Abstract
To investigate the role of osteopontin (OPN) in the formation of hepatolith, we have immunohistochemically studied the involvement of OPN in hepatolithiasis in the intrahepatic bile ducts and the intramural and extramural glands, and in stones. In our hospital, 17 hepatic resections for hepatolithiasis were carried out from June 2006 to October 2009. Immunohistochemistry was performed on 17 liver specimens with hepatolith and on 24 control liver specimens without hepatolith. We compared the osetopontin expression in the epithelia of the intrahepatic bile ducts and peribiliary glands or periglandular macrophages. Staining for OPN was positive in the cytoplasm of the epithelial cells of stone-containing intrahepatic bile ducts and the intramural and extramural glands, and in stones. But there were no significant differences between the hepatolithiasis group and the control group for the OPN immunoreactivity of the luminal surface of the epithelial cells of the intrahepatic bile ducts and intramural and extramural glands. The stone-containing intrahepatic bile ducts were infiltrated by macrophages that showed intense staining for OPN. The core and matrix of the stones showed OPN immunoreactivity. The degree of OPN from the cytoplasm of the epithelia of the intrahepatic bile ducts and peribiliary glands or periglandular macrophages was different between hepatolithiasis and control groups. Our result suggests that the OPN from the intrahepatic bile ducts and peribiliary glands plays a role in the formation of intrahepatic stone.
Keywords: Osteopontin, Hepatolithiasis, Intrahepatic bile duct, Immunohistochemistry
Introduction
Hepatolithiasis is a commonly encountered disease in the East Asia, including Korea. The relative frequency of hepatolithiasis in gallstone disease is still high at about 11–15 % in Korea [1]. This disease is characterized by frequent cholangitis due to long-standing residual stone. Histologically, the ducts that contain stones show fibrosis, proliferation of mucous and serous glands, and inflammatory cell infiltration in their walls and the periductal tissue [2–4]. The abundant mucin, which is composed of high molecular weight glycoprotein, is produced and secreted into the biliary lumen by the biliary lining epithelial cells and peribiliary glands of the large bile ducts, and this mucin is seen within the biliary sludge and is considered to play an important role in the initiation and development of hepatolithiasis [3–5]. The presence of osteopontin (OPN), which was originally isolated from mineralized rat bone matrix, has been demonstrated in many organs, such as bone, kidney, lung, breast, smooth muscle, and gallbladder [5, 6]. It is rich in acidic amino acids and it possesses strong calcium-binding properties [7]. We conducted immunohistochemical studies to determine whether OPN is related with stone formation in the intrahepatic duct.
Methods
This study was performed with approval of the Kyung Hee University Hospital at Gangdong Institutional Review Board (KHNMC IRB 2012-083).
Liver specimens were obtained from 17 patients who underwent surgical treatment for hepatolithiasis, and the control liver specimens were obtained from 24 patients who were without hepatoliths between June 2006 and October 2009. The patient with acute cholangitis and cholecystitis were excluded from both groups. The hepatolithiasis group consisted of 17 hepatectomy specimens from 17 patients who underwent surgical treatment for hepatolithiasis with a mean age of 60.8 years (43–79). The male-to-female ratio was 5:12. Two cases of intrahepatic cholangiocarcinoma were included in this group. The control group consisted of 24 specimens from 24 patients who underwent hepatectomy for trauma and colorectal liver metastasis and they had a mean age of 60.5 years (range 31–76). The male-to-female ratio was 14:10.
The immunohistochemistry procedures were carried out on 4-μm tissue sections using the Bond Polymer Intense Detection system (VisionBioSystems, VIC, Australia) according to the manufacturer's instructions with minor modifications. In brief, 4-μm sections of the formalin-fixed and paraffin-embedded tissues were deparaffinized using Bond Dewax Solution (VisionBioSystems), and an antigen retrieval procedure was done using Bond ER Solution (VisionBioSystems) for 30 min at 100 °C. The endogenous peroxidase was quenched by incubation with hydrogen peroxide for 5 min. The sections were incubated for 15 min at ambient temperature with primary monoclonal Osteopontin antibody (1:200, OP-3N; Leica Microsystems, Newcastle, UK) using the biotin-free polymeric horseradish peroxidase (HRP)–linker antibody conjugate system in a Bond-max automatic slide stainer (VisionBioSystems). The nuclei were counterstained with hematoxylin. The percentage of immunoreactive cells was assessed on all the sections without the scorer having knowledge of the other results. We interpreted the absence of immunoreactivity or slight immunoreactivity (<10 %) of the immunoreactive cells as negative staining and focal or diffuse immunoreactivity (>10 %) of the immunoreactive cells as positive staining.
