Abstract
Drainage after laparoscopic cholecystectomy (LC) for acute calculous cholecystitis (ACC) is used without evidence of its efficacy. The present pilot study was designed to address this issue. After laparoscopic gallbladder removal, 15 patients were randomized to have a drain positioned in the subhepatic space (group A) and 15 patients to have a sham drain (group B). The primary outcome measure was the presence of subhepatic fluid collection at abdominal ultrasonography, performed 24 h after surgery. Secondary outcome measures included postoperative abdominal and shoulder tip pain, use of analgesics, and morbidity. Abdominal ultrasonography did not show any subhepatic fluid collection in eight patients (53.3 %) in group A and in five patients (33.3 %) in group B (P = 0.462). If present, median (range) subhepatic collection was 50 mL (20–100 mL) in group A and 80 mL (30–120 mL) in group B (P = 0.573). No significant differences in the severity of abdominal and shoulder pain and use of parenteral ketorolac were found in either group. Two biliary leaks and one subhepatic fluid collection occurred postoperatively. The present study was unable to prove that the drain was useful in LC for ACC, performed in a selected group of patients.
Keywords: Cholecystectomy, Laparoscopy, Drainage, Cholecystitis, Postoperative complications
Introduction
Laparoscopic cholecystectomy (LC) is the standard of care for the surgical treatment of acute calculous cholecystitis (ACC) [1]. The role of routine drainage after LC to decrease postoperative morbidity is still an issue of considerable debate. The main reason to use drains in LC is to avoid bile and blood collection requiring subsequent open procedures. In elective LC for nonacutely inflamed gallbladder, a Cochrane Database Systematic Review concluded that there is no evidence to support the use of drains [2]. Two recent randomized studies confirmed this conclusion [3, 4].
In a national survey over the surgical management of acute cholecystitis, the use of abdominal drainage was reported by a vast majority of the surgeons [5]. However, there is no evidence supporting the routine use of drains in LC for ACC, and further trials were claimed [2].
The goal of the present pilot study was to assess the role of drains in LC, performed for ACC. In particular, the efficacy of a drain in preventing postoperative abdominal fluid collections and improving surgery outcome was evaluated.
Materials and Methods
From September 2011 to March 2012, 45 patients aged 18 years and older were submitted to LC for ACC. Diagnosis of ACC was made according to the Tokyo criteria [6, 7]. ACC was suspected if sonographic findings of cholecystolithiasis or sludge, wall thickening ≥4 mm, and a positive sonographic Murphy sign were present. In addition to the sonographic findings, one of the following clinical criteria was required: epigastric or right upper quadrant pain, fever >38.0 °C, or a white blood cell count >10,000/mm3. Definitive diagnosis of ACC was made according to the macroscopic and histological examination of the gallbladder. Patients were excluded from randomization if: (1) they had symptoms present for >1 week, (2) gangrenous or emphysematous cholecystitis was present, (3) they had had previous upper abdominal surgery, (4) they had significant medical diseases that rendered them unfit for laparoscopic surgery, or (5) they had coexisting common bile duct stones with ductal dilatation, acute cholangitis, or acute pancreatitis. After approval by the local bioethics committee, informed consent was obtained. On admission, all patients were administered intravenous antibiotic therapy with amoxicillin clavulanate (Augmentin) 1 g IV every 8 h. Patients allergic to penicillin were treated with a combination of ciprofloxacin 400 mg IV every 12 h and metronidazole 500 mg IV every 8 h.
Surgical Management
All operations were performed by surgeons with a previous minimum experience of 50 LC. Under general anesthesia, the abdomen was insufflated with CO2 after the introduction of the first 10-mm trocar with the Hasson technique through an infraumbilical incision. The other 10-mm and two 5-mm trocars were inserted through appropriate subxiphoid, subcostal midclavicular, and subcostal anterior axillary incisions. The pneumoperitoneum pressure and CO2 flow rate were set at 10 mmHg and 2 L/min, respectively. A standard retrograde cholecystectomy with previous isolation and section between 10-mm clips of the cystic duct and artery was always performed. The gallbladder was always bagged and retrieved through the umbilical port. A bile sample was collected to perform cultural examination. Topical application of rifamycin on port wounds was performed at the end of operation and applied at 12, 24, 36, 48, and 72 h after LC. The duration of the operation (from infraumbilical skin incision to pulling off the trocars), bile spillage, and additional complications were also recorded.
