Abstract
We aimed to analyze the diagnostic value of mean platelet volume and platelet distribution width, which are also known as the markers of platelet count, in acute and perforated appendicitis. The data of 202 patients who applied to general surgery clinic in Mustafa Kemal University Hospital from 2007 to 2012 with acute appendicitis were analyzed retrospectively. The findings were separated to two groups due to the perforation status (perforated vs. non-perforated). Age, sex, leukocyte, hemoglobin, hematocrit, mean platelet volume, and platelet distribution width were examined. The mean age of the patients was 35.8. Twenty-one of all cases were perforated appendicitis (10.4 %), and the rest was acute appendicitis (non-perforated) (n = 181, 89.6 %). The mean platelet volume value was 9.8 ± 2.1 fL; mean thrombocyte count, 340.9 × 109/L; and mean platelet distribution width value, 18.3 %. There were statistically significant differences between sex and age, hemoglobin, hematocrit, leukocyte, mean platelet volume, and platelet distribution width. There was a positive correlation between mean platelet volume, platelet distribution width, and platelet. Age, leukocyte, platelet, mean platelet volume, and platelet distribution width were higher in cases with perforation as a comparison with non-perforated cases. We think that mean platelet volume and platelet distribution width may be valuable markers to detect the risk of perforation in early periods of acute appendicitis.
Keywords: Acute appendicitis, Perforated appendicitis, Mean platelet volume, Platelet distribution width, Complete blood count
Introduction
Reginald Fitz described the appendicitis for the first time in 1886, and a few years later, McBurney defined the clinical features, findings of perforation, and surgical techniques of appendicitis [1]. Acute appendicitis is a disease which requires urgent surgery, and its diagnosis is mainly based on anamnesis, clinical examination, and simple laboratory findings. It can be seen in all age groups [2]. Lymphoid tissue maturation starts with birth, and nearly 200 lymph follicles are consistent at the age of 12–20 [3]. Perforation risk is about 14–31 %, and mortality risk is 0.02–0.8 % for all ages. Mortality for children is 0.1–1 %; at the other hand, risk of mortality increases up to 20 % or more for the patients who are older than 70 years [2, 4–6]. Radiological tests are used to decide urgent surgery in suspicious cases [7]. For this reason, some easy, cheap, practical, and applicable in anywhere tests are needed.
Mean platelet volume (MPV) and platelet distribution width (PDW) are markers of the platelet activation. Thrombocyte size is related with the function and activation of thrombocyte [8]. Volume parameters such as MPV and PDW have been used since 1980 [9, 10]. Mean platelet volume is one of the routine parameters in complete blood count (CBC) test and signs the average volume of circulating thrombocytes. Mean platelet volume increases as the thrombocyte production increases [11]. Bigger thrombocytes are more active than smaller ones, and they produce more thromboxane A2 [12]. Mean platelet volume has been analyzed for some diseases as an inflammatory marker and reported as increased in situations like sepsis, myeloproliferative diseases, massive hemorrhage, leukemia, vasculitis, and postsplenectomy [13, 14].
Relationship with MPV and acute appendicitis is only analyzed in one adult and one childhood study [15, 16]. We studied the diagnostic importance of MPV and PDW for acute appendicitis.
Materials and Method
The data of 202 patients who applied to general surgery clinic in Mustafa Kemal University Hospital from January 2007 to January 2012 with acute appendicitis were analyzed retrospectively. The data were classified into two groups according to the perforation status (perforated vs. non-perforated). Age, sex, hemoglobin (Hb), hematocrit (HCT), white blood cell (WBC), thrombocyte, platelet distribution width, and mean platelet volume were analyzed. Ultrasonographic examinations were performed by radiologists with 3.5 MHz convex and 7.5 MHz sector transducer. The criteria for the diagnosis of acute appendicitis counseled by USG were as follows: (1) the anteroposterior diameter of more than 6 mm, (2) periappendicular anechoic fluid, (3) periappendicular hypo-echoic inflammation, (4) significant thickness of the wall of the cecum and terminal ileum than the wall of the other intestinal segments, (5) appendicoliths, and (6) noncompressible and nonperistaltic appendix [17, 18]. Appendectomy was performed to all of the patients. All the specimens were analyzed by pathologists. During histopathological examination, at least two samples from the specimen were analyzed.
