during this period. Open wounds were drawn on acetate paper with a marker pen on the third, seventh, tenth, and fourteenth days and photographed with a digital camera. The surface area of wounds was measured with planimetric program on the computer by scanning the acetate sheets. Percentage of contraction was calculated by the following formula:
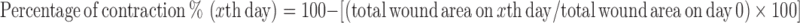 |
Total wound area on xth day was contraction percentage of wound area obtained by planimetric program on the third, seventh, tenth, and fourteenth days. The original wound area on day 0 was assumed to be 100 % for all wounds.
Histopathological Analysis
All rats were sacrificed with high-dose ketamine hydrochloride on postoperative 14th day. The wound area was excised en bloc together with the scar tissue. All specimens were fixed in 10 % phosphate-buffered formaldehyde solution for 24 h at room temperature. Histopathological assays were performed in a blind manner by a pathologist. Specimens were washed in tap water and dehydrated through graded alcohol series. After passing through the routine histological series, tissues were embedded in paraffin blocks. Five-micrometer sections were cut, deparaffinized, and rehydrated. Sections were counterstained with hematoxylin and eosin (H&E), Masson trichrome, and reticulin stain (method of silvering). The intensities of polymorphonuclear leukocytes and mononuclear leukocytes and the degrees of fibroblast proliferation and vascular proliferation were evaluated by inflammatory scoring to determine the general characteristics of scar tissue in the sections stained with H&E. The qualitative assessment of total collagen deposition was performed by using the Mason trichrome stain. The collagen fibers were identified as blue color stained with Mason trichome. In reticulin stain, the fibers observed in the form of thin black fibers were determined as type III and yellow fibers as type I collagen.
The number of polymorphonuclear leukocyte and mononuclear leukocyte, and the degrees of vascular proliferation and fibroblast proliferation were measured by numerical scale from 0 to 3 for determination of inflammatory scores.
The structural density of collagen was scored by numerical scale from 1 to 5: (0) indicates the lack of collagen; (1) indicates the presence of collagen in the form of single fiber; (2) indicates the presence of collagen in the form of a few fibers; (3) indicates more intense but loose collagen; (4) indicates that collagens overlay a microscopic field but have gaps between them; and (5) indicates that collagens overlay a microscopic field and have very dense structure.
Statistical Analysis
Multiple comparisons between the groups were performed with one-way analysis of variance and post hoc tests. Differences between the groups were analyzed with the Mann–Whitney U test. Statistical analysis was performed with the SPSS 15.0 for Windows (SPSS Inc., Chicago). Values of p < 0.05 were considered to be significant.
Results
In the study group, when Permacol® implant was removed from the wound base on the third postoperative day, it was seen that the wounds had been filling up with pink granulation tissue. On 14th day, the wound closure had almost been completed. In the control group, the granulation tissue had been slowly moved from the wound base to the wound surface.
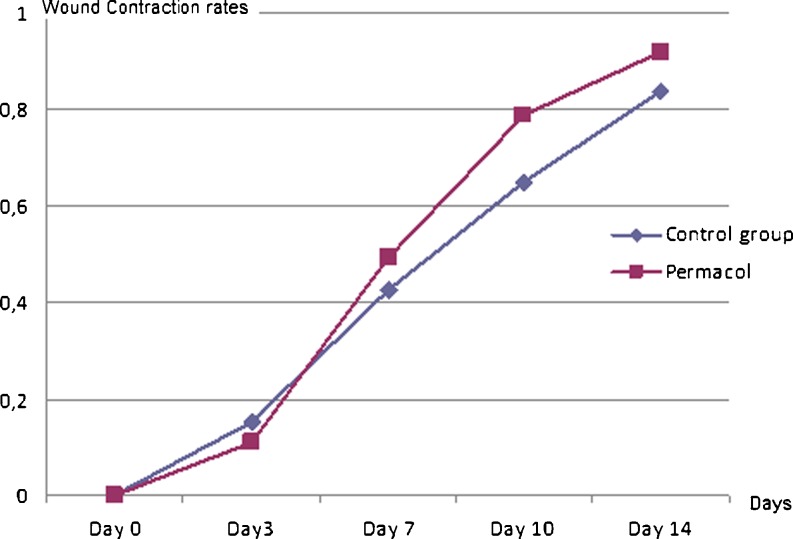
The wound contraction progressed more slowly in control group. Wound contraction rates were evaluated in the form of mean and standard deviation. The wound contraction rates were 0.92 ± 0.01 in the study group and 0.836 ± 0.02 in the control group. As a result of statistical analysis, wound contraction rate was significantly higher in study group than the control group during and at the end of the experiment (p < 0.05) (Table 1 and Fig. 1). Wound healing was better in study group than in control group on third, seventh, and fourteenth postoperative days (Figs. 2, 3, and 4).