Randomization
After gallbladder removal with a containing bag, the patients, who had no serious intraoperative complications, such as significant biliary and/or vascular injury or bleeding (>100 mL), were randomly allocated to undergo the placement of a drain in the subhepatic space (group A) or a sham drain (group B). Randomization was computer generated, using numbered and sealed envelopes, which were opened in the operating room at the end of surgery before drain fixation to the skin. The polyethylene, 5.7-mm, multiparous tube drain was threaded through the most lateral 5-mm trocar. In group B, after the surgeon inserted the drain, a nurse of the operating room pulled out the drain outside the port, shortened the tube, and fixed the end to the skin with a tape after blocking the tip with a bead. All drains in both groups were connected to a 500-mL reservoir. This way, the operator, the patients, and the assessors were blinded to the intervention.
Postoperative Monitoring
Patients were given a standard deep vein thrombosis prophylaxis. Postoperative antibiotic therapy was adjusted according to the results of the antibiogram. Postoperative pain was evaluated as follows: (1) parenteral diclofenac requirements were recorded after the patient was instructed to ask for pain relief liberally; (2) a visual analog scale [8] from 0 (no pain) to 10 (worst pain imaginable) was completed by each patient 24 h after surgery and at least 2 h after any eventual diclofenac assumption regarding either abdominal or shoulder pain. An abdominal ultrasonography was routinely performed on the first postoperative day with the goal to detect any fluid collection. If present, the volume of subhepatic collection was calculated. Ultrasound examinations were performed using an Aloka Prosound Alpha 10® with a 1- to 5-MHz convex probe by experienced radiologists.
The drain was removed 24 h after surgery, unless there was bile (any amount) or 100 mL of blood in the drain bag. In case the drain had to stay in place for bile leak, it was not removed, unless the leak had completely ceased. In case the drain had to stay in place for bleeding, it was removed when the amount was 100 mL/24 h and the patient was hemodynamically stable with stable hemoglobin (no decrease >1 g/dL). Intra-abdominal fluid collections >50 mL were followed up with serial ultrasonographic examinations, and patients were discharged if no increase was detected.
Postoperative problems and complications were recorded within 4 weeks after operation. Patients were reviewed at 1 and 4 weeks postoperatively. An upper abdomen ultrasonography was routinely performed 1 week after surgery. Outcome assessors were unaware of patients’ allocation.
Statistical Analysis
The primary outcome measure was the presence of subhepatic fluid collection at ultrasonographic examination of the abdomen 24 h after surgery. Secondary outcome measures included postoperative abdominal and shoulder tip pain, use of analgesics, and morbidity.
Fisher’s exact test was used for categorical data. The Mann–Whitney U test was used to compare not normally distributed samples. All tests were two tailed, and the level of significance was 0.05. All data were compiled by an independent participant unaware of patients’ allocation, and the results were analyzed using Medcalc® version 12.2 (Frank Schoonjanas, Broekstraat, Belgium).