The exclusion criteria were the following: being less than 15 years of age, history of previous abdominal operation, having acute or chronic infectious disease, unconsciousness and negative cooperativity, comorbidities (respiratory, renal, cardiac, endocrinal, vascular diseases, cancer, etc.), history of blood transfusion within last 1 year, history of severe anemia or hematological disease, and history of medication (analgesics, anticoagulant, iron supplements, oral contraceptives, antimetabolites, etc.). Also, after the histopathologic examination of appendix, patients with normal appendicitis were excluded from the study.
Complete blood counts were performed from the venous blood samples. All the samples were obtained with ethylenediaminetetraacetic acid (EDTA) anticoagulation. Automatic complete blood counter devices with international norm results were used to determine CBC. Number of leukocyte and platelet, Hb, HCT, MPV, and PDW were analyzed via CBC. Mean platelet volume and PDW were examined by a device working according to the openness of the impedance which was branded as Beckman Coulter Gen-S. Normal values of all the samples were determined by the reference values of various laboratories. Reference values according to the openness of the impedance were accepted as 8.0–13.0 fl for MPV and 9.0–14.0 fl for PDW [11, 19]. We did not need to get a control group, since the reference values for PDW and MPV are standard, and age and sex are not associated with these values.
Statistical Analyses
All tests were performed using SPSS for Windows 13.0. The parameters with normal distribution were expressed as mean and standard deviation. Comparisons of means were performed with Student’s t test. Intercorrelations between parameters were computed through the Pearson's correlation analysis. Comparisons of nonparametrics were analyzed by using chi-squared test. Logistic regression analysis was used to detect risk factors. A p value of <0.05 was accepted as statistical significance.
Results
Two hundred and two patients who were undergone appendectomy with acute appendicitis in the last 5 years at Mustafa Kemal University Education and Research Hospital were included in the study. A hundred of all patients were male (49.5 %), and 102 of all were female (50.5 %). The mean age of cases was 35.8 ± 12.0 (15–78) years. Twenty-one of all cases were perforated appendicitis (10.4 %), and the rest (n = 181, 89.6 %) was non-perforated acute appendicitis. The mean Hb value was 12.9 ± 1.6 g/dL (8.8–16.6); mean HCT, 38.3 ± 4.8 % (20.2–27.5); mean number of WBC, 12.7 ± 4.2 × 103/L (2.7–26.5 × 103); mean MPV result, 9.8 ± 2.1 fL (5.9–13.5); mean number of platelet, 340.9 ± 118.4 × 103/L (162.1–662 × 103); and mean PDW value, 18.3 ± 2.3 % (11.0–23.4).
The mean ages of male and female patients were 33.8 ± 11 and 37.8 ± 12.6 years, respectively (p = 0.01). The mean Hb levels were higher in males than females, and this was statistically significant (13.9 ± 1.4 vs. 11.8 ± 1.2 g/dL, p = 0.0001). The mean HCT was 41.3 ± 4.1 % in men and 35.4 ± 3.4 % in women (p = 0.0001). The mean WBC was also higher in men than women (13.5 ± 3.7 × 103 vs. 11.9 ± 4.5 × 103/L, p = 0.007). The mean MPV was 10.2 ± 2.1 fL in males and 9.3 ± 2.0 fL in females (p = 0.002). The mean thrombocyte count was 364.4 × 103 ± 122.3 × 103/L in men and 335.6 × 103 ± 114.8 × 103/L in women (p > 0.05). The mean PDW was 18.7 ± 2.4 % in male patients and 17.9 ± 2.1 % in female patients (p = 0.02). Age, Hb, HCT, WBC, MPV, and PDW showed statistically significant differences when they were compared according to sex.
