Abstract
Diabetic foot wounds present a great challenge to surgeons. They are difficult to heal and are a significant risk factor for non-traumatic foot amputation besides being a huge financial burden. NPWT systems commercially available (VAC™ system, KCI Inc., USA) are costly precluding widespread use. To determine whether negative-pressure wound therapy (NPWT) would afford quicker wound recovery as compared to saline-moistened gauze in the treatment of diabetic foot wounds. Sixty patients were randomized into either the experimental NPWT group or conventional dressing group (control). All patients were given medical therapy for diabetes and antibiotics given according to culture and sensitivity patterns. All foot ulcers were surgically debrided prior to initiation of NPWT or conventional treatment. In the NPWT group, dressings were changed every 48–72 h. In the control group, conventional dressings were applied at the time of surgical debridement and changed twice a day thereafter. End point of study was when wound was ready for either skin grafting or secondary suturing. End point was achieved in the NPWT group in 17.2(SD ± 3.55) days, compared to 34.9 (SD ± 5.96) days in the control group (p < 0.001). Number of dressing applied were 7.46(SD ± 2.25) in NPWT group versus 69.8(SD ± 11.93) in conventional dressing group (p < 0.001). Ninety percent cases were successfully treated in NPWT Group as compared to 76.6 % in conventional group. Rate of healing of ulcer is faster in NPWT group as compared to conventional group. Economically modified NPWT is more cost-effective to the patients in our setup.
Keywords: Negative-pressure wound therapy, Dressing, Improvised, Cost-effective
Introduction
Diabetics are estimated to have 15 % lifetime risk of developing foot ulcers with some data showing that lifetime incidence rates may be as high as 25 %. These ulcers are very difficult to treat. And often do not heal satisfactorily or take very long time to heal. Often, these ulcers get worse and result in amputation. Diabetes is the leading cause of non-traumatic foot amputations worldwide [1, 2].
Various dressing materials and solutions are used in treatment of diabetic foot ulcers with limited efficacy. Dressings are required for many days or weeks and it bears significant cost to the patient as well as healthcare system.
Negative-pressure wound therapy is the latest technology in management of diabetic foot ulcers. It is claimed to have faster healing rates and is cost-effective as well. This study is undertaken to test these claims and to check the efficacy of our modification to the standard VAC therapy kit by KCI Inc., USA.
We have modified the proprietary VAC therapy system by KCI Inc., USA, to suit demands of our institute. We have a huge number of poor patients requiring care of foot ulcers. The modified model does not require costly equipment and can be applied with material widely available in general wards at a much cheaper cost with simplified method. Besides confirming clinical efficacy of NPWT, our study focuses cheap and simple yet equally efficacious model for NPWT suitable for large-volume use at a general hospital.
Methods
A total of 60 patients having diabetic foot were included in this study. The study period was from July 2011 to July 2012 and it was conducted in the Department of Surgery, Civil Hospital, Ahmedabad. Patients were having ulcers on dorsum of foot of size > 10 cm2. Adequate blood circulation was assessed by doing lower limb arterial Doppler. Patients with osteomyelitis, peripheral vascular disease, or malignancy were excluded. All these patients initially underwent surgical debridement for removal of necrotic patch or slough. All patients were given standard medical therapy for diabetes and anti-microbials were given according to culture sensitivity reports. Thirty patients were dressed with NPWT dressing and 30 patients were dressed with conventional dressing. Here, NPWT was applied by modified technique. NPWT system consisted of four components: A usual suction machine generating pressure of −80 to −150 mmHg, Ryle’s tube, piece of foam cut according to size and shape of ulcer, and adhesive transparent dressing (OpSite by Smith & Nephews, UK). The suction was applied 30 min on and 30 min off. Dressings changed every 48–72 h. Conventional dressing was done by cleaning with povidine iodine solution with or without hydrogen peroxide and applying moist gauze to wound and dressing closed by cotton bandage. Dressing changed twice a day. Patients were examined daily. The dressings continued till the ulcer bed had healthy granulation tissue and was ready for skin grafting. The total number of days till end point achieved, total number of dressings required and average cost of treatment calculated. Patients with failure of dressings treated with other methods of dressing. Remaining patients were managed with split-thickness graft. Failure of either dressing was considered when no response was obtained for 2 weeks of treatment, there was worsening of condition or development of complications. In this study, only material cost is taken into consideration.