Results
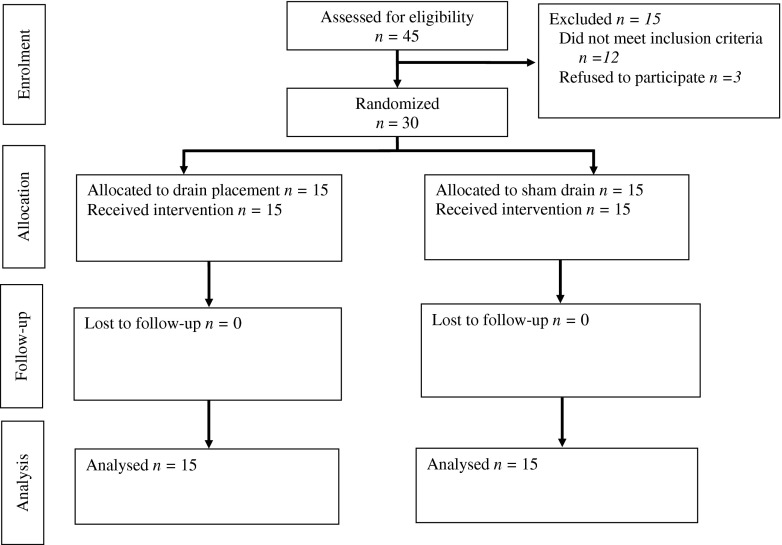
The profile of the trial is shown in Fig. 1. Both groups were comparable regarding sex, age, ASA, median operative time, and median postoperative hospital stay (Table 1). No significant intraoperative morbidity occurred.
Fig. 1.
Profile of the trial
Table 1.
Characteristics of patients
| Characteristic | Group A (n = 15) | Group B (n = 15) |
|---|---|---|
| Sex | ||
| M | 6 (40.0) | 4 (26.7) |
| F | 9 (60.0) | 11 (73.3) |
| Age median (range), years | 59.0 (36–84) | 67.5 (37–88) |
| ASA | ||
| I | 3 (20.0) | 6 (40.0) |
| II | 9 (60.0) | 8 (53.3) |
| III | 3 (20.0) | 1 (6.7) |
| Operative median time (range), min | 95.0 (72 to 120) | 91.0 (80.4 to 110) |
| Median (range) postoperative hospital stay, days | 3 (2–6) | 4 (2–5) |
Values are given as number (percentage) of patients unless otherwise indicated
Abdominal ultrasonography did not show any subhepatic fluid collection in eight patients (53.3 %) in group A and in five patients (33.3 %) in group B (P = 0.462, Fisher’s exact test). If present, median (range) subhepatic collection was 50 mL (20–100 mL) in group A and 80 mL (30–120 mL) in group B (P = 0.573; Mann–Whitney U test).
Median (range) abdominal pain scores 24 h after operation were 4 (2–5) in group A and 3 (2–3) in group B (P = 0.351; Mann–Whitney U test). Median (range) shoulder pain scores 24 h after operation were 1 (0–3) in group A and 0 (0–0) in group B (P = 0.232; Mann–Whitney U test). Median (range) parenteral ketorolac consumed was 120 mL (30–180 mL) in group A and 120 mL (30–120 mL) in group B (P = 0.643; Mann–Whitney U test).
Three (10.0 %) significant postoperative complications occurred. One patient in group A was readmitted on the sixth postoperative day because of abdominal pain with tenderness and fever. Ultrasound scan of the abdomen showed a 150-mL collection in the subhepatic region. A percutaneous drain was positioned under ultrasound scan guidance, documenting the presence of bile. Because of the persisting biliary leakage exceeding 50 mL/day, endoscopic retrograde cholangiography was performed 9 days after operation, documenting a duct of Luschka injury. An endoscopic stenting was performed. The patient improved subsequently, and the stent was removed after 4 weeks. Another patient in group A reported heaviness and abdominal pain without tenderness and fever on the seventh postoperative day. Ultrasonography of the abdomen showed subhepatic collection, and therapeutic aspiration was performed, documenting biloma. One patient in group B showed a 100-mL fluid collection in the subhepatic space at the control ultrasound scan performed 7 days after operation. No abdominal symptoms or signs were present. The patient was submitted to serial ultrasonographic examinations of the abdomen, documenting the progressive reduction in the collection, which disappeared 1 month after operation.
Discussion
In the setting of ACC, the main concern with LC is that the procedure carries a significant risk of complications. The rate of complications is related to the severity of the disease, defined as the presence of gangrenous and emphysematous gallbladder inflammation [9]. In particular, conversion rate is relevant with a threefold increase when severe cholecystitis is present [9]. Our study group includes selected patients without severe cholecystitis. Moreover, we only analyzed the results of patients in whom no important intraoperative complication occurred. However, we observed a significant rate of surgery-related complications. In a recent large survey, local complications occurred in 4.8 % of successfully performed LC for cholecystitis. In the subgroup requiring conversion, local complication rate was 26.7 % [5].
