Abstract
The aim of this study was to prepare a profile of diabetic foot ulcer (DFU) complications and its management and to assess the outcome of the surgical interventions. A prospective study was carried out in 60 patients with DFU during the period of January 2009 to October 2010 to categorize them based on Meggit–Wagner system and to find out the complications, management, below knee amputation rate, and mortality rate. Majority of the patients (30 %, n = 18) presented with Wegner grade 3 DFU. Only three patients (5 %) presented with grade 0 DFU. Split skin grafting was the most frequently done intervention, comprising 29 % of the time. Below knee amputation was required in 10 % of cases and mortality rate was 12 %. Lack of awareness about diabetes mellitus and its lower limb complications, poor compliance to the treatment, poorly controlled blood sugar levels, delay in diagnosis, and late presentation to the tertiary care center are all factors which led to occurrence of DFU at an age earlier than that seen in other studies.
Keywords: Diabetic foot ulcer, Complications, Management, Wagner grade
Introduction
The World Health Organization defines diabetic foot as the lower limb of a diabetic patient that has the potential risk of pathological consequences, including infection, ulceration, and/or destruction of deep tissues associated with neurologic abnormalities, various degrees of peripheral vascular disease, and/or metabolic complications of diabetes. The world is facing a major epidemic of diabetes. About 194 million people worldwide or 5.1 % in the age group of 20 to 79 were estimated to have diabetes in 2003 [1]. This estimate is expected to increase to some 333 million or 6.3 % of the adult population by 2025 [1]. India is the world capital of known diabetes. There are currently more than 30 million people living with diabetes in India. There is also an increasing number of young people and children with type 2 diabetes, especially among ethnic minority groups. This increase in diabetes is mainly attributed to modernization or westernization of the world’s societies [2]. In the USA, diabetes is expected to increase by 60 % over the next 22 years, while in Europe, diabetes is expected to increase by 16 %. Diabetes is expected to increase in Australia by 59 %, in South America by 88 %, and in Africa, Middle East, and Asia by a tremendous 98 % [3]. Foot ulcers develop in about 15 % of patients with diabetes and foot disorders are the leading cause of hospitalization for patients with diabetes [4–6]. The lifetime risk of a person with diabetes developing a foot ulcer could be as high as 25 % [7]. Up to 70 % of all leg amputations in the USA are performed on people with diabetes [8], and approximately 85 % of lower limb amputations in patients with diabetes are preceded by foot ulceration [5], highlighting the importance of prevention and appropriate management of foot lesions. Worldwide, a lower limb is lost every 30 s as a consequence of diabetes [9]. Among persons with diabetes, 15 % develop foot ulcers during their lifetime. Their risk of lower extremity amputation increases by a factor of 8 once an ulcer develops. There is a mortality rate of 36 % at 2 years following transtibial amputation [10] for diabetic foot ulcer. In fact, every year, approximately 5 % of diabetics develop foot ulcers and 1 % requires amputation. Diabetic peripheral neuropathy, present in 60 % of diabetic persons and 80 % of diabetic persons with foot ulcers, confers the greatest risk of foot ulceration and microvascular disease, and suboptimal glycemic control also contributes. Even after successful management resulting in ulcer healing, the recurrence rate is 66 % and the amputation rate rises to 12 %. Half of all nontraumatic amputations are the result of diabetic foot complications, and the 5-year risk of needing a contralateral amputation is 50 % [11]. Diabetes occurs in 3 to 6 % of Americans. Of these, 10 % have type 1 diabetes and are usually diagnosed when they are younger than 40 years. Among middle-aged adults, the prevalence of diabetes is about 10 % (of these, 90 % have type 2 diabetes). Diabetic neuropathy tends to occur about 10 years after the onset of diabetes, and, therefore, diabetic foot deformity and ulceration occur sometime thereafter. Charcot foot (neuropathic osteoarthropathy) is most commonly observed in diabetes mellitus, affecting about 2 % of diabetic persons.
