Abstract
Many clinical conditions such as shock, sepsis, mesenteric thrombosis, necrotizing enterocolitis, and bowel transplantation can cause intestinal ischemia–reperfusion (IR) injury. This study was designed to determine the effects of leptin on intestinal IR injury. Thirty rats were divided into three groups, each containing ten rats: group A (IR group), group B (treatment group), and group C (sham group). After 1 h of intestinal ischemia, the clamp was removed in order to perform reperfusion. In group B, 100 mg/kg leptin was administered subcutaneously 30 min before reperfusion. In groups A and C, 0.1 ml physiologic saline was injected. In group A, serum and tissue nitric oxide (NO) levels were significantly decreased, and malondialdehyde levels were significantly increased compared to sham group (p < 0.05). Histopathologic injury was significantly lower in sham group compared to group A. In group B, serum and tissue malondialdehyde levels were significantly decreased (p < 0.05), but serum and tissue NO levels were significantly increased compared to group A (p < 0.05). Histopathologic injury was significantly lower in group B compared to group A (p < 0.05). The results of the present study demonstrated that leptin decreases intestinal IR injury by increasing NO production, rearranging mucosal blood flow, and inhibiting polymorphonuclear leukocyte infiltration.
Keywords: Leptin, Ischemia, Reperfusion injury, Intestine, Nitric oxide, Malondialdehyde
Introduction
Interruption of the blood supply to the intestine results in intestinal ischemia. The initial injury caused by ischemia is further worsened by reperfusion, paradoxically [1]. The enhanced generation of oxygen radicals and the activation of phospholipase are the possible mechanisms that cause ischemia–reperfusion (IR) injury.
Leptin is a 16-kDa non-glycosylated peptide hormone, encoded by the obese gene, which is located on human chromosome 7 and mainly produced by adipocytes [2]. Its plasma levels are directly correlated with adipose tissue mass and act at hypothalamic central level as a satiety factor inducing a decrease in food intake and an increase in energy consumption [3]. Several studies have demonstrated that acute infection, sepsis, and a wide range of inflammatory mediators increase serum leptin levels, suggesting that leptin is a part of the immune response and host defense mechanisms [4–6]. It has been reported that circulating leptin levels are highly and promptly increased in experimental models of acute inflammation [7]. Previous studies showed that leptin activates monocytes and T lymphocytes and it has interactions with inflammatory mediators such as interleukin-1β, tumor necrosis factor-α, and C-reactive protein [2, 8–10].
Previously, it was demonstrated that leptin increases nitric oxide (NO) synthesis by activating nitric oxide synthase in Wistar-albino rats [11]. Although the effect of leptin treatment on tissue NO and malondialdehyde (MDA) levels in intestinal IR injured rats was reported, its effect on serum levels of NO and MDA has not been researched yet.
This study aims to research the effect of leptin on remodeling of intestinal IR injury by detecting serum and tissue levels of NO and MDA, and also to determine the histopathologic grades in Wistar-albino rats.
Materials and Methods
Preparation
With permission obtained from the Animal Ethics Committee of Gaziantep University, 140 ± 14.5 g Wistar-albino rats were used in the study. The rats were kept in constant room temperature and humidity during 12-h daylight and 12-h dark periods. They were maintained on a standard laboratory dry feed and provided water ad libitum.
Thirty female Wistar-albino rats were randomly allocated into three groups according to procedures performed, each group containing ten rats:
IR group (group A): Only surgery was performed, no medication. 0.1 mL SF.
Treatment group (group B): Leptin was administered before reperfusion.
Sham group (group C): Only laparotomy was performed, no medication. 0.1 mL SF.
The rats were anesthetized using ketamine hydrochloride (Ketalar, Eczacıbaşı, Turkey) and all procedures were performed under sterile technique after a 12-h starvation period. The superior mesenteric artery was occluded with an atraumatic vascular clamp through a midline incision. The intestine was replaced into the peritoneal cavity and the laparotomy incision was closed continuously with 5/0 silk (Silk, Ethicon, UK). After 1 h of intestinal ischemia, the clamp was removed to perform reperfusion. Reperfusion of the superior mesenteric arteries was determined by the return of pulsation and color.