The expression of osteopontin between the hepatolithiasis group and the control group was evaluated using the Pearson’s chi-squared test. SPSS 12.0 was used for the statistical analysis. Differences were considered to be significant if the P value was less than 0.05.
Results
-
Osteopontin expression in the intrahepatic bile duct cells
Histologically, the stone-containing bile ducts showed luminal dilatation and the epithelial cells of the bile ducts showed marked proliferation. Of the 17 hepatolithiasis specimens, two showed cholangiocarcinoma.
The osteopontin expression in the cytoplasm of the intrahepatic duct cells was negative in 19 and positive in five in the control group, and negative in 0 and positive in 17 in the hepatolithiasis group. There was a significant difference between the groups for the osteopontin expression in the cytoplasm of the intrahepatic duct cells (P < 0.001).
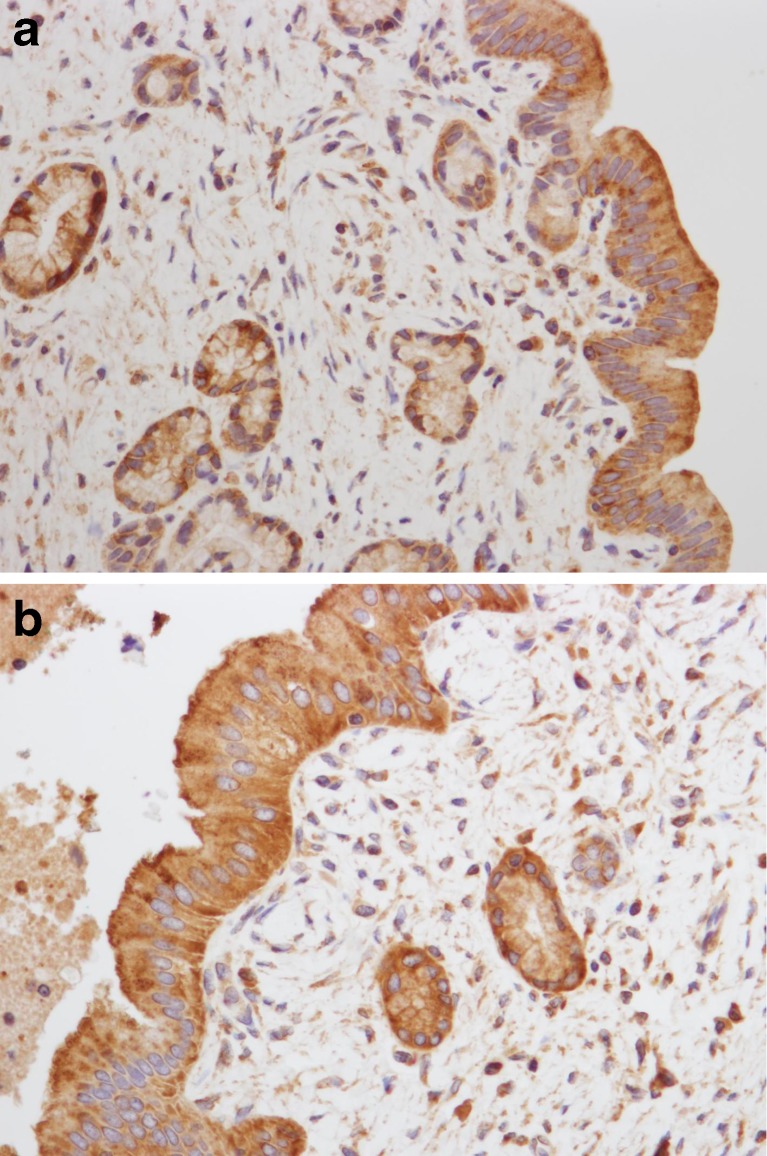
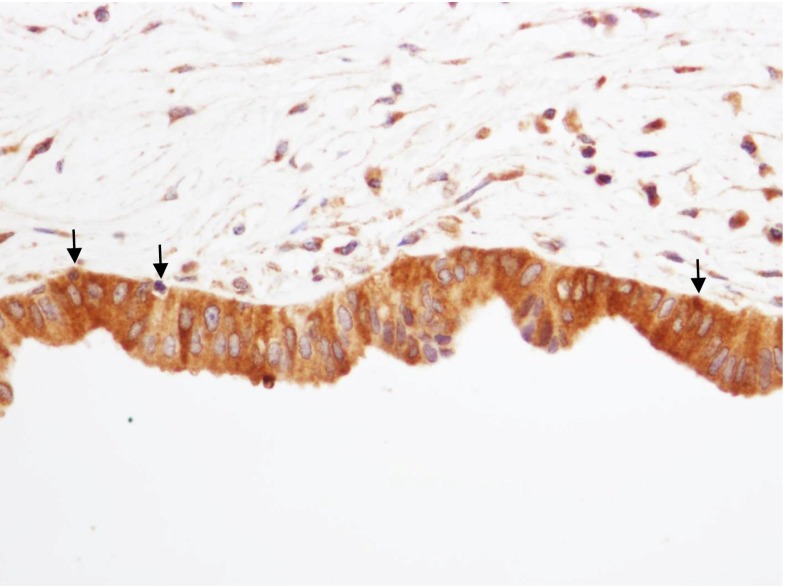
The osteopontin expression on the luminal surface of the intrahepatic duct cells was negative in 20 and positive in four in the control group, and negative in 11 and positive in six in the hepatolithiasis group. There was not a significant difference between the groups for the osteopontin expression on the luminal surface of the intrahepatic duct cells (P = 0.159) (Table 1; Figs. 1 and 2a, b).
-