Table 1.
Wound contraction rates of the groups (%)
| Days | Groups | Mean (%) |
|---|---|---|
| Day 0 | Control | 0 |
| Permacol® | 0 | |
| Day 3 | Control | 15.1 |
| Permacol® | 10.9 | |
| Day7 | Control | 42.5 |
| Permacol® | 49.3 | |
| Day 10 | Control | 64.8 |
| Permacol® | 78.7 | |
| Day14 | Control | 83.6 |
| Permacol® | 92.0 |
Fig. 1.
The comparison of wound contraction rates between the groups
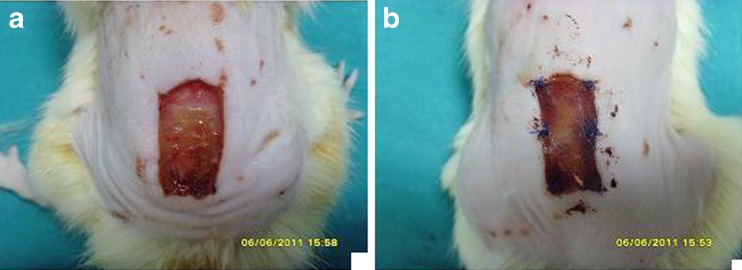
Fig. 2.
The appearance of the wounds on third postoperative day (control group (a), Permacol® group (b))
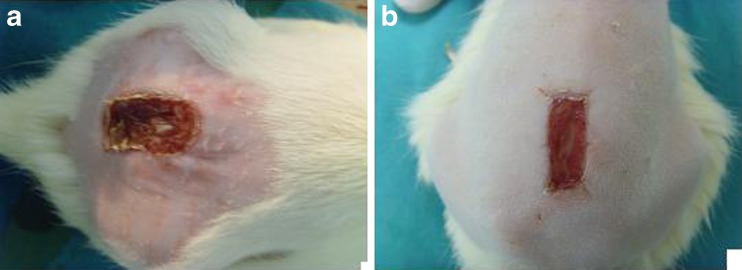
Fig. 3.
The appearance of the control (a) and Permacol® (b) groups on seventh postoperative day
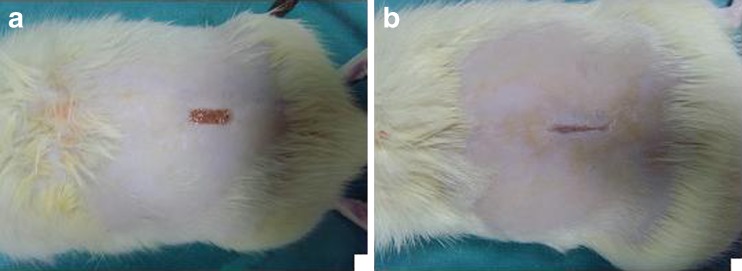
Fig. 4.
The appearance of the wounds on fourteenth postoperative day (control group (a), Permacol® group (b))
The mean inflammatory scores of the groups were given in Table 2. According to these scores, a significant difference was found between the control and study groups (p < 0.05). The scores were better in study group than in the control group.
Table 2.