As 100 μg/kg leptin (Sigma-Aldrich, Germany) and 0.1 mL physiologic saline were administered subcutaneously in group B, 0.1 mL physiologic saline was injected in groups A and C 30 min before the reperfusion. Relaparotomy was performed on all rats 4 h after the reperfusion. A 5-cm-long terminal ileum was harvested and placed on ice. This segment was rinsed fully with physiologic saline and divided into two equal pieces: one of them was used to measure tissue NO and MDA concentration and was immediately placed in deep freezer at −80 °C, and the other was placed in 10 % formalin solution for histopathologic examination. Serum samples were collected by puncturing their hearts to analyze serum NO and MDA levels.
Measurement of Serum and Tissue NO
After the homogenization of the intestinal tissue, it was centrifuged at 4,000×g for 5 min. Supernatant was assayed by a modification of the cadmium reduction method as Cortas and Wakid reported for serum NO levels [12]. The results were expressed as micrometer per liter of protein for the serum and micrometer per microgram of protein for the tissue levels.
Measurement of Serum and Tissue MDA
The intestinal tissue was homogenized and centrifuged. MDA levels were determined by thiobarbituric acid in supernatant similar to serum. The results were expressed as micrometer per liter of protein for the serum and micrometer per microgram of protein for the tissue levels.
Histopathologic Examination
Terminal ileum segments were embedded in paraffin, and 4-μm-thick sections were stained with hematoxylin and eosin. The degree of intestinal tissue injury was evaluated under a light microscope by a blinded pathologist and it was graded from 0 to 5 according to Chiu scale as follows: grade 0—mucosa without changes; grade 1—subepithelial space at the tips of the villi; grade 2—extension of the subepithelial space; grade 3—massive desquamation from the tips of the villi; grade 4—entirely desquamated mucosa with markedly capillary congestion; and grade 5—derangement of lamina propria with ulceration.
Statistical Analysis
The statistical analyses were performed using SPSS for Windows 10.0 (SPSS Inc. Software, Chicago, Illinois, USA). The results were expressed as mean ± standard deviation. One-way analysis of variance was used to compare serum and tissue NO and MDA levels among groups. The histopathologic difference between groups was evaluated by chi-square test. p < 0.05 was accepted as statistically significant.
Results
Evaluation of Biochemical Measurements
Although tissue and serum NO levels were significantly lower in group A compared to group C, tissue and serum MDA levels were significantly higher (p < 0.05). Although tissue and serum NO levels were significantly higher in group B compared to group A, tissue and serum MDA levels were significantly lower (p < 0.05) (Table 1).
Table 1.
Effects of leptin on serum and tissue MDA and NO levels
| Groups | Serum MDA | Serum NO | Tissue MDA | Tissue NO |
|---|---|---|---|---|
| (n = 10) | μm/L | μm/L | μm/mg | μm/mg |
| A* | 40.53 ± 26.83 | 207.58 ± 18.69 | 0.83 ± 0.64 | 52.19 ± 21.53 |
| B** | 19.31 ± 8.88 | 312.25 ± 71.32 | 0.20 ± 0.25 | 82.42 ± 40.88 |
| C | 8.94 ± 4.04 | 299.99 ± 54.25 | 0.10 ± 0.17 | 91.96 ± 31.08 |
*p < 0.05 (compared to group C); **p < 0.05 (compared to group A)
Histopathological Examination
Intestinal tissue injury was significantly higher in group A compared to group C (p < 0.05). It was significantly lower in group A compared to group B (p < 0.05) (Table 2; Figs. 1, 2, and 3).
Table 2.
The levels of intestinal injury scores
| Groups(n = 10) | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| A* | – | – | 4 | 4 | 2 | – |
| B** | – | 7 | 2 | 1 | – | – |
| C | 10 | – | – | – | – | – |
*p < 0.05 (compared to group C); **p < 0.05 (compared to group A)
Fig. 1.