Osteopontin expression in the intramural gland cells of the peribiliary gland
The osteopontin expression in the cytoplasm of the intramural gland cells was negative in 22 and positive in two in the control group, and negative in three and positive in 14 in the hepatolithiasis group. There was a significant difference between the groups for the osteopontin expression in the cytoplasm of the intramural gland cells (P < 0.001).
The osteopontin expression on the luminal surface of the intramural gland cells was negative in 23 and positive in one in the control group, and negative in 17 and all negative in the hepatolithiasis group. There was not a significant difference between the groups for the osteopontin expression on the luminal surface of the intramural gland cells (P = 0.585) (Table 1 and Fig. 2a, b).
-
Osteopontin expression in the extramural gland cells of the peribiliary gland
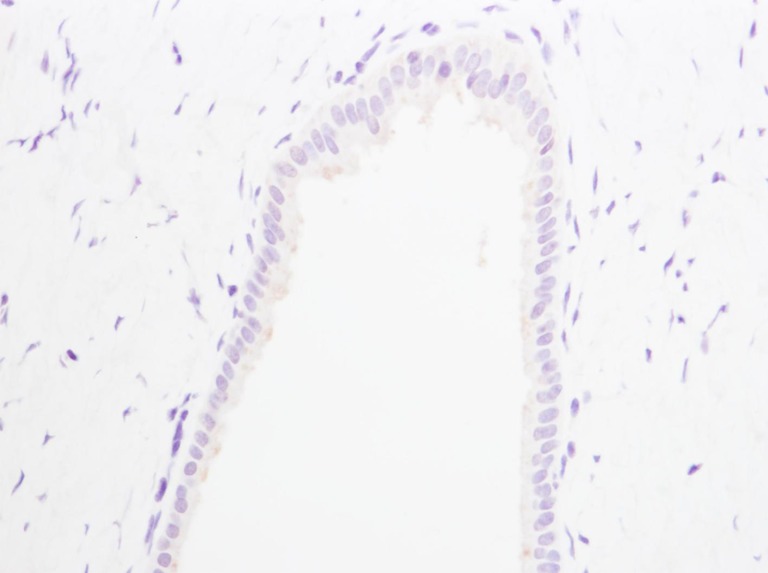
The osteopontin expression in the cytoplasm of the extramural gland cells was negative in 24 and all negative in the control group, and negative in five and positive in 12 in the hepatolithiasis group. There was a significant difference between the groups for the osteopontin expression in the cytoplasm of the extramural gland cells (P < 0.001) (Table 1; Figs. 3 and 4). The osteopontin expression on the luminal surface of the extramural gland cells was negative in both groups.