Results
In this study, majority of patients having diabetes are in middle age group and the average is 56.5 years. Thirty-one patients (56.66 %) presented with cellulitis, 13 patients (21.66 %) presented with necrotic patch, 11 patients (18.33 %) with chronic ulcer and 5 patients (8.34 %) presented with gangrene. Patients underwent surgical debridement and raw area was created. Thirty patients were dressed with NPWT and remaining 30 patients were dressed with conventional method. Results of study are summarized in Table 1.
Table 1.
Summary of results
| Criteria | NPWT group | Conventional group |
|---|---|---|
| Mean no. of dressing | 7.46 (SD ± 2.25) | 69.8 (SD ± 11.93) |
| Mean days of dressing | 17.2 (SD ± 3.55) | 34.9 (SD ± 5.96) |
| Success rate | 90.00 % | 76.66 % |
Satisfactory healing was achieved in mean 7.46 (SD ± 2.25) dressings in NPWT group vs 69.8 (SD ± 11.93) dressings in conventional group (p < 0.001). Mean days of dressings were 17.2 (SD ± 3.55) in NPWT group as compared to 34.9 (SD ± 5.96) days in conventional group (p < 0.001). Success rates of 90 and 76.66 % were achieved in NPWT and conventional groups respectively. Patients, in whom failure or complications occurred, were treated with alternative therapy or amputation. NPWT failed in three patients in whom the therapy discontinued. While conventional therapy failed in seven patients. Failure considered when there was no improvement after 2 week, worsening of condition or development of complication. In NPWT group, two patients showed no response and one patient developed worsening of condition. In conventional dressing group, four patients showed no improvement and three patients developed worsening of wound.
Average cost of NPWT was Rs. 500 approximately and conventional dressing costs Rs. 200 approximately per dressing. Therefore, average cost of NPWT and conventional dressing was Rs. 3,750 and 7,000, respectively. If, cost of daily treatment, hospital stay, and morbidity is taken into account, the cost of conventional dressing will significantly increase. The VAC system by KCI Inc., USA costs Rs. 3–4 lacs and dressing costs Rs. 1,100/day. And rental charges are $100/day. Requirement for analgesics and antibiotics was much less in NPWT group. Patient compliance was also better among NPWT group.
Discussion
Diabetic foot present a significant challenge to treating physicians. Treatment involves multiple modalities including debridement, assessment, and treatment of infection, revascularization if indicated, and sufficient off-loading of the foot. Many dressing materials are used and abused for healing of diabetic foot [3]. A key component of the healing process is debridement because it enables removal of devitalized and necrotic tissue. Debridement is critically important to the initiation of healing [4]. NPWT and other wound healing technologies work in conjunction with debridement as the foundation upon which the wound healing process can begin. It appears that NPWT in addition to established standards of care enhances successful healing and closure of wounds (Fig. 1).
Fig. 1.
Before and after NPWT dressing
The success of NPWT in chronic wounds is associated with removal of excess interstitial fluid, an increase in vascularity and associated decrease of bacterial colonization, and stimulation of granulation tissue formation through the response of wound tissue to the mechanical forces exerted by the application of negative pressure through the foam dressing [5, 6]. Open-pore foam dressing creates micromechanical deformations of the wound surface. These micromechanical deformations are caused when negative pressure draws tissue into the foam pores. This stretches cells and promotes cell division that stimulates granulation tissue formation [7, 8]. However in patients with already compromised vascularity, NPWT may even worsen the situation by compromising blood flow. So NPWT should be used with caution in patients with compromised vascularity, particularly when used circumferentially [9].