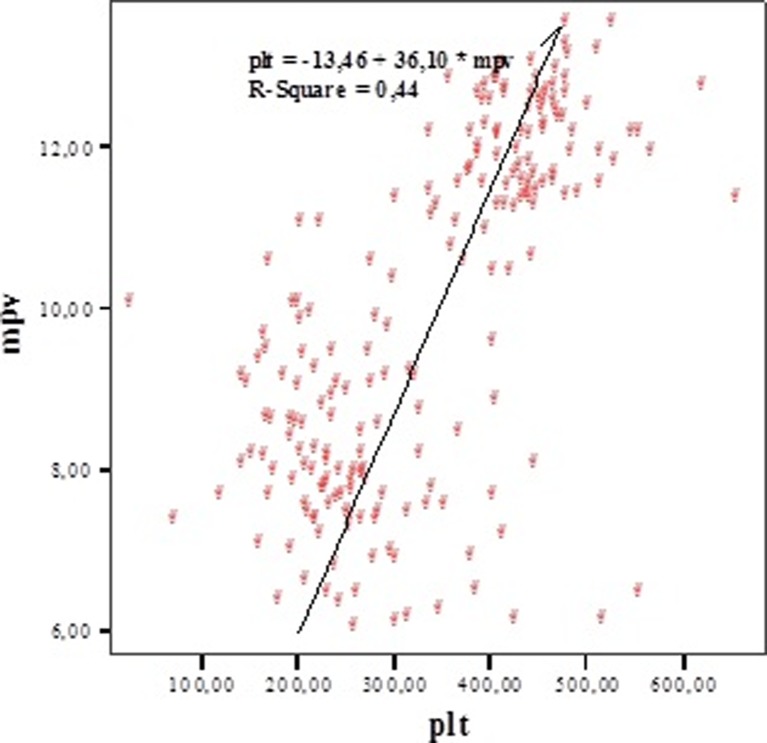
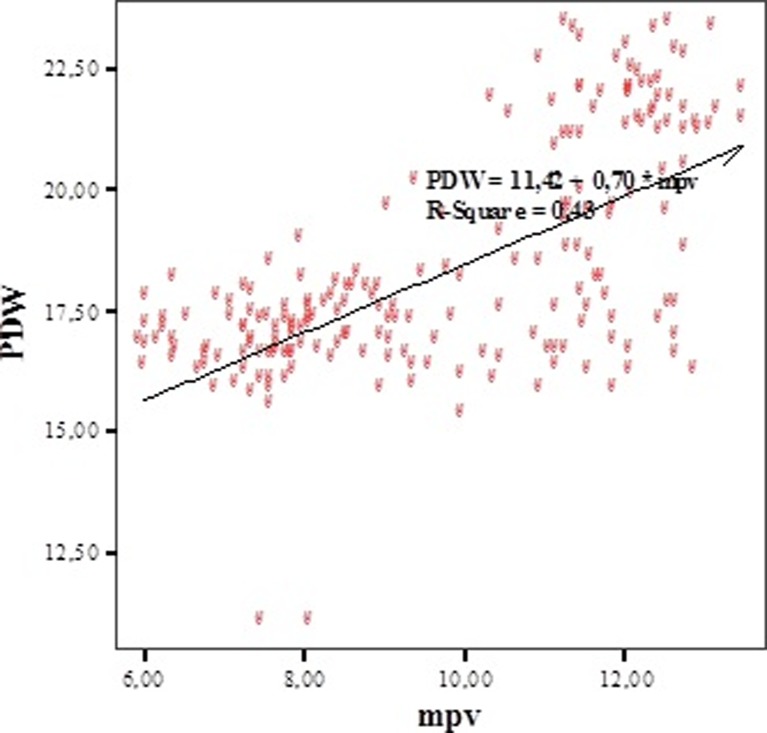
To determine the relation between MPV, thrombocyte count, and PDW, Pearson's correlation analysis test was used. There was a positive correlation between MPV and number of platelets (r = 0.662; p = 0.0001) (Fig. 1). There was a positive correlation between MPV and PDW (r = 0.654; p = 0.0001) (Fig. 2). A positive correlation between thrombocyte and PDW (r = 0.541; p = 0.0001) was detected. There were positive correlations between MPV, PDW, and platelet (PLT) (Table 1).