Materials and Methods
The aim of this study was to prepare a profile of diabetic foot ulcer (DFU) complications and its management and to assess the outcome of the surgical interventions. The objectives were to find out all the cases of DFU, to take a detailed history and do a standard clinical examination and necessary investigations, categorize them into six grades from 0 to 5 based on Meggit–Wagner classification system, find out the complications of DFU in each case, study the different types of management of DFU in those cases, and to find out below knee amputation rate and mortality rate. All the cases of diabetic foot ulcer that were treated in MKCG Medical College, Berhampur during the period of January 2009 to October 2010 were taken as the study population and a descriptive study was carried out in 60 consecutive cases with DFU. Patients with diabetic foot ulcer having other diseases causing ulcer in the foot and those cases which could not be followed up for a minimum period of 6 weeks were excluded from this study. Written informed consents were obtained from the patients and they were evaluated and a follow-up was done at 6 weeks to note the recurrence. All the details of the patients, their examination findings, diagnosis, and investigation reports were maintained as per the proforma. Foot ulcers were categorized into six grades from 0 to 5 based on Meggit–Wagner classification system as follows:
| Grade | Lesion |
| 0 | No open lesions; may have deformity or cellulitis |
| 1 | Superficial diabetic ulcer (partial or full thickness) |
| 2 | Ulcer extension to ligament, tendon, joint capsule, or deep fascia without abscess or osteomyelitis |
| 3 | Deep ulcer with abscess, osteomyelitis, or joint sepsis |
| 4 | Gangrene localized to portion of forefoot or heel |
| 5 | Extensive gangrenous involvement of the entire foot |
A semi-structured questionnaire was developed to record the medical history, examination details, and investigation reports. A pilot study was carried out on five subjects and we found that transcutaneous oxygen tension, ankle–brachial pressure index, and Doppler study could not be carried out in all patients because of the lack of facility and poor financial backup of patients, and those parameters were omitted from the proforma. Neuropathy was assessed by a 128-Hz tuning fork and a 10-g monofilament. Absent pulsations of dorsalis pedis and posterior tibial, popliteal, or femoral vessels were considered to be the sign of ischemia. In cases with bilateral involvement, the foot with higher grade was studied. Septic shock is defined by a fall in systolic BP below 90 mmHg along with any two or more of the following: pulse rate >90/min, respiratory rate >20/min, body temperature >38 or <36 °C, neutrophil count <4,000 or >14,000 mL−3. Ketosis will occur if the urine ketone bodies were positive. Hyperosmolar state is defined by a plasma osmolality >310 mmol/L. Osmolality was calculated using the equation:
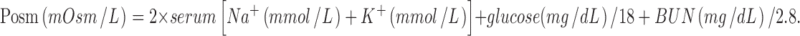 |
Serum sodium concentration below 110 mmol/L was defined as hyponatremia.
Data collection was done using the proforma and the study variables were recorded. The study parameters were age, sex, and occupation of the patient, duration of the symptoms, duration of diabetes mellitus, type of treatment, compliance to treatment, Wegner grade of the DFU, hemoglobin percentage at the time of admission, number of units of blood transfused, complications of DFU, pus culture, modality of management, duration of hospital stay, and recurrence of DFU at 6 weeks after completion of treatment. Age at the time of diagnosis of diabetes mellitus, smoking, alcoholism, awareness of complications, peripheral neuropathy, peripheral vascular disease, pulse rate, systolic and diastolic BP in millimeter of mercury, respiratory rate, random blood sugar (RBS), serum osmolality, axillary temperature in degree Celsius, and serum sodium in millimoles per liter were also recorded during admission. The data were collected and analyzed using descriptive statistics.
Results
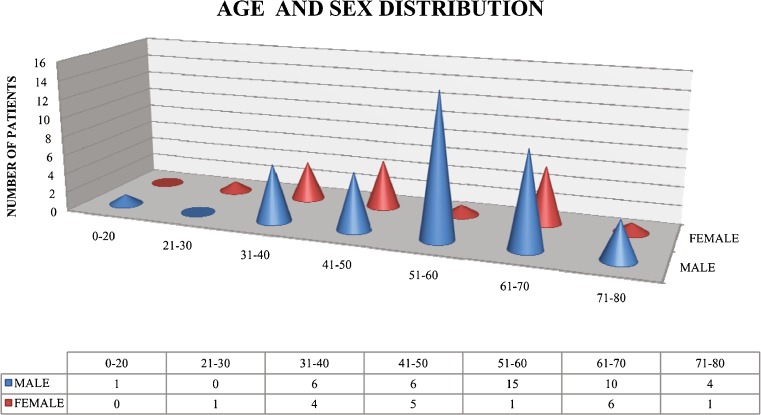
Among the 60 DFU patients studied, 42 (70 %) were males and 18 (30 %) were females. Male-to-female ratio is 2.33:1. The age of patients ranged from 20 to 80 years. Mean age was 54.57 years and the SD was 13 years. Majority of patients (54 %) were in the age group of 51 to 70 years. Majority of male patients were in the age group of 51 to 60 years. Majority of female patients were in the age group of 61 to 70 years (Fig. 1). Seventy-five percent (n = 45) of patients were above 40 years of age, while 25 % (n = 15) were 40 years and below at the time of diagnosis of diabetes mellitus.