Mean histological scores of the groups
| Groups | PMNL | MNL | Vascular proliferation | Fibroblast proliferation |
|---|---|---|---|---|
| Control | 0.30 ± 0.18 | 0.90 ± 0.56 | 2.20 ± 0.63 | 2.00 ± 0.66 |
| Permacol | 0.80 ± 0.42a | 1.50 ± 0.70a | 2.90 ± 0.31a | 2.80 ± 0.42a |
aSignificantly different when compared with control group
The mean collagen scores and type I/type III collagen ratios of the groups were given in Table 3. When these values were compared statistically, significant difference was found between the groups (p < 0.05). The collagen scores and type I/type III collagen ratios were higher in study group than in control group.
Table 3.
Mean collagen scores and type I/type III collagen ratios of the groups
| Groups | Collagen scores | Type I/type III ratios |
|---|---|---|
| Control | 2.40 ± 0.51 | 8,33 |
| Permacol | 3.40 ± 0.51a | 15.00a |
aSignificantly different when compared with control group
Discussion
The process of wound healing is a progressive and dynamic event with predictable stages. Wound healing comprises four primary stages that occur in a sequential cascade of overlapping processes: hemostasis, inflammation, proliferation, and remodeling [1]. Most of skin wounds can heal naturally, but additional surgery necessitates immediate coverage using skin substitutes to aid repair and regeneration when extensive or irreversible damages to skin are caused [4].
There is a wide range of materials being used as dermal substitutes. The materials should protect wounds from infection and fluid loss. The material should be stable enough to function as a provisional matrix and should not elicit immunogenic reactions. Composition, pore size, and degradability of the substitute should support cell migration and function [2]. Commercially available natural biological materials originate four types of tissue: acellular bovine pericardium, human cadaveric dermis, porcine small intestinal submucosa, cross-linked PDC, and non–cross-linked porcine dermal materials [5].
PDC is a biomaterial derived from porcine dermis by enzymatic and chemical removal of cellular components, leaving a cross-linked collagen and its constituent elastin-rich matrix [6]. This architecture very closely resembles the human tissue. A precisely controlled degree of cross-linking is introduced into the structure, making it resistant to the collagenase enzymes responsible for the breakdown and resorption of implanted collagen. Because the biomaterial is acellular, it contains no substance capable of provoking an immunogenic reaction, which is a very important feature in a product designed for implantation into human tissue. PDC implant is able to support host cell infiltration and revascularization, and within a few months, it becomes an integral part of the body. As such, it gradually becomes permanently incorporated into the surrounding tissue, providing strength and contour support [7]. However, in our study, PDC implant was removed on the third postoperative day to limit the contraction. Therefore, we could not evaluate the incorporation of the implant into the surrounding tissue.
Zheng et al. [8] showed that PDC induced a milder inflammatory response, less adhesion formation, more orderly collagen deposition, and neovascularization than polypropylene mesh and reached a comparable tensile strength in 90 days. PDC demonstrated essential properties of an ideal hernia repair material: low inflammation, less elastin, lower adhesion rates, and more contracture (thus, less laxity). Furthermore, it demonstrated near-equivalent result in the categories of vascularity, integration, and peak tensile strength when compared with well-studied human acellular product [9]. Di-isocyanate cross-linking of PDC prevents biodegradation and mineralization in the presence of infection. Furthermore, systemic antibiotic can easily reach the implant because of neovascularization and thereby helps eradicate infective organisms in the implant. This property allows its use in contaminated wounds [10]. It is non-allergic, non-antigenic, and entirely biocompatible. For that reason, it has been used successfully in many different ways, including abdominal wall hernia repairs, incisional and parastomal hernias, as well as for pelvic floor surgery [11]. In an experimental porcine model of fistula-in-ano, Himpson et al. [12] concluded that, when the fistula tract was completely removed and durable infill material used, it was possible to treat fistulas successfully even in presence of infection or contamination. The same authors suggested that PDC provided tissue repair regeneration with stability and facilitated successful wound healing because of the cross-linked nature of the product.