-
Osteopontin expression in the peribiliary duct macrophages
The osteopontin expression in the macrophages of the peribiliary duct was negative in 24 and positive in 0 in the control group, and negative in 2 and positive in 15 in the hepatolithiasis group. There was a significant difference between the groups for the osteopontin expression in the peribiliary duct macrophages (P < 0.001) (Table 2 and Fig. 5).
-
Osteopontin expression in the intrahepatic stone
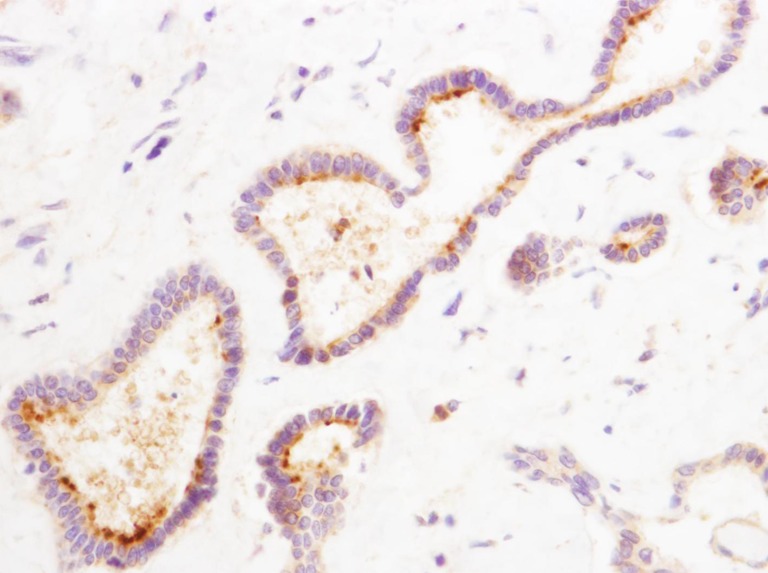
The sections of the calcium bilirubinate stones showed OPN immunoreactivity. A lamellar pattern is demonstrated by OPN immunostaining (Fig. 6).
Table 1.
Comparison of the osteopontin expression in the intrahepatic ducts and peribiliary glands between the hepatolithiasis group and the control group
| Control group (%) | Hepatolithiasis group (%) | P value | |
|---|---|---|---|
| Intrahepatic bile duct cells (cytoplasm) | <0.001 | ||
| Negative | 19 (100) | 0 (0) | |
| Positive | 5 (22.7) | 17 (77.3) | |
| Intrahepatic bile duct cells (luminal surface) | 0.159 | ||
| Negative | 20 (64.5) | 11 (35.5) | |
| Positive | 4 (40) | 6 (60) | |
| Intramural gland cells (cytoplasm) | <0.001 | ||
| Negative | 22 (88) | 3 (12) | |
| Positive | 2 (12.5) | 14 (87.5) | |
| Intramural gland cells (luminal surface) | 0.585 | ||
| Negative | 23 (57.5) | 17 (42.5) | |
| Positive | 1 (100) | 0 (0) | |
| Extramural gland cells (cytoplasm) | <0.001 | ||
| Negative | 24 (82.8) | 5 (17.2) | |
| Positive | 0 (0) | 12 (100) | |
| Extramural gland cells (luminal surface) | |||
| Negative | 24 (58.5) | 17 (41.5) | |
| Positive | 0 | 0 | |
Fig. 1.
Osteopontin immunoreactivity in the control group showed negative immunostaining in the cytoplasm and on the luminal surface of the epithelial cells in the intrahepatic duct (×400)
Fig. 2.
a Positive immunostaining is seen in the cytoplasm and on the luminal surface of the epithelial cells of the intrahepatic duct. Negative immunostaining is seen in the cytoplasm of the intramural glands (×400). b Positive immunostaining is seen in the cytoplasm and on the luminal surface of the epithelial cells of the intrahepatic duct and intramural glands (×400)
Fig. 3.
Cytoplasmic expression of OPN in the extramural gland cells. Negative immunostaining is seen in the cytoplasm and on the luminal surface (×400)
Fig. 4.
