Abstract
The purpose of this study was to find the unusual findings in the childhood appendectomy specimens and their incidence. The clinicopathological data of 1,306 patients whose ages ranged from 3 to 16 were retrospectively collected. Histopathological findings in appendectomy specimens taken from patients who had a prediagnosis of appendicitis were obtained. Incidental appendectomies were not included in the research. Unusual findings were reevaluated in the histopathological assessment of appendectomy specimens. The number of patients whose pathological findings are considered unusual is 25 (1.91 %). Nine of the patients were girls and 16 of them were boys. Their ages ranged from 6 to 15. Pathological results revealed that there were 16 (1.22 %) cases of parasitosis, 3 (0.23 %) cases of granulomatosis, 3 (0.23 %) cases of eosinophilic appendicitis, 2 (0.15 %) cases of carcinoid tumors, and 1 (0.08 %) case of appendiceal non-Hodgkin’s lymphoma. All patients underwent a standard appendectomy. Uncommon histopathological findings in childhood appendectomy specimens are more common than those in adulthood. This kind of certain unexpected lesions of the appendix may require advanced diagnostics, careful clinical care, follow-up for years, and a multidisciplinary approach. Therefore, histopathological examinations of appendectomy specimens must be performed routinely.
Keywords: Unusual, Childhood, Granulomatous appendicitis, Eosinophilic, Carcinoid, Non-Hodgkin’s lymphoma
Introduction
Appendicitis is among the most commonly observed acute surgical conditions. The risk of developing acute appendicitis in a person’s lifetime is around 7 % [1]. It is commonly observed in late adolescence and early 20s when lymphoid development is really fast. Fecaliths and lymphoid hyperplasia are the most well-known reasons of lumen obstruction which is the reason for the disease. However, this condition may develop due to unusual reasons as well [2]. Obstruction may be due to Enterobius vermicularis, granulomatous diseases, eosinophilic appendicitis, carcinoid tumor, or appendiceal non-Hodgkin’s lymphoma [3–12]. The aim of the present study was to evaluate the unusual findings in the childhood appendectomy specimens and their incidence.
Material and Methods
Between October 2007 and October 2012, 1,306 patients with presumed acute appendicitis whose ages ranged from 3 to 16 underwent surgical treatment at the Department of Pediatric Surgery of Gaziantep Children’s Hospital in Turkey.
Data were retrospectively collected from patients who were reported to have unusual pathological appendix findings. The original pathology specimens with unusual findings were evaluated again by an experienced pathologist. Incidental appendectomies were not included in the study.
Results
A total of 1,306 appendectomies were performed on patients who had a prediagnosis of acute appendicitis at Gaziantep Children’s Hospital. All patients underwent standard appendectomy. Histopathological findings revealed 1,110 cases of inflamed appendix (84.22 %), 71 cases of perforated appendicitis (5.44 %), 52 cases of lymphoid hyperplasia (3.98 %), 28 cases of normal appendix vermicularis (2.14 %), 11 cases of obliterated appendix vermicularis (0.84 %), and 9 cases of periappendicitis (0.69 %). Of all the appendectomies performed, 25 (1.91 %) specimens showed incidental abnormal histopathological diagnoses (Table 1). Of the 25 patients, 16 were boys and 9 were girls with ages ranging from 6 to 15 years. Pathological assessment revealed 16 cases of enterobius vermicularis, 3 cases of granulomatosis, 3 cases of eosinophilic appendicitis, 2 cases of carcinoid tumor, and 1 case of non-Hodgkin’s lymphoma (Figs. 1, 2, 3, 4, 5, 6, 7, and 8; Table 2).
Table 1.
Histopathologic findings in appendectomy specimens
| Number | Percent | |
|---|---|---|
| Normal appendix vermiformis | 28 | 2.14 |
| Acute appendicitis | 1,110 | 84.22 |
| Perforated appendicitis | 71 | 5.44 |
| Lymphoid hyperplasia | 52 | 3.98 |
| Periappendicitis | 9 | 0.69 |
| Obliterated appendix vermiformis | 11 | 0.84 |
| Unusual appendicitis | 25 | 1.91 |
Fig. 1.

E. vermicularis inside the appendix lumen (HE, ×4)
Fig. 2.