Fig. 1.
The correlation between MPV and platelet
Fig. 2.
The correlation between MPV and PDW
Table 1.
The correlation between MPV, PDW, and thrombocyte count
| MPV | Thrombocyte count | PDW | |
|---|---|---|---|
| MPV | |||
| r | 1 | 0.662 | 0.654 |
| p | 0.0001 | 0.0001 | |
| Thrombocyte count | |||
| r | 0.662 | 1 | 0.541 |
| p | 0.0001 | 0.0001 | |
| PDW | |||
| r | 0.654 | 0.541 | 1 |
| p | 0.0001 | 0.0001 | |
All the patients were analyzed according to their perforation status. Twenty-one of all cases were perforated appendicitis (11 males and ten females), and the rest of 181 cases was acute appendicitis without perforation. The risk of perforation in male patients was not increased [odds ratio (OR) 1.13, p > 0.05). The mean age of patients with perforated appendicitis was 42.3 ± 12.5, and the mean age of patients without perforation was 35.1 ± 11.7 (p = 0.008). The average age of males with perforated appendicitis was 41.2 ± 15.1, and it was 43.6 ± 9.6 in females with perforation (p > 0.05). In our study, the age as a variable was a risk factor in terms of perforation in acute appendicitis (OR 1.054, p = 0.008). The mean Hb levels in patients with and without perforation were 13.4 ± 1.8 and 12.8 ± 1.6 g/dL, respectively (p > 0.05). The mean HCT in patients with perforated appendicitis (39.7 ± 5.1 %) was higher than that in patients with non-perforated appendicitis (38.1 ± 4.7 %), but there was no statistically significant difference (p > 0.05). However, the difference between mean WBC and perforation status was statistically significant. The mean WBC in patients with perforated appendicitis was 20.1 ± 2.5 × 103/L, and the mean WBC in non-perforated cases was 11.9 ± 3.5 × 103/L (p = 0.0001). The mean MPV levels in patients with and without perforation were 12.5 ± 0.5 and 9.5 ± 2 fL, respectively (p = 0.0001). The mean PLT in patients with perforated appendicitis was 448.7 × 103 ± 48.1 × 103/L, and the mean PLT for patients with non-perforated appendicitis was 328.4 × 103 ± 117.8 × 103/L (p = 0.0001). The mean PDW value was 20.8 ± 1.8 % in cases with perforation and 18.0 ± 2.2 % in cases without perforation (p = 0.0001). Age, WBC, PLT, MPV, and PDW values were higher in patients with perforated appendicitis (Table 2).
Table 2.
The comparison of cases according to perforation status
| Perforation | Number | Mean | SD | p value | |
|---|---|---|---|---|---|
| Age | − | 181 | 35.11 | 11.74 | 0.008 |
| + | 21 | 42.38 | 12.53 | ||
| Hb | − | 181 | 12.86 | 1.66 | >0.05 |
| + | 21 | 13.47 | 1.89 | ||
| HCT | − | 181 | 38.18 | 4.78 | >0.05 |
| + | 21 | 39.75 | 5.18 | ||
| WBC | − | 181 | 11.90 | 3.51 | 0.0001 |
| + | 21 | 20.18 | 2.53 | ||
| MPV | − | 181 | 9.50 | 2.06 | 0.0001 |
| + | 21 | 12.51 | 0.55 | ||
| Platelet | − | 181 | 328.45 | 117.86 | 0.0001 |
| + | 21 | 448.71 | 48.19 | ||
| PDW | − | 181 | 18.02 | 2.20 | 0.0001 |
| + | 21 | 20.84 | 1.86 |
Discussion
Appendicitis is seen in 1/1,000 of the general population [20]. The classical symptoms for acute appendicitis are abdominal pain (begins around the periumbilical area and then settles to right lower quadrant within 24 h), nausea, vomiting, lack of appetite, and diarrhea. However, these symptoms occur in only 60 % of all patients [21]. Even though the morbidity and mortality of acute appendicitis are very low as compared to the previous years, the differential diagnose may still be difficult. Mortality rate due to perforated appendicitis is rapidly declining during the past 50 years; however, the complication rate is still high [22]. The most seen complication after the operation for perforated appendicitis is surgical area infection. This prolongs the hospitalization period and causes the loss of cost-effectiveness [23].