Fig. 1.
Age and sex distribution
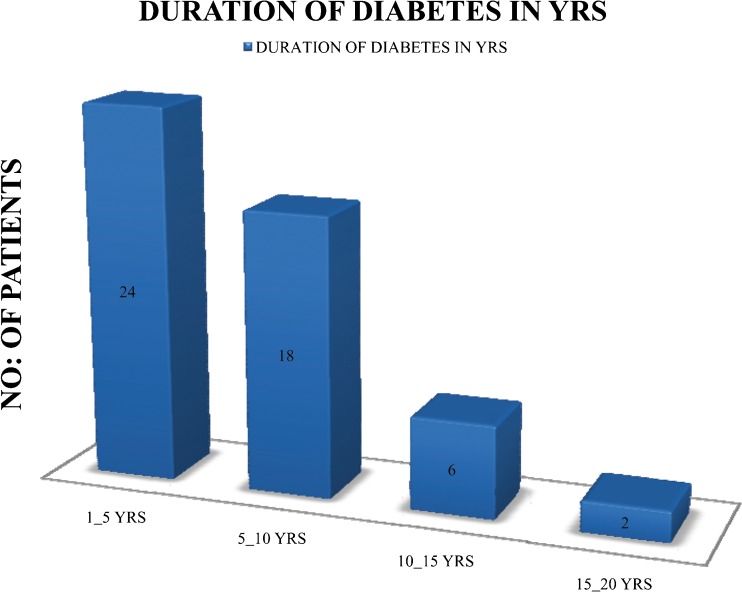
Only eight patients (13.33 %) were presented to the tertiary care center within 5 days of onset of symptoms; 20 patients (34 %) were presented within 30 days of onset of symptoms, whereas majority of patients (66 %) were presented after 1 month of onset of symptoms. Eighty-three percent (n = 50) of cases were known cases of diabetes; 17 % (n = 10) were newly diagnosed cases and were presented with DFU as the initial symptom. In majority of patients (48 %, n = 24), diabetes was diagnosed within 5 years only (Fig. 2). Mean duration of diabetes was 5.975 years and the SD was 5.265 years.
Fig. 2.
Duration of diabetes in years
Only 20 patients (40 %) were taking drugs regularly, while 30 patients (60 %) were on irregular treatment; 24 patients (48 %) were on subcutaneous insulin, 25 patients (50 %) were taking oral hypoglycemic drugs, and 1 patient (2 %) was not taking any medication; 22 % of the patients were dependents and 29 % were doing either office job, teaching, or business; 22 % were housewives; and 20 % were carpenters or other moderate physical activity workers. Only 7 % of the patients were manual laborers or agricultural workers; 18 % (n = 11) of the patients were aware of lower limb complications of diabetes, whereas 82 % (n = 49) were unaware of it; and 18 patients (30 %) were smokers and 15 % (n = 9) were alcoholics. Neuropathy was present in 38 patients (63 %) and peripheral vascular disease (PVD) was present in 21 patients (35 %). The mean RBS was 278.25 mg/dL and the SD was 88.26 mg/dL; 82 % of patients were having RBS more than 200 mg/dL, that means with uncontrolled diabetes mellitus; 15 % of the patients (n = 9) were having hemoglobin (Hb) of 6 g/dL and below, whereas another 15 % (n = 9) were having Hb between 6.1 and 7.9 g/dL. Majority of patients (70 %, n = 42) were having Hb of 8 g/dL or more. The mean Hb was 9.11 g/dL and the SD was 2.14 g/dL. Blood transfusion was given to 52 % (n = 31) of the patients, among whom 23 % (n = 7) received 1 unit, 29 % (n = 9) received 2 units, 23 % (n = 7) received 3 units, 19 % (n = 6) received 4 units, 3 % (n = 1) received 5 units, and 3 % (n = 1) received 6 units.
Out of the 60 pus samples collected, 3 samples (5 %) were sterile; 13 samples (22 %) showed polymicrobial flora and 44 samples (73 %) showed monomicrobial flora. Out of the 68 isolated and cultured organisms, 61 (90 %) were bacteria and 7 (10 %) were fungi. Sixty-two percent of isolates in grade 5 DFU were polymicrobial and none of the samples were sterile. Eighty percent of the isolates in grade 4 were monomicrobial, 30 % were polymicrobial, and 7 % were sterile. Twenty-eight percent (n = 17) Pseudomonas aeruginosa, 25 % (n = 15) Staphylococcus aureus, 18 % (n = 11) Escherichia coli, 11 % (n = 7) Proteus, 6 % (n = 4) Enterococcus faecalis, 5 % (n = 3) Klebsiella pneumoniae, 5 % (n = 3) Acinetobacter, and 2 % (n = 1) Citrobacter were the isolated bacteria in the pus culture. Pseudomonas was isolated in 28 % of the samples and Staphylococcus in 25 % of the samples. Polymicrobial flora is seen more in grade 5 compared to other grades. Out of the seven fungal isolates, Candida was present four times, Aspergillus was present twice, and Fusarium was present only once. Polymicrobial flora is seen more in grade 5 compared to grade 4. P value is 0.0147.