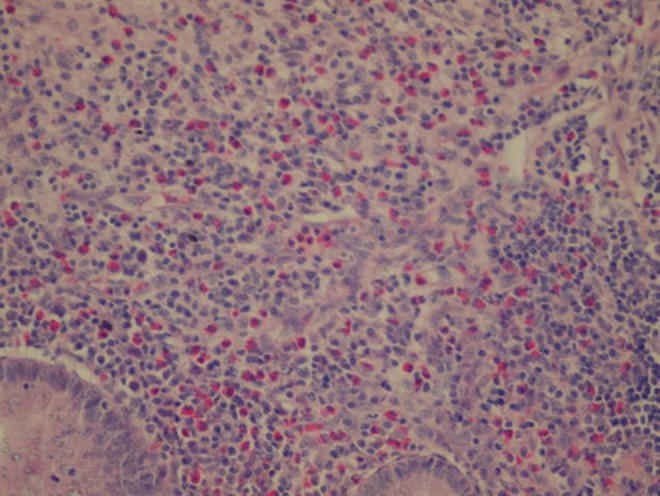
Appendix mucosa and focal granuloma (HE, ×20)
Fig. 3.
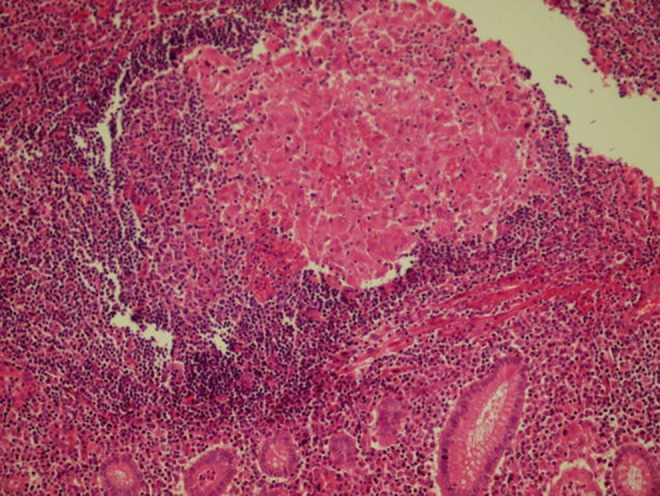
Numerous eosinophilic leucocyte infiltration through the appendix mucosa (HE, ×20)
Fig. 4.

Diffuse eosinophilic leucocyte infiltration inside the appendix muscular layer (HE, ×20)
Fig. 5.

Carcinoid tumor. A tumoral growth of monomorphic cells demonstrating insular structures and mitotic figures which fill the lumen at the tip of the appendix, infiltrating the muscular tissue, and approaching to the serosa (HE, ×20)
Fig. 6.

Carcinoid tumor. Appendicitis tissue intact from the tumor cells (HE, ×4)
Fig. 7.

Primer appendiceal non-Hodgkin’s lymphoma. Atypical lymphoid infiltration in muscular tissue of the appendix vermiformis (HE, ×100)
Fig. 8.

Primer appendiceal non-Hodgkin’s lymphoma. Diffuse atypical lymphoid proliferation and desquame appendix vermiformis mucosa (right) (HE, ×200)
Table 2.
Distribution of the 25 unusual histopathological findings in all appendectomy specimens
| Number | Percent | |
|---|---|---|
| Enterobius vermicularis | 16 | 1.22 |
| Idiopathic granulomatous appendicitis | 3 | 0.23 |
| Eosinophilic appendicitis | 3 | 0.23 |
| Carcinoid tumor | 2 | 0.15 |
| Appendiceal non-Hodgkin’s lymphoma | 1 | 0.08 |
The number of histopathological examinations of patients with E. vermicularis with acute inflammation was 7 and that with no evidence of any pathological change was 9. After obtaining the pathology reports, the patients with enterobiasis were given 100 mg of mebendazole, which was repeated 7–10 days later.