Grade 0—normal intestinal mucosa in group C rats (H&E, ×100).
Fig. 2.
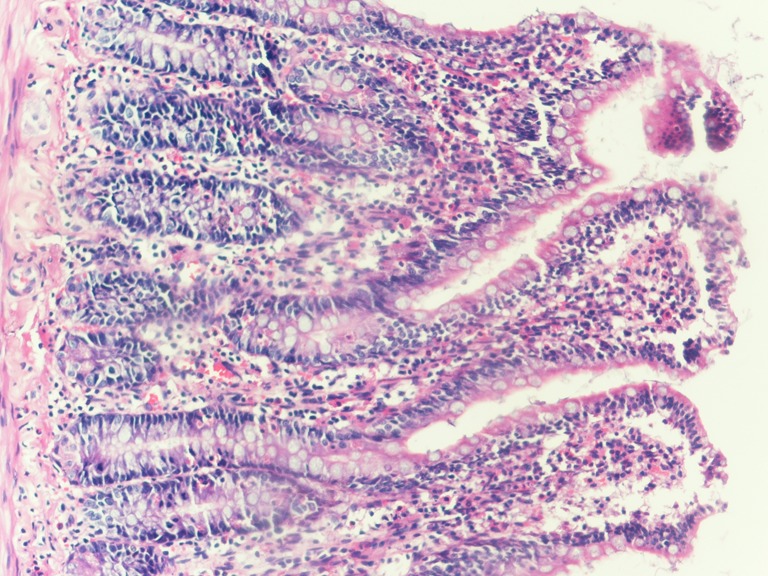
Grade 1—development of a subepithelial space at the tip of a villus in group B rats (H&E, ×100).
Fig. 3.

Grade 4—structural destruction of the villosities, formed by inflamed cells and necrotic material in group A rats (H&E, ×100).
Discussion
The intestine is highly sensitive to IR injury caused by various clinical conditions such as shock, sepsis, midgut volvulus, neonatal necrotizing enterocolitis, mesenteric thrombosis, and bowel transplantation [13]. It is associated with a high morbidity and mortality. In the ischemic phase of IR injury, hypoxia induces intestinal mucosal cell damage. Reoxygenation of the hypoxic tissue causes the second phase of injury by inducing reactive oxygen species (ROS) production.
During reperfusion, hypoxanthine is metabolized to xanthine by xanthine oxidase. In this process, superoxide radical (O2·−) is generated and converted to hydrogen peroxide (H2O2) or a hydroxyl radical (.OH). The conversion of xanthine dehydrogenase to xanthine oxidase by intracellular calcium, which is accumulated in microvascular endothelial cells during ischemia, is markedly seen in the ileum [14]. Therefore, the terminal ileum was preferred to evaluate the effects of the IR injury.
Increased lipid peroxidation in the intestine has been demonstrated with markedly increased MDA levels in rats with intestinal IR injury [15, 16]. Therefore, MDA is accepted as a reliable index of lipid peroxidation and oxidative tissue injury. NO plays a significant role in the maintenance of mucosal integrity and vascular homeostasis. The failure of NO production in the mesenteric endothelium is thought to be the primary mechanism in microvascular deterioration in IR injury [17]. In this study, tissue and serum NO levels markedly decreased after a 1-h mesenteric ischemia and a 4-h reperfusion compared to laparotomy performed group. Furthermore, tissue and serum MDA levels markedly increased in the ischemic group compared to the nonischemic group. Histopathological analysis revealed that intestinal tissue injury markedly increased in the group A compared to group C. These findings showed that it is a suitable model for intestinal IR injury in rats.
The development of intestinal tissue damage in IR injury depends on the balance between ROS and protective mechanisms, including NO produced by the constitutive NO synthase and indigenous probiotics such as Bifidobacterium infantis. Early stage of acute intestinal ischemia that is characterized by mucosal erosions and hemorrhagic ulcerations could be reversible, if stopped with vasodilator drugs.