Tissue damage and inflammation about thrombocyte are the topics of interest recently. Thrombocytes are heterogeneous, small disk-shaped elements in terms of volume, density, age, and metabolic functions. Thrombocyte volume heterogeneity occurs due to the production factors in the bone marrow. The maturation does not happen during circulation. Mean platelet volume, which is formed as a response to the thrombopoietic stress, is with increased growth of megakaryocytes. Bigger thrombocytes are known as stress thrombocytes. The degree of stimulation of megakaryocyte as reflected by DNA content is the most important marker of thrombocyte volume [19, 24–27].
Evaluation of the thrombocyte volume and structure may be helpful for the diagnosis of some thrombocyte diseases [28]. Thrombocyte volume parameters, thrombocytopenia, and thrombocytosis are valuable in diagnosis. The routine analysis of volume parameters helps to detect abnormal thrombocyte production even if the number of thrombocyte is normal. MPV distinguishes the excess of destruction or lack of production, hypersplenism, and myeloproliferative or thalassemia diseases [29]. Thrombocytosis is common in infectious and inflammatory situations. We detected higher mean thrombocyte numbers in cases of perforated appendicitis in our study, and this signs the severity of infection. In this study, there were positive correlations between the number of thrombocytes and MPV and PDW. At the same time, MPV and PDW values were higher in perforated cases than those in non-perforated ones, and this signs that the increase in MPV and PDW values in correlation with the number of thrombocytes may make physicians one step closer to the diagnosis in suspicious cases.
Thrombocyte volume can be evaluated by impedance or light disturbance technology tools routinely. Thrombocytes in EDTA form isovolumetric spherical shapes. Thrombocytes in citrate form discoid shapes. Thrombocytes in EDTA cause significant increase in volume when they are measured by impedance, and then, log-normal volume distribution is obtained. PDW is a function of standard deviation of log volume and is also known as volume change coefficient. It is equal to geometric standard deviation which is amplified by a permanent [2, 9–11]. The reference values of MPV and PDW due to impedance openness are 8.0–13.0 and 9.0–13 fL, respectively. According to optical system, the values are (for MPV) 7.4–11.2 fL and (for PDW) 44–56 % [19]. We detected the measurement system of MPV and PDW as impedance openness when we retrospectively studied the medical records of cases with acute appendicitis. We did not plan any control group differently from acute and perforated appendicitis cases. The normal ranges of WBC, Hb, HCT, MPV, PDW, and platelet are well-known. PDW is an index of thrombocyte volume heterogeneity similar to erythrocyte distribution. The examination of PDW with MPV provides a better definition of thrombocyte volume disturbance. The heterogeneity of thrombocyte volume occurs due to heterogenic demarcation of megakaryocytes rather than the aging of circulating thrombocytes.
Idiopathic thrombocytopenic purpura (ITP) or dissemine intravascular coagulation with increased thrombocyte turnover, Bernard–Soulier syndrome, May–Hegglin anomaly, sepsis, myeloproliferative disorders, massive hemorrhage, leukemia, history of splenectomy, and vasculitis should be remembered when MPV is increased, and macro-thrombocytes are present. Normal MPV signs infiltrated or hypocellular bone marrow in malign diseases. Low MPV levels are usually with some uncommon situations like Wiskott–Aldrich syndrome and thrombocytopenia-absent radius syndrome [30].