The first of the patients with carcinoid tumors was a 10-year-old girl. The patient had tenderness in the right lower quadrant but did not have any complaints of nausea and vomiting. Her WBC count was 4.15 × 103/μL (4.5–11), and her CRP count was 0.10 mg/dL (0.0–0.8). The other girl was 11.5 years old and had symptoms of acute appendicitis. Her WBC count was 18.20 × 103/μL (4.5–11), and her CRP count was 4.6 mg/dL (0.0–0.8). Carcinoid tumors had an average diameter of 8 mm (range 6–10 mm), and they appeared on the distal end of the appendix. In both patients, the tumors were not in surgical boundaries. The first one infiltrated the surrounding fat tissue and lymphatic vessel. Both carcinoid tumors were diagnosed clinically with acute appendicitis, and neither of them had symptoms of carcinoid syndrome nor was preoperatively diagnosed with an appendicular tumor. After the pathological confirmation of the diagnosis, the patients underwent abdominal ultrasonography (US) and computed tomography. At first, they underwent clinical examination once every 3 months. No complication has developed on follow-up (mean 26 months).
A 6-year-old boy was diagnosed with non-Hodgkin’s lymphoma after a histopathological assessment. On admission, the boy had abdominal pain, vomiting, and swelling on the face and lips. His CRP count was 0.18 mg/dL (0.0–0.8), and his WBC count was 8.11 × 103/μL (4.5–11). Appendectomy was performed following the detection of appendicitis in the abdominal US. Grossly, the appendix was diffusely enlarged and thickened by a fleshy intramural tumor.
Nonspecific changes including fibrous obliteration were observed in 11 of the 1,306 (0.84 %) appendices (Figs. 9 and 10).
Fig. 9.
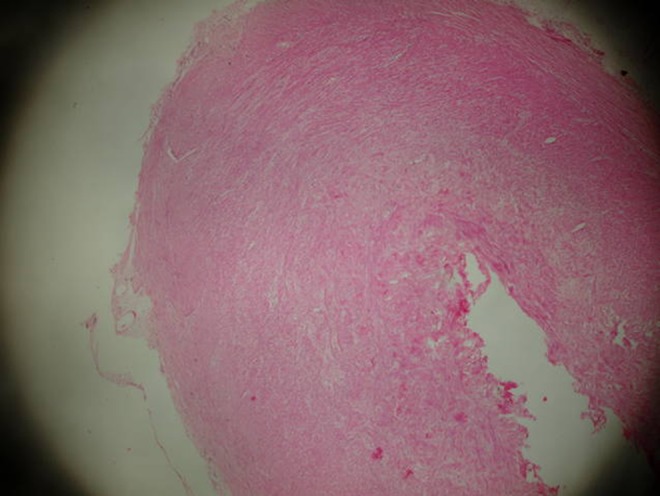
Distal end of the appendix with fibrous proliferation (HE, ×4)
Fig. 10.
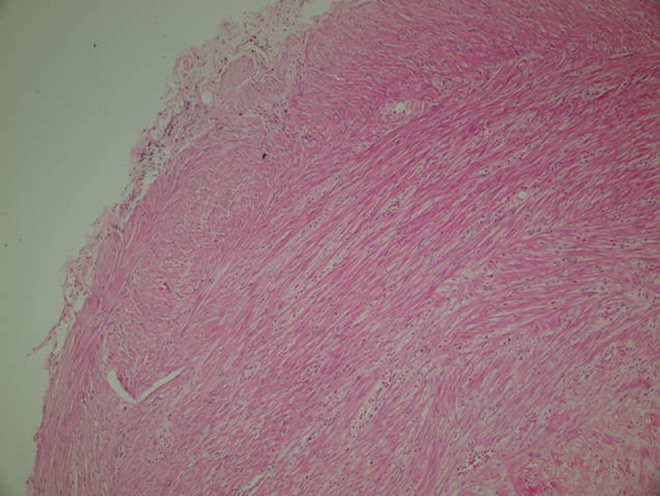
Distal end of the appendix with fibrous proliferation (HE, ×20)
Discussion
The most common emergency operation is appendectomy. Appendectomy is annually performed on 70,000 child patients in the USA. This figure accounts for 10 % of all abdominal operations. Although the most well-known reasons for appendicitis are lymphoid hyperplasia and fecaliths, unexpected and surprising causes can influence the condition [13, 14].