Leptin is an active protein, mainly secreted by adipose tissue in mice, rats, and humans, which consists of 167 amino acids. The similarity of leptin precursor in humans, mice, and rats is very high; that is, human leptin is 83–84 % identical to mouse and rat leptin [18]. Structurally, it belongs to the type 1 superfamily. Leptin is an anorexic peptide that acts as a hypothalamic modulator of food intake, body weight, and fat stores [19]. It also induces angiogenesis [20]. Leptin regulates vascular tone through local mechanisms involving NO release [21, 22]. Kimura et al. have suggested that arterial relaxation by leptin is mediated by NO released from the endothelium [23]. The protective effect of leptin in intestinal IR injury has recently been demonstrated [24, 25]. Hacioglu et al. performed intestinal IR injury in adult Wistar rats weighting 200–250 g, but we used 140 ± 14.5 g rats, aged 8 weeks in our study [24]. In contrast to our study, they did not report any significant difference in intestinal injury to the ileum between IR + leptin group and IR group. This may be because of the short (2 h) reperfusion they performed [24]. Sukhotnik et al. showed the protective effect of leptin in rats that underwent 30-min mesenteric ischemia followed by 24 h of reperfusion, but they administered leptin at a dose of 50 μg/kg once a day for 48 h before and 24 h following IR [25]. Differently, we showed the protective effect of single-dose leptin administered 30 min before reperfusion in our study.
A recent study has shown that supplementing NO substrate, l-arginine, before reperfusion reduces serum MDA and enhances serum NO production in IR injury [26]. Leptin has a time-dependent response to acute inflammatory stimuli and acts as an anti-inflammatory cytokine [4]. Accumulation of polymorphonuclear leukocyte (PMNL) on postcapillary venular endothelium plays an important role in IR injury. Bozkurt et al. reported the anti-inflammatory effect of leptin involving the reduction in colonic neutrophil infiltration in rats with colitis [27]. In the present study, tissue and serum NO levels markedly increased in leptin-administered group compared to the IR group. Also, histopathological analysis revealed lower intestinal tissue injury in group B. Leptin may inhibit PMNL accumulation by inducing NO production. To the best of our knowledge, it is the first reported study that demonstrates the protective effect of leptin in intestinal IR injury by analyzing serum and tissue NO and MDA levels and histopathologic grades together in Wistar-albino rats.
In conclusion, the results of the present study indicated that leptin may protect intestinal mucosa against IR injury, probably by increasing NO production, rearranging mucosal blood flow, and inhibiting PMNL infiltration.
References
- 1.Granger DN, Hollwarth ME, Parks DA. Ischemia–reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- 2.Santos-Alvarez J, Goberna R, Sanchez Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 3.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- 4.Lin J, Yan GT, Wang LH, Hao XH, Zhang K, Xue H. Leptin fluctuates in intestinal ischemia–reperfusion injury as inflammatory cytokine. Peptides. 2004;25:2187–2193. doi: 10.1016/j.peptides.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 6.Francis J, MohanKumar PS, MohanKumar SM, Quadri SK. Systemic administration of lipopolysaccharide increases plasma leptin levels: blockade by soluble interleukin-1 receptor. Endocrine. 1999;10:291–295. doi: 10.1007/BF02738628. [DOI] [PubMed] [Google Scholar]