Out of the 60 cases, 5 % (n = 3) were Wagner grade 0, 10 % (n = 6) were grade 1, 17 % (n = 10) were grade 2, 30 % (n = 18) were grade 3, 25 % (n = 15) were grade 4, and 13 % (n = 8) were grade 5. A majority of the patients (30 %, n = 18) presented with Wagner grade 3 DFU. Only three patients (5 %) presented with grade 0 DFU. Out of the 60 cases, 38.33 % (n = 23) were presented with various complications, 35 % (n = 8) had septic shock, 4 % (n = 1) had serum hyperosmolarity, 13 % (n = 3) had ketosis, 26 % (n = 6) had both septic shock and hyperosmolarity, 9 % (n = 2) had septic shock and ketosis, and 13 % (n = 3) had hyponatremia. Septic shock (70 %) was the most commonly encountered complication being present in 35 % of the cases alone and along with other complications in another 35 % of the cases.
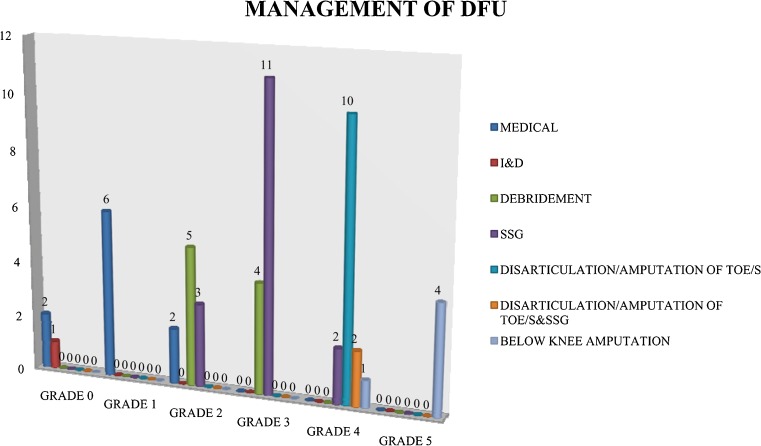
Out of the 53 patients who survived, 19 % (n = 10) were treated by medical means, 2 % (n = 1) by incision and drainage, 17 % (n = 9) by debridement, 29 % (n = 16) by debridement and split skin graft (SSG), 19 % (n = 10) by disarticulation/amputation of toe/s, 4 % (n = 2) by disarticulation/amputation of toe/s and SSG, and 10 % (n = 5) by below knee amputation. SSG (29 %) was the most frequently done intervention for the management of DFU. In grade 0 and 1 DFU, medical management alone was successful in 88.9 % of cases (Fig. 3). Amputation was required in 89.5 % of cases of grade 4 and 5 DFU. Below knee amputation rate is high in grade 5 compared to grade 4 DFU. P value is 0.0164. Fifty-one percent of the patients had to stay in the hospital for more than 20 days. Out of the 53 patients who were treated successfully, 7 patients had recurrence at the 6-week follow-up visit. Of the seven patients who died, septic shock was present in one patient, septic shock and hyperosmolar state were present in three patients, septic shock and ketosis were present in two patients, and hyponatremia was present in one patient.
Fig. 3.
Management of diabetic foot ulcer in different grades
Discussion
Average age of DFU in our study was 2.5 years that is less than Bansal et al. [12] study (Table 1). In our study, more female patients were present as the male-to-female ratio is 2.33 only. Majority of patients had duration of diabetes of less than 5 years. Awareness to lower limb complications was very less in our study subjects compared to the Bansal et al. study. Majority of patients were noncompliant to the treatment and revealed poor glycemic control. In the present study, neuropathy was more and PVD was less prevalent. Gram-positive-to-gram-negative ratio is almost same in both studies. Pseudomonas was the predominant isolate in both studies, but shows higher percentage in the present study. Out of the 60 patients, 12 % (n = 7) mortality was observed in our study. Out of the remaining 53 patients who were treated successfully, 7 patients had recurrence at the 6-week follow-up visit. Polymicrobial flora was seen more in grade 5 compared to grade 4 (P value is 0.0147). Amputation rate is high in grade 5 compared to grade 4 (P value is 0.0164).