The histological results of the majority of the studies in which PDC implant was used exhibited minimal inflammatory response, whereas two studies showed moderate to strong inflammatory response [5]. The polymorphic neutrophils migrate to the site of local injury. These inflammatory cells phagocytize bacteria and assist in the removal of devitalized tissue [1]. During this process, especially in proliferation phase, monocytes differentiate into macrophages, which then release numerous growth factors responsible for angiogenesis and granulation tissue formation. The macrophages are responsible for amplifying, coordinating, and sustaining the wound healing response [1]. In our study, mean histological scores of both polymorphonuclear and mononuclear leukocytes were higher in the study group than in the control group.
Vascularization of dermal substitutes is critical for high take rate of these substances [2]. The application of collagen/elastin scaffolds showed increased vascularization 1 week after wounding in a porcine excisional wound model [13]. Implant infiltration by endothelial cells and subsequent vessels formation is a favorable mesh attribute [5]. In our study, vascularization scores were higher in the study group in the control group.
Fibroblasts are a heterogeneous population of cells found in numerous tissues and are of mesenchymal origin. Dermal fibroblasts have numerous functions, only in synthesizing and depositing extracellular matrix components, but also proliferation and migration in response to chemotactic, mitogenic, and modulatory cytokines [14]. The fibroblast proliferation scores were also higher in the study group than in the control group.
Collagen, which is beneficial for endoepidermal growth to promote healing, is a major functional extracellular matrix protein of the dermal layer of skin [15]. Type I collagen is the predominant collagen of the dermis and forms collagen fibers that maintain dermal configuration [16]. Collagen matrices have been shown to promote fibroblast repopulation in a controlled way in the wound area, decreasing wound contraction and scarring [17]. In our study, the collagen scores and type I/type III collagen ratios were higher in the study group than in the control group.
All these parameters that were important for effective wound healing, including polymorphonuclear leukocyte, mononuclear leukocyte, vascularization, and fibroblast proliferation were better in the study group than in the control group. Therefore, we concluded that the positive effects of PDC on wound healing might be attributable to these useful histopathological alterations.
PDC is non-allergenic, non-antigenic, and entirely biocompatible [11]. Because of smooth surface and lack of foreign body reaction, PDC can be placed in contact with the bowel and with adipose tissue. It does not facilitate the formation of a biofilm and thus is ideal for use in operations with a high risk of infection. Systemic antibiotics will have access to the infection site via blood vessels growing through this material [7].
Because of the above-mentioned effects and advantages of PDC on wound healing and abdominal wall hernia repair, PDC may effectively and safely be used on full-thickness wounds as a current dermal substitute. Further studies are needed to completely evaluate the effects of PDC on wound healing.
References
- 1.Brissett AE, Hom DB. The effects of tissue sealants, platelet gels, and growth factors on wound healing. Curr Opin Otolaryngol Head Neck Surg. 2003;11:245–250. doi: 10.1097/00020840-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Van der Veen VC, Boekema BK, Ulrich MM, Middelkoop E. New dermal substitutes. Wound Repair Regen. 2011;19:59–65. doi: 10.1111/j.1524-475X.2011.00713.x. [DOI] [PubMed] [Google Scholar]