- 7.Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, 3rd, Flier JS, Lowell BB, Fraker DL. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas P, Straub RH, Bedoui S, Nave H. Peripheral but not central leptin treatment increases numbers of circulating NK cells, granulocytes and specific monocyte subpopulations in non-endotoxaemic lean and obese LEW-rats. Regul Pept. 2008;151:26–34. doi: 10.1016/j.regpep.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, Fantuzzi G. Leptin deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci U S A. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI200421134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehebik N, Jaubert AM, Sabourault D, Giudicelli Y, Ribière C. Leptin-induced nitric oxide production in white adipocytes is mediated through PKA and MAP kinase activation. Am J Physiol Cell Physiol. 2005;289:C379–C387. doi: 10.1152/ajpcell.00320.2004. [DOI] [PubMed] [Google Scholar]
- 12.Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440–1443. [PubMed] [Google Scholar]
- 13.Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia–reperfusion injury. Anesthesiology. 2001;94:1133–1138. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 14.Qu XW, Rozenfeld RA, Huang W, Bulkley GB, Hsueh W. The role of xanthine oxidase in platelet activating factor induced intestinal injury in the rat. Gut. 1999;44:203–211. doi: 10.1136/gut.44.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma NS. Changes in malondialdehyde contents in serum and tissues after ischemia and reperfusion of the bowel in dogs. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1992;8:127–129. [PubMed] [Google Scholar]
- 16.Van Ye TM, Roza AM, Pieper GM, Henderson J, Jr, Johnson CP, Adams MB. Inhibition of intestinal lipid peroxidation does not minimize morphologic damage. J Surg Res. 1993;55:553–558. doi: 10.1006/jsre.1993.1183. [DOI] [PubMed] [Google Scholar]
- 17.Salzman AL. Nitric oxide in the gut. New Horiz. 1995;3:33–45. [PubMed] [Google Scholar]
- 18.Taouis M, Chen JW, Daviaud C, Dupont J, Derouet M, Simon J. Cloning the chicken leptin gene. Gene. 1998;208:239–242. doi: 10.1016/S0378-1119(97)00670-7. [DOI] [PubMed] [Google Scholar]
- 19.Paracchini V, Pedotti P, Taioli E. Genetics of leptin and obesity: a HuGE review. Am J Epidemiol. 2005;162:101–114. doi: 10.1093/aje/kwi174. [DOI] [PubMed] [Google Scholar]
- 20.Anagnostoulis S, Karayiannakis AJ, Lambropoulou M, Efthimiadou A, Polychronidis A, Simopoulos C. Human leptin induces angiogenesis in vivo. Cytokine. 2008;42:353–357. doi: 10.1016/j.cyto.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B, Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51:168–173. doi: 10.2337/diabetes.51.1.168. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi N, Waelput W, Guisez Y. Leptin is an endogenous protective protein against the toxicity exerted by tumor necrosis factor. J Exp Med. 1999;189:207–212. doi: 10.1084/jem.189.1.207-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura K, Tsuda K, Baba A, Kawabe T, Boh-oka S, Ibata M, Moriwaki C, Hano T, Nishio I. Involvement of nitric oxide in endothelium-dependent arterial relaxation by leptin. Biochem Biophys Res Commun. 2000;273:745–749. doi: 10.1006/bbrc.2000.3005. [DOI] [PubMed] [Google Scholar]
- 24.Hacioglu A, Algin C, Pasaoglu O, Pasaoglu E, Kanbak G. Protective effect of leptin against ischemia–reperfusion injury in the rat small intestine. BMC Gastroenterol. 2005;5:37. doi: 10.1186/1471-230X-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sukhotnik I, Helou H, Lurie M, Khateeb K, Bejar J, Coran AG, Mogilner JG, Shiloni E. The effect of leptin on intestinal recovery following ischemia–reperfusion injury in a rat. Pediatr Surg Int. 2007;23:273–478. doi: 10.1007/s00383-006-1863-9. [DOI] [PubMed] [Google Scholar]
- 26.Fu TL, Zhang WT, Zhang L, Wang F, Gao Y, Xu M. L-arginine administration ameliorates serum and pulmonary cytokine response after gut ischemia–reperfusion in immature rats. World J Gastroenterol. 2005;11:1070–1072. doi: 10.3748/wjg.v11.i7.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozkurt A, Cakir B, Ercan F, Yegen BC. Anti-inflammatory effects of leptin and cholecystokinin on acetic acid-induced colitis in rats: role of capsaicin-sensitive vagal afferent fibers. Regul Pept. 2003;116:109–118. doi: 10.1016/S0167-0115(03)00194-0. [DOI] [PubMed] [Google Scholar]