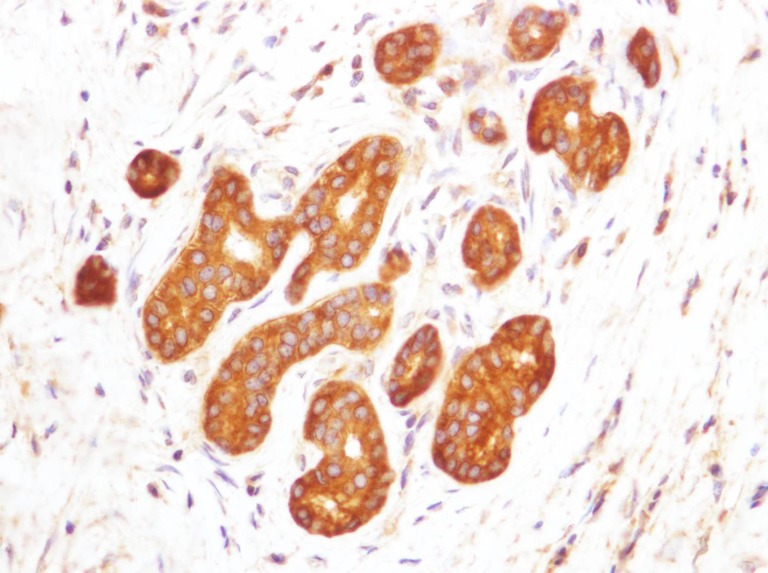
Cytoplasmic expression of OPN in the extramural gland cells. Positive immunostaining is seen in the cytoplasm and on the luminal surface (×400)
Table 2.
Comparison of the osteopontin expression in the periductal macrophages between the hepatolithiasis group and the control group
| Control group (%) | Hepatolithiasis group (%) | P value | |
|---|---|---|---|
| Periductal macrophage | <0.001 | ||
| Negative | 24 (92.3) | 2 (7.7) | |
| Positive | 0 (0) | 15 (100) |
Fig. 5.
OPN-positive macrophages (arrow) are seen in the epithelial layer of the intrahepatic duct. OPN immunostaining (×400)
Fig. 6.
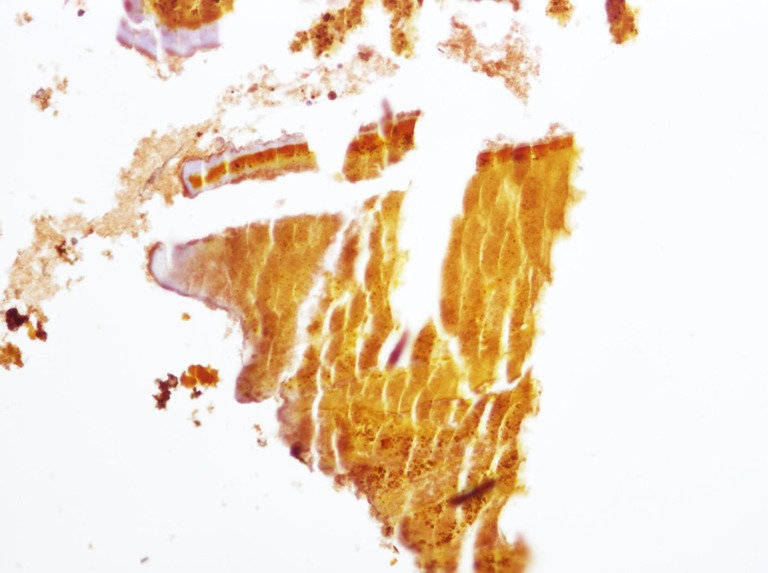
Immunohistochemical staining of calcium bilirubinate stone. The lamellar pattern is demonstrated by OPN immunostaining (×200)
Discussion
Hepatolithiasis is prevalent in the Far East, including Korea, and the majority of intrahepatic stones are calcium bilirubinate stones. Calcium salts are often present in the center of all types of gallstones, and this suggests the role of calcium in stone formation and growth [8]. Matrix proteins are known to be essential for biomineralization, and they may also be important in the formation and growth of gallstones [9]. Mucin is a high molecular weight glycoprotein, and it is the predominant matrix protein [10–12]. Mucin is found within the central nidus and throughout the matrices of gallstones, and it is apparently the structural protein of gallstones [13, 14]. Mucin secreted from stone-laden intrahepatic bile ducts and peribiliary glands, such as intramural and extramural glands, is regarded as an important factor in stone formation [10]. This mucin and bile pigment form a complex, which results in generating the nucleus of a stone [11, 12].