Sepsis, preeclampsia, or ITP causing increased thrombocyte destruction should be thought in cases of thrombocytopenia or thrombocytosis with high MPV levels. At the other hand, low MPV levels sign decreased hypoplasic thrombocyte production or hypersplenism [31]. We detected higher mean thrombocyte number and higher mean MPV levels in perforated appendicitis when compared with non-perforated ones. This reveals increased thrombocyte destruction in patients with perforated appendicitis, so the number of platelet becomes high due to increased production of young thrombocytes. Mean platelet volume is an important marker in thrombopoiesis like reticulocytes in erythropoiesis [31–34]. In our study, we could not reach all the CBC results of postoperative periods. For this reason, we think that patients with thrombocytopenia or thrombocytosis in preoperative period may have normal thrombocyte count and normal MPV and PDW levels after appendectomy. There is a great need of prospective studies to proof this.
The increase of some cytokines in acute appendicitis may cause to change the thrombocyte volume. In many studies, interleukin-6 (IL-6) enhancement in acute appendicitis is reported [35, 36]. Some studies reveal the possible effect of an important proinflammatory cytokine, IL-6, on thrombocyte volume [37, 38]. Yoon et al. reported that IL-6 levels in patients with perforated appendicitis were higher than those in non-perforated ones [39]. We found significant high levels of MPV, PDW, and thrombocyte in perforated cases as these previous studies. We agree the suggestion of increased thrombocyte volume by IL-6 in perforated appendicitis.
There are two studies analyzing the relation between MPV and acute appendicitis in the literature. One of them studied pediatric patients [15], and the other one studied adult patients [16]. Both studies were performed within 1 year, and they found MPV as low in acute appendicitis. Also, they could not exhibit the relation between acute appendicitis and PDW and thrombocyte count. They did not study the difference on perforated or non-perforated appendicitis. We detected that high MPV value was a significant risk factor for perforation in our study. So, because of the high number of cases; the presence of significantly high levels of thrombocyte, MPV, and PDW in cases with perforated appendicitis; and the positive correlations between these parameters, our study is an important, valuable, and unique report in the literature. Both MPV and PDW are markers of platelet immaturity, and an increase in both as compared to controls suggests that young platelets are coming into the peripheral circulation. As compared to normal appendicitis, there is more platelet utilization in the periphery, and thus, these may be early surrogate indicators of perforation. The correlated increase of these three parameters (thrombocyte, MPV, and PDW) in appendicitis should set physicians thinking perforation and early surgery. So, the contribution of these parameters on the diagnosis of perforated appendicitis makes this study valuable. Both these previous studies interestingly found MPV decreased in acute appendicitis. The mean Hb levels were in normal ranges in our study, and also, we excluded the patients with severe anemia from the study. We think that these two factors make our study more efficient. In adult study, there were no data about Hb and HCT levels, and severe anemia was not an exclusion criterion. This might affect the results of study. There were no data about Hb and HCT levels in pediatric study as similar to the adult one. Iron-deficiency anemia is common in childhood, and there are some studies pointing the effect of severe anemia on MPV levels [19]. Also, the number of cases was low in childhood study (n = 100).
As a result, we claim that MPV and PDW which are routinely calculated on all automated hematology cell counters may be important markers for the early detection of perforation risk in acute appendicitis. However, there is a great need for multicentric and prospective studies with high number of cases.
Acknowledgments
Conflict of Interest
The authors declare that there is no conflict of interest in this study.