In the present study, 90 % patients (27 out of 30) attained success with NPWT and 76.66 % patients (23 out of 30) attained success in conventional dressing group. Mean number of dressing was 7.5 in NPWT versus 69.8 in conventional group. Average days required to reach the end point of treatment were 17.2 in NPWT versus 34.8 in conventional group (Table 1). This result parallels the findings by Fleishmann et al. [10], Armstrong et al. [11], McCallon et al. [12], Blume et al. [13], and Thomas [14].
Fleishman et al. reported similar results. Three hundred thirteen patients with acute and chronic infections were treated by vacuum sealing (VS). The average duration of VS treatment was 16.7 days, and there was an average of 3.1 changes in the VS system. In acute infections (n = 203), the wounds were closed by secondary suturing (65.5 %), spontaneous epithelialization (17.2 %), skin grafting (12.3 %). and flap transfer (2 %). Six patients died (3 %). Infection recurred in 3.9 % and was cured by another VS treatment. Unstable scar formations (1 %) were treated by free flap transfers. When compared with standard open-wound treatment, the low-cost VS technique offers great advantages with regard to hospital hygiene, patient comfort, and therapeutic results [10].
Armstrong et al. in their study reported that 43 (56 %) of 77 patients randomly assigned to the NPWT group achieved complete closure during the 16-week assessment (median time to closure 56 days) and 33 (39 %) of 85 patients randomly assigned to the control group achieved complete closure (77 days). The time to reach 76–100 % granulation tissue for patients receiving NPWT (42 days) was faster than that for controls (84 days) [11].
In their study McCallon et al. reported, satisfactory healing in the VAC group was achieved in 22.8 days, compared to 42.8 days in the control group. Surface area changes of 28.4 % average decrease in wound size in the VAC group, compared to a 9.5 % average increase in the control group during measurement period [12].
Blume et al. showed a greater proportion of foot ulcers achieved complete ulcer closure with NPWT (73 of 169, 43.2 %) than with AMWT (48 of 166, 28.9 %) within the 112-day active treatment phase (P = 0.007). NPWT patients experienced significantly (P = 0.035) fewer secondary amputations. In assessing safety, no significant difference between the groups was observed in treatment-related complications such as infection, cellulitis, and osteomyelitis at 6 months [13].
Moues et al. showed in a prospective RCT of level 1 evidence that the costs of VAC therapy were similar when compared to conventional therapy (moist gauze) in the management of full-thickness wounds that required surgical closure. Cost analysis showed significantly higher mean material expenses for wounds treated with VAC therapy ($520.65) compared with conventional therapy ($18.86) but significantly lower mean nursing expenses ($41.50 and $104.38) for VAC therapy and conventional therapy, respectively. Hospitalization costs were lower in the VAC therapy group ($2,248.59) than in the conventional treatment group ($3,102.50) due to an average shorter duration until they were ready for surgical closure. There was no significant difference in total costs per patient between the two therapies ($2,810 for VAC therapy versus $3,225 for conventional therapy). The authors concluded that the lower number of time-consuming dressing changes and the shorter duration until they were ready for surgery compensated for the higher material costs of VAC Therapy. As a result, VAC therapy was equally as expensive as conventional moist gauze [15].
Philbeck et al. evaluated 1,170 pressure ulcers and other chronic wounds that failed to respond to previous interventions and were subsequently treated at home with negative-pressure wound therapy. Reductions in wound area and volume were compared and costs analyzed. The average 22.2-cm2 wound treated with conventional therapy would take 247 days to heal and cost $23,465. Using negative-pressure wound therapy, the wound would heal in 97 days and cost $14,546. The study concluded that negative-pressure wound therapy is an efficacious and economical treatment modality for a variety of chronic wounds [16].