- 3.Shevchenko RV, Sibbons PD, Sharpe JR, James SE. Use of a novel porcine collagen paste as a dermal substitute in full-thickness wounds. Wound Repair Regen. 2008;16:198–207. doi: 10.1111/j.1524-475X.2008.00360.x. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramani M, Kumar TR, Babu M. Skin substitutes: a review. Burns. 2001;27:534–544. doi: 10.1016/S0305-4179(01)00018-3. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou SA, Pointner R, Granderath FA. Hiatal hernia repair with the use of biologic meshes. Surg Laparosc Endosc Percutan Tech. 2011;21:1–9. doi: 10.1097/SLE.0b013e31820ad56c. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh FM, Giri SK, Durrani S, Waldron D, Grace PA. Experience with porcine acellular dermal collagen implant in one-stage tension-free reconstruction of acute and chronic abdominal wall defects. World J Surg. 2007;31:1966–1972. doi: 10.1007/s00268-007-9174-4. [DOI] [PubMed] [Google Scholar]
- 7.Catena F, Ansaloni L, Gazzotti F, Gagliardi S, Di Saverio S, D’Alessandro L, Pinna AD. Use of porcine dermal collagen graft (Permacol) for hernia repair in contaminated fields. Hernia. 2007;11:57–60. doi: 10.1007/s10029-006-0171-6. [DOI] [PubMed] [Google Scholar]
- 8.Zheng F, Lin Y, Verbeken E, Claerhout F, Fastrez M, De Ridder D, Deprest J. Host response after reconstruction of abdominal wall defects with porcine dermal collagen in a rat model. Am J Obstet Gynecol. 2004;191:1961–1970. doi: 10.1016/j.ajog.2004.01.091. [DOI] [PubMed] [Google Scholar]
- 9.Stanwix MG, Nam AJ, Hui-Chou HG, Ferrari JP, Aberman HM, Hawes ML, Keledjian KM, Jones LS, Rodriguez ED. Abdominal ventral hernia repair with current biological prostheses: an experimental large animal model. Ann Plast Surg. 2011;66:403–409. doi: 10.1097/SAP.0b013e3181e051ed. [DOI] [PubMed] [Google Scholar]
- 10.Harper C. Permacol: clinical experience with a new biomaterial. Hosp Med. 2001;62:90–95. doi: 10.12968/hosp.2001.62.2.2379. [DOI] [PubMed] [Google Scholar]
- 11.Sileri P, Franceschilli L, Del Vecchio BG, Stolfi VM, Angelucci GP, Gaspari AL. Porcine dermal collagen matrix injection may enhance flap repair surgery for complex anal fistula. Int J Colorectal Dis. 2011;26:345–349. doi: 10.1007/s00384-010-1066-7. [DOI] [PubMed] [Google Scholar]
- 12.Himpson RC, Cohen CR, Sibbons P, Phillips RK. An experimentally successful new sphincter-conserving treatment for anal fistula. Dis Colon Rectum. 2009;52:602–608. doi: 10.1007/DCR.0b013e31819ece3e. [DOI] [PubMed] [Google Scholar]
- 13.Lamme EN, de Vries HJ, van Veen H, Gabbiani G, Westerhof W, Middelkoop E. Extracellular matrix characterization during healing of full-thickness wounds treated with a collagen/elastin dermal substitute shows improved skin regeneration in pigs. J Histochem Cytochem. 1996;44:1311–1322. doi: 10.1177/44.11.8918906. [DOI] [PubMed] [Google Scholar]
- 14.Wong T, McGrath JA, Navsaria H. The role of fibroblasts in tissue engineering and regeneration. Br J Dermatol. 2007;156:1149–1155. doi: 10.1111/j.1365-2133.2007.07914.x. [DOI] [PubMed] [Google Scholar]
- 15.Medalie DA, Tompkins RG, Morgan JR. Evaluation of acellular human dermis as a dermal analog in a composite skin graft. Am Soc Artif Intl J. 1996;42:455–462. doi: 10.1097/00002480-199609000-00030. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Chen JY, Shang HT, Liu CE, Wang Y, Niu R, Wu J, Wei H. Light microscopic, electron microscopic, and immunohistochemical comparison of Bama minipig (Sus scrofa domestica) and human skin. Comp Med. 2011;60:142–148. [PMC free article] [PubMed] [Google Scholar]
- 17.Wehrhan F, Nkenke E, Melnychenko I, Amann K, Schlegel KA, Goerlach C, Zimmermann WH, Schultze-Mosgau S. Skin repair using a porcine collagen I/III membrane-vascularization and epithelization properties. Dermatol Surg. 2010;36:919–930. doi: 10.1111/j.1524-4725.2010.01569.x. [DOI] [PubMed] [Google Scholar]