References
- 1.Saidi HS, Chavda SK. Use of a modified Alvorado score in the diagnosis of acute appendicitis. East Afr Med J. 2003;80:411–414. doi: 10.4314/eamj.v80i8.8732. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum BA, Wilson SR. Appendicitis at the millennium. Radiology. 2000;215:337–338. doi: 10.1148/radiology.215.2.r00ma24337. [DOI] [PubMed] [Google Scholar]
- 3.Shelton T, McKilay R, Scwartz RW. Acute appendicitis: current diagnosis and treatment. Curr Surg. 2003;60:502–505. doi: 10.1016/S0149-7944(03)00131-4. [DOI] [PubMed] [Google Scholar]
- 4.Bendeck SE, Nino-Murcia M, Berry GJ, Jeffrey RB. Imaging for suspected appendicitis: negative appendectomy and perforation rates. Radiology. 2002;225:131–136. doi: 10.1148/radiol.2251011780. [DOI] [PubMed] [Google Scholar]
- 5.Dunn EL, Moore EE, Elerding SC. The unnecessary laparotomy for appendicitis—can it be decreased? Am Surg. 1982;48:320–323. [PubMed] [Google Scholar]
- 6.Binnebösel M, Otto J, Stumpf M, Mahnken AH, Gassler N, Schumpelick V. Acute appendicitis. Modern diagnostics surgical ultrasound Chirurg. 2009;80:579–587. doi: 10.1007/s00104-009-1684-1. [DOI] [PubMed] [Google Scholar]
- 7.Fox JC, Solley M, Anderson CL, Zlidenny A, Lahham S, Maasumi K. Prospective evaluation of emergency physician performed bedside ultrasound to detect acute appendicitis. Eur J Emerg Med. 2008;15:80–85. doi: 10.1097/MEJ.0b013e328270361a. [DOI] [PubMed] [Google Scholar]
- 8.Martin JF, Trowbridge EA, Salmon G. The biological significance of platelet volume: its relationship to bleeding time, thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res. 1983;32:443–460. doi: 10.1016/0049-3848(83)90255-4. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy JT, Petty RE, Laxer RM, Lindsley CB. Periodic fever syndromes in children. Philadelphia: Elsevier; 2005. pp. 657–690. [Google Scholar]
- 10.Gershoni-Baruch R, Shinawi M, Leah K, Badarnah K, Brik R. Familial Mediterranean fever: prevalence, penetrance and genetic drift. Eur J Hum Genet. 2001;9:3–7. doi: 10.1038/sj.ejhg.5200672. [DOI] [PubMed] [Google Scholar]
- 11.Van der Loo B, Martin JF. Megakaryocytes and platelets in vascular disease. Baillieres Clin Haematol. 1997;10:109–123. doi: 10.1016/S0950-3536(97)80053-4. [DOI] [PubMed] [Google Scholar]
- 12.Trowbridge EA, Martin JF. The platelet volume distribution: a signature of the prethrombotic state in coronary heart disease. Thromb Haemost. 1987;58:714–717. [PubMed] [Google Scholar]
- 13.Doğru T, Taşçı İ, Naharcı Mİ, Sönmez A, Erdem G, Kiliç S. Mean platelet volume levels in metabolic syndrome. AJCI. 2007;1:99–105. [Google Scholar]
- 14.Arıca S, Ozer C, Arıca V, Karakuş A, Celik T, Güneşaçar R. Evaluation of the mean platelet volume in children with familial Mediterranean fever. Rheumatol Int. 2011;32(11):3559–3563. doi: 10.1007/s00296-011-2251-x. [DOI] [PubMed] [Google Scholar]
- 15.Bilici S, Sekmenli T, Göksu M, Melek M, Avci V. Mean platelet volume in diagnosis of acute appendicitis in children. Afr Health Sci. 2011;3:427–432. [PMC free article] [PubMed] [Google Scholar]
- 16.Albayrak Y, Albayrak A, Albayrak F, Yildirim R, Aylu B, Uyanik A, et al. Mean platelet volume: a new predictor in confirming acute appendicitis diagnosis. Clin Appl Thromb Hemost. 2011;4:362–366. doi: 10.1177/1076029610364520. [DOI] [PubMed] [Google Scholar]
- 17.Rioux M. Sonographic detection of the normal and abnormal appendix. AJR Am J Roentgenol. 1992;158:773–778. doi: 10.2214/ajr.158.4.1546592. [DOI] [PubMed] [Google Scholar]
- 18.Siegel MJ. Acute appendicitis in childhood: the role of US. Radiology. 1992;185:341–342. doi: 10.1148/radiology.185.2.1410335. [DOI] [PubMed] [Google Scholar]