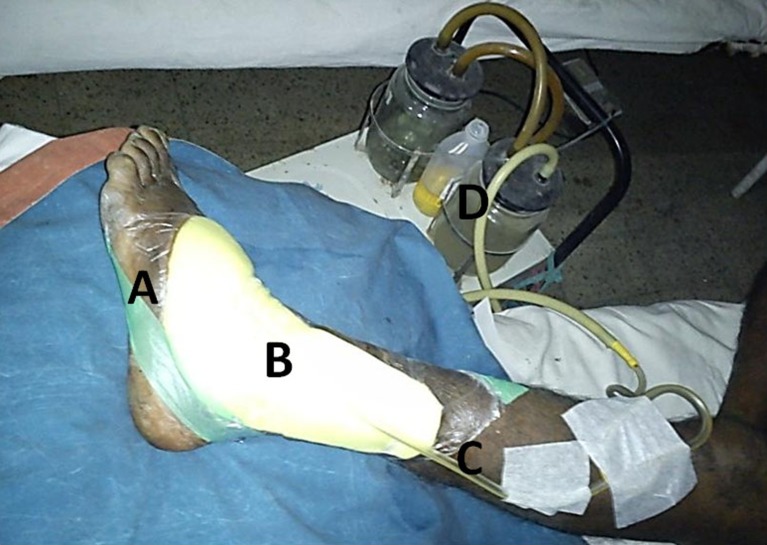
Cost of NPWT using the original VAC system developed by KCI Inc., USA, is very costly. But we have it modified to suit our institution. This is much cheaper and requires commonly available materials (OpSite, foam, Ryle’s tube, and simple suction machine) and hence can be used by anybody at any place [17, 18] (Fig. 2).
Fig. 2.
Our modification of original VAC system. A: OpSite, B: foam, C: Ryle’s tube, d: suction machine
The cost of dressing with VAC System is Rs. 1,100/dressing and cost of equipment is approximately 5 lacs. When this instrument is used on rental basis it costs $100 (Rs. 5,000/day). Due to budgetary constraints, widespread use of this equipment is not feasible at our institute. So we have developed a modified system to deliver NPWT using materials available in general wards which costs approximately Rs. 500/dressing (OpSite Rs. 250–350, Ryle’s tube Rs. 50, foam Rs. 100).
Shalom et al. in their study using “homemade” NPWT system obtained results similar to what one could expect with the VAC™ system in all parameters. Complications encountered were few and minor. Cost per day using our negative-pressure system for a 10-cm2 wound is about US$1, as compared to US$22, utilizing the VAC™ system [19].
NPWT has higher cost per dressing but as lesser number of dressings required for lesser number of days, the overall cost is much lower than conventional dressing. It also results in decreased need for antibiotics and analgesics which further reduces cost and improves patient compliance. The NPWT dressing is less painful as compared to conventional dressing as we have observed during out study. The NPWT reduces pain and suffering of patient. Rapid recovery results in less days of indoor treatment which means lesser expenses and saving of man power. According to Government of Gujarat estimates, Civil Hospital, Ahmedabad, spend Rs. 1.7 lacs/year or Rs. 465/day per bed [19]. By reducing days of treatment the burden on health care system is also reduced. To the individual, rapid recovery reduces financial loss and improves quality of life [20].
Conclusions
NPWT is a safe, efficacious, and cost-effective treatment for diabetic foot wounds, and could lead to a higher proportion of healed wounds, faster healing rates at a lesser cost. When applied using method described in present study, it brings down the total cost of treatment with equal efficacy and safety.