Nakanuma et al. [3] reported that abundant mucin and bile duct dilatation are important factors of stone formation, and they demonstrated that mucin from stone-laden peribiliary glands was found in bile sludge and calcium bilirubinated stone.
OPN is an acidic noncollagenous bone matrix mucinous glycoprotein that is sialated and phosphorylated [7]. OPN acts as a chemoattractant cytokine for the recruitment of macrophages, and OPN plays an important role in the production of autoantibodies and in inflammatory diseases [15]. The OPN expression in the proliferated bile ductules is a key marker of the degree of liver inflammation [16]. OPNs have been found in many organs such as bone, kidney, lung, breast, smooth muscle, gallbladder, and intrahepatic bile duct epithelium [5, 6, 17, 18]. Nalbone et al. [19] and Okido et al. [20] have isolated the same anionic calcium-binding, low molecular weight glycoprotein from hepatic bile, gallbladder bile, cholesterol gallstones, and pigment gallstones, which may be a fragment of OPN.
Nakai et al. [21] recently demonstrated over-expressed immunohistochemical staining for OPN in the intramural glands and extramural glands of bile ducts that contain stone, but there was weak and diffuse immunohistochemical staining for OPN in the epithelial cells of the normal bile duct. They also suggested that OPN may protect the epithelial surfaces, with decreased production in pathologic conditions, and this favors degeneration of the epithelium. A year later, Ichikawa et al. [18] with the same research group reported that OPN from epithelial cells may play an important role in the nidation of cholesterol gallstones. In the present study, strong immunoreactivity was also seen in the cytoplasm of the bile duct and intramural and extramural gland cells of the hepatolithiasis, but the osteopontin expression of the luminal surface of the bile duct and intramural and extramural cells of the hepatolithiasis group was not different from that of the control group. Yet it is uncertain if these findings are an effect or the cause of degeneration.
Hepatolithiasis is often associated with cholangiocarcinoma. Some authors have suggested that in hepatolithiasis, chronic mechanical irritation and damage of the biliary epithelium lead to repeated exfoliation and regeneration of the epithelium, and this induces carcinogenesis in the bile duct epithelial cells through hyperplasia, dysplasia, noninvasive adenocarcinoma, and invasive adenocarcinoma [22]. Recent studies have reported that the overexpression of OPN is correlated with tumorigenesis, tumor aggressiveness, and a poor prognosis for several types of human cancer [23–26].
Terashi et al. [27] have reported that the decreased expression of OPN is a reliable indicator of tumor aggressiveness and the clinical outcome in patients with intrahepatic cholangiocarcinoma. Yet in the present study, only two cases showed negative staining for OPN in the macrophages in the peribiliary glands of the hepatolithiasis group, and these two cases had associated intrahepatic cholangiocarcinoma. This indicates that the OPN expression may not be correlated with tumorigenesis. The present study has a significant limitation in that the number of patients was insufficient, and so further study is needed to confirm and expand our results.
It has reported that the plasma OPN levels were significantly higher in hepatocellular carcinoma, hepatitis, and liver cirrhosis patients than that in healthy patients [28, 29]. Harada et al. [15] have reported that OPN is an important molecule in the portal tracts, and OPN contributes to the immune process in primary biliary cirrhosis and autoimmune hepatitis. Many articles have focused on OPN and cholangitis and cholangiocarcinoma, but there is no report regarding the relationship between OPN and hepatolithiasis except for that of Nakai et al. [21]. In the present study, the cytoplasm of the intrahepatic bile duct cells and intramural and extramural gland cells with hepatolithiasis expressed more intense OPN reactivity than that of the control group, but the luminal surface of the intrahepatic bile duct cells and the intramural and extramural gland cells, irrespective of the presence of stone, showed no immunoreactivity in almost all the specimens. OPN was expressed in the macrophages in 15 of 17 hepatolithiasis patients. On the contrary, no OPN immunoreactive macrophages were demonstrated in the control group. In the stones, we found that the core and matrix demonstrated OPN immunoreactivity.
In conclusion, the degree of OPN expression from the cytoplasm of epithelial cells in the intrahepatic ducts and intramural and extramural glands or macrophages was different between control and hepatolithiasis group. But the degree of OPN expression from the luminal surface of epithelial cells in the intrahepatic ducts and intramural and extramural glands or macrophages was not different between control and hepatolithiasis group.