- 19.Dow RB. The clinical and laboratory utility of platelet volume parameters. Australian Jnl Med Sci. 1994;15:12–15. [Google Scholar]
- 20.Pieper R, Kagel L. The incidence of acute appendicitis: an epidemiological study of 971 cases. Acta Chir Scand. 1982;148:45–49. [PubMed] [Google Scholar]
- 21.Fales WD, Overton DT, editors. A study guide in emergency medicine. 4. McGraw-Hill: Dallas; 1996. [Google Scholar]
- 22.Pearl RH, Hale DA, Molloy M, Schutt DC, Jaques DP. Pediatric appendectomy. J Pediatr Surg. 1995;30:173–181. doi: 10.1016/0022-3468(95)90556-1. [DOI] [PubMed] [Google Scholar]
- 23.Fishman SJ, Pelosi L, Klavon SL, O'Rourke EJ. Perforated appendicitis: prospective outcome analysis for 150 children. J Pediatr Surg. 2000;35:923–926. doi: 10.1053/jpsu.2000.6924. [DOI] [PubMed] [Google Scholar]
- 24.Rowan RM. Platelet size distribution analysis: principles techniques and potential clinical utility. Hematol Rev. 1986;1:109–144. [Google Scholar]
- 25.Gewirtz AM, Poncz M. Megakaryocytopoiesis and platelet production. In: Hoffman R, Benz EJ Jr, Shattil SJ, Furie B, Cohen H, editors. Hematology: basic principles and practice. New York: Churchill Livingstone; 1991. pp. 1148–1157. [Google Scholar]
- 26.Tong M, Seth P, Penington DG. Proplatelets and stress platelets. Blood. 1987;69:522–528. [PubMed] [Google Scholar]
- 27.Thompson CB, Jakubowski JA. The pathophysiology and clinical relevance of platelet heterogeneity. Blood. 1988;72:1–8. [PubMed] [Google Scholar]
- 28.Holme S, Simmonds M, Ballek R, Murphy S. Comparative measurements of platelet size by coulter counter microscopy of blood smears, and light transmission studies, relationship between platelet size and shape. J Lab Clin Med. 1981;97:610–622. [PubMed] [Google Scholar]
- 29.Bessman JD, Gilmer PR, Gardner FH. Use of mean platelet volume improves detection of platelet disorders. Blood Cells. 1985;11:127–135. [PubMed] [Google Scholar]
- 30.Lanzkowsky P. Disorders of platelet. Manual of pediatric hematology and oncology. 3. Boston: Academic Press; 2000. pp. 233–85. [Google Scholar]
- 31.Nelson RB, Kehl D. Electronically determined platelet indices in thrombocytopenic patients. Cancer. 1981;48:954–956. doi: 10.1002/1097-0142(19810815)48:4<954::AID-CNCR2820480417>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Tomita E, Akatsuka J, Kokubun Y. Differential diagnosis of various thrombocytopenias in childhood by analysis of platelet volume. Pediatr Res. 1980;14:133–137. doi: 10.1203/00006450-198002000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Odell TT, Murphy JR, Jackson CW. Stimulation of megakaryocytopoiesis by acute thrombocytopenia in rats. Blood. 1976;48:765–5. [PubMed] [Google Scholar]
- 34.Kim KY, Kim KE, Kim KH. Mean platelet volume in the normal state and in various clinical disorders. Yonsei Med J. 1986;27:219–226. doi: 10.3349/ymj.1986.27.3.219. [DOI] [PubMed] [Google Scholar]
- 35.Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci. 2000;917:608–617. doi: 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- 36.Cruickshank AM, Fraser WD, Burns HJ. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci. 1990;79:161–165. doi: 10.1042/cs0790161. [DOI] [PubMed] [Google Scholar]
- 37.Kaser A, Brandacher G, Steurer W. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.V98.9.2720. [DOI] [PubMed] [Google Scholar]
- 38.Van Gameren MM, Willemse PH, Mulder NH, et al. Effects of recombinant human interleukin-6 in cancer patients: a phase I–II study. Blood. 1994;84:1434–1441. [PubMed] [Google Scholar]
- 39.Yoon DY, Chu J, Chandler C, Hiyama S, Thompson JE, Hines OJ. Human cytokine levels in nonperforated versus perforated appendicitis: molecular serum markers for extent of disease? Am Surg. 2002;68:1033–1037. [PubMed] [Google Scholar]