Table 1.
Comparison between the present study and the Bansal et al. [12] study
| Parameter | Present study | Bansal et al. study |
|---|---|---|
| Average age in years | 54.57 | 57.05 |
| Male-to-female ratio | 2.33 | 3.68 |
| Duration of diabetes (DOD) <5 years | 48 % | 25.24 % |
| DOD 5–10 years | 36 % | 26.21 % |
| DOD >10 years | 16 % | 48.54 % |
| Smoking | 30 % | 20.39 % |
| Alcoholism | 15 % | 36.89 % |
| No awareness of lower limb complications | 82 % | 77 % |
| Noncompliant to treatment of diabetes | 60 % | 37.11 % |
| Neuropathy | 63 % | 57.28 % |
| PVD | 35 % | 75.73 % |
| Poor glycemic control >200 mg/dL | 82 % | 67 % |
| Gram positive | 25 % | 24 % |
| Gram negative | 75 % | 76 % |
| Pseudomonas | 28 % | 21.67 % |
| Staphylococcus | 25 % | 18.88 % |
| E. coli | 18 % | 18.18 % |
DFU is a disease seen exclusively in diabetic patients and it develops usually in the sixth and the seventh decades of life. It usually develops at a 5- to 10-year duration of diabetes mellitus. The main predisposing factors were peripheral neuropathy and PVD. Other contributory risk factors include obesity, sedentary life style, smoking, and alcoholism. Slight male predominance was observed. Lack of awareness about diabetes mellitus and its lower limb complications, poor compliance to treatment, delay in diagnosis, and late presentation to the tertiary care center are all factors which led to the occurrence of DFU at a younger age than that was observed in other studies. DFU is a polymicrobial infection and its polymicrobial nature is more evident in higher grades of 4 and 5. Majority of isolates were gram negative. Pseudomonas was the most common organism isolated and Staphylococcus was the most common gram-positive isolate. Nonpathogenic fungi cause opportunistic infection in diabetic foot patients. Candida was the most commonly isolated fungus. DFU is associated with systemic complications such as septic shock, diabetic ketoacidosis, hyperglycemic hyperosmolar state, and hyponatremia which can be life threatening if not recognized and treated promptly. Debridement and SSG were the most frequently performed surgical intervention for DFU. The disease is a financial burden to the patient as the average hospital stay is 25 days. DFU carries 33 % amputation rate and 12 % mortality.
References
- 1.International Diabetes Federation. Diabetes Atlas (2006) Available at: http://www.eaqtlas.idf.org/Prevalence/All_diabetes. Accessed 17 May 2006
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention National Diabetes Fact Sheet 2005. Available at: http://www.edc.gov/diabetes/pubs/estimates05.htm#prev. Accessed 17 May 2006
- 4.Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM. Preventive foot care in people with diabetes. Diabetes Care. 1998;21(12):2161–2177. doi: 10.2337/diacare.21.12.2161. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo PJ, Melton LJ III (1985) Peripheral vascular disease and diabetes. In: Diabetes in America: Diabetes Data Complete 1984. Washington, DC: Government Printing Office; XV-I–XV-21
- 6.Consensus Development Conference on Diabetic Foot Wound Care. 7–8 April 1999. Boston, Massachusetts: American Diabetes Association Diabetes Care. 1999; 2298:1354–1360 [DOI] [PubMed]
- 7.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 8.Reiber GE, Ledous WR. Epidemiology of diabetic foot ulcers and amputations evidence for prevention. In: Williams R, Herman W, Kinmonth A, Wareham NJ, editors. The evidence base for diabetes care. Hoboken: Wiley; 2002. pp. 642–665. [Google Scholar]
- 9.International Diabetes Federation (2005) Time to act. Diabetes and foot care. Brussels, Belgium: International Diabetes Federations
- 10.Michael S Pinzur, MD (2010) In: Diabetic foot. Available at: http://emedicine.medscape.com/article/1234396-overview. Accessed 20 Oct 2012
- 11.Richard M Stillman, MD (2010) FACS. Diabetic ulcers. Updated: 7 Jun 2010. Available at: http://emedicine.medscape.com/article/460282-overview Accessed 20 Oct 2012
- 12.Bansal E, Garg A, Bhatia S, Attri AK, Chander J. Spectrum of microbial flora in diabetic foot ulcers. Indian J Pathol Microbiol. 2008;51:204–208. doi: 10.4103/0377-4929.41685. [DOI] [PubMed] [Google Scholar]