Acknowledgment
This work was supported by a grant from Kyung Hee University in 2009 (KHU-20090576).
Funding
This work was supported by a grant from Kyung Hee University in 2009 (KHU-20090576).
Ethical approval
This work was performed with approval of the Kyung Hee University Hospital at Gangdong Institutional Review Board (KHNMC IRB 2012-083).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Footnotes
Sung-Jig Lim performed the majority of the experiments; Bum-Soo Kim and Sun-Hyung Joo provided the collection of the human material and were involved in editing the manuscript; Kwang-Ro Joo advised for the manuscript.
References
- 1.Park YH, Park SJ, Jang JY, Ahn YJ, Park YC, Yoon YB, et al. Changing patterns of gallstone disease in Korea. World J Surg. 2004;28(2):206–210. doi: 10.1007/s00268-003-6879-x. [DOI] [PubMed] [Google Scholar]
- 2.Nakanuma Y, Yamaguchi K, Ohta G, Terada T. Pathologic features of hepatolithiasis in Japan. Hum Pathol. 1998;19(10):1181–1186. doi: 10.1016/S0046-8177(88)80150-3. [DOI] [PubMed] [Google Scholar]
- 3.Nakanuma Y, Sasaki M, Terada T, Harada K. Intrahepatic peribiliary glands of humans. II. Pathological spectrum. J Gastroenterol Hepatol. 1994;9(1):80–86. doi: 10.1111/j.1440-1746.1994.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto K. Intrahepatic periductal glands and their significance in primary intrahepatic lithiasis. Jpn J Surg. 1982;12(3):163–170. doi: 10.1007/BF02469582. [DOI] [PubMed] [Google Scholar]
- 5.Brown LF, Berse B, Van de Water L, Papadopoulos-Sergiou A, Perruzzi CA, Manseau EJ, et al. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992;3(10):1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu H, Brown LF, Senger DR, Geng LL, Dvorak HF, Dvorak AM. Ultrastructural immunogold localization of osteopontin in human gallbladder epithelial cells. J Histochem Cytochem. 1994;42(3):351–361. doi: 10.1177/42.3.8308252. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Bal BS, Gorski JP. Calcium and collagen binding properties of osteopontin, bone sialoprotein, and bone acidic glycoprotein-75 from bone. J Biol Chem. 1992;267(24):871–878. [PubMed] [Google Scholar]
- 8.Kaufman HS, Magnuson TH, Pitt HA, Frasca P, Lillemoe KD. The distribution of calcium salt precipitates in the core, periphery and shell of cholesterol, black pigment and brown pigment gallstones. Hepatology. 1994;19(5):1124–1132. doi: 10.1002/hep.1840190509. [DOI] [PubMed] [Google Scholar]
- 9.Kestell MF, Sekijima J, Lee SP, Park HZ, Long M, Kaler EW. A calcium-binding protein in bile and gallstones. Hepatology. 1992;16(6):1315–1321. doi: 10.1002/hep.1840160602. [DOI] [PubMed] [Google Scholar]
- 10.Lee KT, Sheen PC, Liu YE. Histomorphometric evaluation of mucin content in stone-containing intrahepatic bile ducts. J Formos Med Assoc. 1998;97(11):788–790. [PubMed] [Google Scholar]
- 11.Messing B, Bories C, Kunstlinger F, Bernier JJ. Does total parenteral nutrition induce gallbladder sludge formation and lithiasis? Gastroenterology. 1983;84(5):1012–1019. [PubMed] [Google Scholar]
- 12.Lee KT, Liu TS. Altered mucin gene expression in stone-containing intrahepatic bile ducts and cholangiocarcinomas. Dig Dis Sci. 2001;46(10):2166–2172. doi: 10.1023/A:1011906830301. [DOI] [PubMed] [Google Scholar]
- 13.Been JM, Bills PM, Lewis D. Microstructure of gallstones. Gastroenterology. 1979;76(3):548–555. [PubMed] [Google Scholar]
- 14.Smith BF, LaMont JT. Identification of gallbladder mucin–bilirubin complex in human cholesterol gallstone matrix. Effects of reducing agents on in vitro dissolution of matrix and intact gallstones. J Clin Invest. 1985;76(2):439–445. doi: 10.1172/JCI111991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada K, Ozaki S, Sudo Y, Tsuneyama K, Ohta H, Nakanuma Y. Osteopontin is involved in the formation of epithelioid granuloma and bile duct injury in primary biliary cirrhosis. Pathol Int. 2003;53(1):8–17. doi: 10.1046/j.1440-1827.2003.01426.x. [DOI] [PubMed] [Google Scholar]