Traditionally, drains in LC are used for the early detection of bile leaks and any unsuspected hemorrhage and to evacuate abdominal fluid collections without the need for more invasive procedures. A large retrospective series reported the following main reasons to insert a drain after open cholecystectomy: (1) operation for cholecystitis, (2) intraoperative bile spillage, and (3) excessive blood loss during the operation [10]. However, experimental studies showed that when a drain is inserted in the peritoneal cavity that contains no fluids, it is quickly surrounded by omentum and completely occluded within 48 h [11]. Bile leak and bile duct injury are the two most feared complications of LC for acute cholecystitis. The reported incidence for bile leak after LC for acute cholecystitis is approximately 2–3 % [5, 12, 13]. Studies from the era of open cholecystectomy showed that most patients who underwent laparotomy for postcholecystectomy bile peritonitis had drains placed, suggesting that drain placement does not detect this complication effectively [6, 14–16]. Drains are also not effective to treat bile leak in elective LC [3, 4]. In the present study, both bile leaks occurred after LC with drainage, suggesting that drains may be also useless after LC for ACC in selected patients. In cases of excessive intraoperative blood loss, drains are not a substitute for adequate hemostasis and do not facilitate detection of hemorrhage unless bleeding is immediate and brisk [17]. Moreover, severe bleeding may be rapidly diagnosed because of postoperative hypotension, acute blood loss anemia, and intra-abdominal hypertension. Drains are also not effective to treat bleeding in elective LC [3, 4].
Wound infection is a frequent complication after LC for ACC. The reported rate in a large survey is 2.3 % [5]. The rate increases if severe cholecystitis is present [9]. We had no wound infection. We adopt the routine use of prophylactic antibiotic therapy, adjusted according to the results of the routine cultural examination of the bile, as recommended by the Infectious Diseases Society of America. Moreover, we used topical administration of rifamycin, which was showed to reduce wound infection after LC [18].
The effect of subhepatic drain on postoperative pain is controversial with reported conflicting results after elective LC [3, 19]. Our data were unable to prove that the drain has any effect on either abdominal or shoulder tip pain in the setting of ACC.
Conclusion
Drainage after LC for ACC is used without evidence of its efficacy. The present pilot study was unable to prove that the drain was useful in a selected group of patients. A randomized trial with adequate blinding and number of patients enrolled is recommended to confirm these preliminary results.
References
- 1.Strasberg SM. Clinical practice. Acute calculous cholecystitis. N Engl J Med. 2008;358:2804–2811. doi: 10.1056/NEJMcp0800929. [DOI] [PubMed] [Google Scholar]
- 2.Gurusamy KS, Samraj K, Mullerat P, Davidson BR. Routine abdominal drainage for uncomplicated laparoscopic cholecystectomy (review) Cochrane Database Syst Rev. 2007;4:CD006004. doi: 10.1002/14651858.CD006004.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Tzovaras G, Liakou P, Fafoulakis F, Baloyiannis I, Zacharoulis D, Hatzitheofilou C. Is there a role for drain use in elective laparoscopic cholecystectomy? A controlled randomized trial. Am J Surg. 2009;197:759–763. doi: 10.1016/j.amjsurg.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Picchio M, De Angelis F, Zazza S, Di Filippo A, Mancini R, Pattaro G, Stipa F, Adisa AO, Marino G, Spaziani E (2012) Drain after elective laparoscopic cholecystectomy. A randomized multicentre controlled trial. Surg Endosc. doi:10.1007/s00464-012-2252 [DOI] [PubMed]