References
- 1.Center for Disease Control and Prevention (2005) 2005 National Diabetes Fact Sheet: general information and national estimates on diabetes in the United States. Center for Disease Control and Prevention , Atlanta
- 2.Brem H, Shehan P, Rosenberg HJ, Schnieder JS, Boulton AJM. Evidence based protocol for diabetic foot ulcers. Plast Reconstr Surg. 2006;117:193S–207S. doi: 10.1097/01.prs.0000225459.93750.29. [DOI] [PubMed] [Google Scholar]
- 3.Day MR, Fish SE, Day RD. The use and abuse of wound care materials in the treatment of diabetic ulcerations. Clin Podiatr Med Surg. 1998;15(1):139–150. [PubMed] [Google Scholar]
- 4.Eneroth M, van Houtum WH. The value of debridement and vacuum-assisted closure (V.A.C.) therapy in diabetic foot ulcers. Diabetes Metab Res Rev. 2008;24(Suppl 1):S76–S80. doi: 10.1002/dmrr.852. [DOI] [PubMed] [Google Scholar]
- 5.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38(6):563–576. doi: 10.1097/00000637-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Morykwas MJ, Argenta LC. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Borgquist O, Ingemansson R, Malmsjö M. Wound edge microvascular blood flow during negative-pressure wound therapy: examining the effects of pressures from −10 to −175 mmHg. Plast Reconstr Surg. 2010;125(2):502–509. doi: 10.1097/PRS.0b013e3181c82e1f. [DOI] [PubMed] [Google Scholar]
- 8.The science behind VAC therapy. http://www.kci-medical.in/IN-ENG/sciencebehindvactherapy. Accessed 1 Dec 2012
- 9.Kairinos N, Voogd AM, Botha PH, Kotze T, Kahn D, Hudson DA, Solomons M. Negative-pressure wound therapy II: negative-pressure wound therapy and increased perfusion. Just an illusion? Plast Reconstr Surg. 2009;123(2):601–612. doi: 10.1097/PRS.0b013e318196b97b. [DOI] [PubMed] [Google Scholar]
- 10.Fleischmann W, Lang E, Russ M. Treatment of infection by vacuum sealing. Unfallchirurg. 1997;100(4):301–304. doi: 10.1007/s001130050123. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704–1710. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]
- 12.McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum assisted closure versus saline moistened gauze in healing of postoperative diabetic foot wounds. Ostomy Wound Management. 2000;46:28–34. [PubMed] [Google Scholar]
- 13.Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2007;31(4):631–636. doi: 10.2337/dc07-2196. [DOI] [PubMed] [Google Scholar]
- 14.Thomas S (2001) An introduction to the use of vacuum assisted closure. http://www.worldwidewounds.com/2001/may/Thomas/Vacuum-Assisted-Closure.html. Accessed 1 Nov 2012
- 15.Mouës CM, van den Bemd GJ, Meerding WJ, Hovius SE. An economic evaluation of the use of TNP on full-thickness wounds. J Wound Care. 2005;14(5):224–227. doi: 10.12968/jowc.2005.14.5.26776. [DOI] [PubMed] [Google Scholar]
- 16.Philbeck TE, Jr, Whittington KT, Millsap MH, Briones RB, Wight DG, Schroeder WJ. The clinical and cost effectiveness of externally applied negative pressure wound therapy in the treatment of wounds in home healthcare Medicare patients. Ostomy Wound Manage. 1999;45:41–50. [PubMed] [Google Scholar]
- 17.Bui TD, Huerta S, et al. Negative pressure therapy with off the shelf components. Am J Surg. 2006;2006:235–237. doi: 10.1016/j.amjsurg.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Shalom A, Eran H, Westreich M, Friedman T. Our experience with a “homemade” vacuum-assisted closure system. Isr Med Assoc J. 2008;10(8–9):613–616. [PubMed] [Google Scholar]
- 19.Sahni A: A study of Medical services infrastructure and utilization in Gujarat State. http://medind.nic.in/haa/t00/i1/haat00i1p96g.pdf. Accessed 1 Dec 2012
- 20.Andros G, Armstrong DG et al. (2006) Consensus statement on negative pressure wound therapy (V.A.C. therapy) for the management of diabetic foot wounds. Ostomy Wound Manage 2006 Jun; Suppl:1–32 [PubMed]