- 16.Tajiri T, Tate G, Kunimura T, Endo Y, Inoue K, Mitsuya T, et al. Osteopontin expression in proliferated bile ductules: the correlation with liver damage in fulminant hepatitis. Dig Dis Sci. 2005;50(1):188–195. doi: 10.1007/s10620-005-1299-4. [DOI] [PubMed] [Google Scholar]
- 17.Haylock DN, Nilsson SK. Osteopontin: a bridge between bone and blood. Br J Haematol. 2006;134(5):467–474. doi: 10.1111/j.1365-2141.2006.06218.x. [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa H, Imano M, Takeyama Y, Shiozaki H, Ohyanagi H. Involvement of osteopontin as a core protein in cholesterol gallstone formation. J Hepatobiliary Pancreat Surg. 2009;16(2):197–203. doi: 10.1007/s00534-009-0043-4. [DOI] [PubMed] [Google Scholar]
- 19.Nalbone G, Lafont H, Vigne JL, Domingo N, Lairon D, Chabert C, et al. The apoprotein fraction of the bile lipoprotein complex: isolation, partial characterization and phospholipid binding properties. Biochimie. 1979;61(9):1029–1041. doi: 10.1016/S0300-9084(80)80257-4. [DOI] [PubMed] [Google Scholar]
- 20.Okido M, Shimizu S, Ostrow JD, Nakayama F. Isolation of a calcium-regulatory protein from black pigment gallstones: similarity with a protein from cholesterol gallstones. Hepatology. 1992;15(6):1079–1085. doi: 10.1002/hep.1840150618. [DOI] [PubMed] [Google Scholar]
- 21.Nakai A, Imano M, Takeyama Y, Shiozaki H, Ohyanagi H. An immunohistochemical study of osteopontin in hepatolithiasis. J Hepatobiliary Pancreat Surg. 2008;15(6):615–662. doi: 10.1007/s00534-007-1320-8. [DOI] [PubMed] [Google Scholar]
- 22.Terada T, Nakanuma Y, Ohta T, Nagakawa T. Histological features and interphase nucleolar organizer regions in hyperplastic, dysplastic and neoplastic epithelium of intrahepatic bile ducts in hepatolithiasis. Histopathology. 1992;21(3):233–240. doi: 10.1111/j.1365-2559.1992.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 23.Oates AJ, Barraclough R, Rudland PS. The role of osteopontin in tumorigenesis and metastasis. Invasion Metastasis. 1997;17(1):1–15. [PubMed] [Google Scholar]
- 24.Cao DX, Li ZJ, Jiang XO, LumYL KE, Lee NP, et al. Osteopontin as potential biomarker and therapeutic target in gastric and liver cancers. World J Gastroenterol. 2012;14(80):3923–3930. doi: 10.3748/wjg.v18.i30.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao XQ, Dong JH, Zhang WZ, Liu Z. Prognosis of ampullary cancer based on immunohistochemical type and expression of osteopontin. Dign Pathol. 2011;6:98–104. doi: 10.1186/1746-1596-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shevde LA, Das S, Clark DW, Samant RS. Osteopontin: an effector and an effect of tumor metastasis. Curr Mol Med. 2010;10(1):71–81. doi: 10.2174/156652410791065381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terashi T, Aishima S, Taguchi K, Asayama Y, Sugimachi K, Matsuura S, et al. Decreased expression of osteopontin is related to tumor aggressiveness and clinical outcome of intrahepatic cholangiocarcinoma. Liver Int. 2004;24(1):38–45. doi: 10.1111/j.1478-3231.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Ki SS, Lee SD, Han CJ, Kim YC, Park SH, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101(9):2051–2059. doi: 10.1111/j.1572-0241.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 29.Seth D, Gorrell MD, Cordoba S, McCaughan GW, Haber PS. Intrahepatic gene expression in human alcoholic hepatitis. J Hepatol. 2006;45(2):306–320. doi: 10.1016/j.jhep.2006.04.013. [DOI] [PubMed] [Google Scholar]