- 5.Navez B, Ungureanu F, Michiels M, Claeys D, Muysoms F, Hubert C, Vanderveken M, Detry O, Detroz B, Closset J, Devos B, Kint M, Navez J, Zech F, Gigot JF, The Belgian Group for Endoscopic Surgery (BGES) and the Hepatobiliary and Pancreatic Section (HBPS) of the Royal Belgian Society of Surgery (2012) Surgical management of acute cholecystitis: results of a 2-year prospective multicenter survey in Belgium. Surg Endosc. doi:10.1007/s00464-012-2206-2207 [DOI] [PubMed]
- 6.Takada T, Kawarada Y, Nimura Y, Yoshida M, Mayumi T, Sekimoto M, Miura F, Wada K, Hirota M, Yamashita Y, Nagino M, Tsuyuguchi T, Tanaka A, Kimura Y, Yasuda H, Hirata K, Pitt HA, Strasberg SM, Gadacz TR, Bornman PC, Gouma DJ, Belli G, Liau KH. Tokyo guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Surg. 2007;14:1–10. doi: 10.1007/s00534-006-1150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayumi T, Takada T, Kawarada Y, Nimura Y, Yoshida M, Sekimoto M, Miura F, Wada K, Hirota M, Yamashita Y, Nagino M, Tsuyuguchi T, Tanaka A, Gomi H, Pitt HA. Results of the Tokyo Consensus Meeting Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:114–121. doi: 10.1007/s00534-006-1163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127. doi: 10.1016/S0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 9.Borzellino G, Sauerland S, Minicozzi AM, Verlato G, Di Pietrantonj C, de Manzoni G, Cordiano C. Laparoscopic cholecystectomy for severe acute cholecystitis. A meta-analysis of results. Surg Endosc. 2008;22:8–15. doi: 10.1007/s00464-007-9511-6. [DOI] [PubMed] [Google Scholar]
- 10.Zaydfudim V, Russell RT, Feurer ID, Wright JK, Pinson CW. Drain use after open cholecystectomy: is there a justification? Langenbecks Arch Surg. 2009;394:1011–1017. doi: 10.1007/s00423-009-0549-x. [DOI] [PubMed] [Google Scholar]
- 11.Agrama HM, Blackwood JM, Brown CS, Machiedo GW, Rush BF. Functional longevity of intraperitoneal drains: an experimental evaluation. Am J Surg. 1976;132:418–421. doi: 10.1016/0002-9610(76)90409-8. [DOI] [PubMed] [Google Scholar]
- 12.Garber S, Korman J, Cosgrove J, Cohen J. Early laparoscopic cholecystectomy for acute cholecystitis. Surg Endosc. 1997;11:347–350. doi: 10.1007/s004649900360. [DOI] [PubMed] [Google Scholar]
- 13.Unger W, Glick G, Landeros M. Cystic duct leak after laparoscopic cholecystectomy: a multi-institutional study. Surg Endosc. 1996;10:1189–1193. doi: 10.1007/s004649900276. [DOI] [PubMed] [Google Scholar]
- 14.Monson RTJ, Keane BVF, Brenman GT. Cholecystectomy is safer without drainage: the results of a prospective, randomized clinical trial. Surgery. 1991;109:740–746. [PubMed] [Google Scholar]
- 15.Farha JG, Chang CF, Mathews HE. Drainage in elective cholecystectomy. Am J Surg. 1981;142:678–680. doi: 10.1016/0002-9610(81)90310-X. [DOI] [PubMed] [Google Scholar]
- 16.Budd CD, Cochran CR, Fouty JW. Cholecystectomy with and without drainage. Am J Surg. 1982;143:307–309. doi: 10.1016/0002-9610(82)90097-6. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty SH, Simmons RL. The biology and practice of surgical drains. Part II. Curr Probl Surg. 1992;29:633–730. doi: 10.1016/0011-3840(92)90028-2. [DOI] [PubMed] [Google Scholar]
- 18.Neri V, Fersini A, Ambrosi A, Tartaglia N, Valentino TP. Umbilical port-site complications in laparoscopic cholecystectomy: role of topical antibiotic therapy. JSLS. 2008;12:126–132. [PMC free article] [PubMed] [Google Scholar]
- 19.Hawasli A, Brown E. The effect of drains in laparoscopic cholecystectomy. J Laparoendosc Surg. 1994;4:393–398. doi: 10.1089/lps.1994.4.393. [DOI] [PubMed] [Google Scholar]