The most common unusual cause in our research was E. vermicularis. In endemic areas, it is shown that it can cause inflammation of the appendix in 13 to 37 % of the population [15]. In other studies, it is reported that this factor can present a normal histology or chronic appendicitis findings [16, 17]. It is detected in 0.2 to 4.18 % of appendectomy specimens. In our case, series rates were found to be similar to that in the literature (1.22 %). No clinical features or diagnostic laboratory tests distinguish helminthic from bacterial appendicitis preoperatively. These patients should be started on antihelminthic treatment, and the treatment should be repeated 7–10 days later.
Granulomatous appendicitis (GA) is a rare condition especially in pediatric patients. A patient who is thought to have GA can only be diagnosed following a pathological examination of the specimen. Its incidence ranges between 0.31 and 0.95 %. Since our series only consisted of pediatric age group, our rate was 0.23 %. Various infections and systemic diseases such as Crohn’s disease or sarcoidosis can cause the condition. In the past, it was thought that Crohn’s disease could always cause GA, while this applies to only 5–10 % of the cases. The differential diagnosis of Crohn’s disease at the early stages of the disease is difficult, since the disease localizes only on the appendix. Therefore, additional gastroenterological tests and a careful clinical follow-up are necessary following the pathological assessment [18–20]. Crohn’s disease is a chronic disorder that presents itself with transmural inflammation in which there is epithelioid granulation in the intestinal wall. Among its symptoms are right lower quadrant pain and fever, which are the most likely demonstrations of acute appendicitis. Crohn’s disease, which localizes in the appendix, requires the exclusion of many diseases. Actinomyces, Yersinia, Schistosoma, Mycobacterium tuberculosis, Histoplasma capsulatum, and Campylobacter are just a few of the causative agents of GA. Crohn’s disease which is limited to the appendix has good prognosis with low rate of fistula formation [6, 13]. In our cases, appendectomy would suffice in the treatment.
Eosinophils are normal constituents of the lamina propria and submucosa of the appendix, but the existence of eosinophils only in the muscular layer has been previously described as eosinophilic appendicitis. This condition was also named as subacute appendicitis [21]. Jona and Aravindan stated that mural eosinophilic infiltration was the only finding in their appendicitis series and this development was attributed to type 1 hypersensitivity reaction [22, 23]. This is again in contrast with the previous belief, supporting that the eosinophilic infiltration does not lead to subacute or chronic appendicitis [21, 24]. In our series, eosinophilic appendicitis was 0.23 %.
Appendiceal carcinoid tumors (ACT) are very difficult to identify preoperatively. The diagnosis is generally made after pathological examination. They are the most common of the malignant lesions of the appendix, since they account for almost 60 % of all appendiceal tumors [10]. In various series, it is reported to be detected in 0.3 to 2.27 % of appendectomy specimens [10, 11]. In our series, owing to the pediatric age group, the numbers are smaller (0.15 %). It should be kept in mind that this tumor can behave aggressively according to its type, size, and whether or not it involves the mesoappendix. Of cases which are smaller than 1 cm, 70 to 95 % have a metastasis risk of nearly zero, and therefore, simple appendectomy would be sufficient [10, 11]. But an ACT larger than 2 cm should be managed with a formal right hemicolectomy because of the metastasis risk of up to 85 %. In our cases, ACT had a diameter of ≤1 cm, so we only performed the appendectomy.
Extranodal lymphoma most commonly presents in the gastrointestinal system and localizes in the stomach. That which localizes in the appendix is almost always non-Hodgkin’s B cell lymphoma. They are categorized among malignant appendix tumors. This extremely uncommon type of neoplasm was first defined by Warren in 1899 [25]. The diagnosis is usually done following a postoperative histopathological examination. Male–female ratio is 1.5:1 and the average onset time is 18 years of age [26]. Our case was just a 6.5-year-old boy. This disease is observed in only 0.015 % of appendectomy specimens [12]. In our series, the percentage was 0.08 %. Clinical features resemble acute appendicitis. The primary symptom is pain in the right lower quadrant continuing for a few months. However, though rare, a mass in this area may be an admission complaint. In imaging studies, the appendix shows significant enlargement, whereas it preserves the vermiform look [27]. On ultrasound, the diffuse thickening is hypoechoic and often resembles the cystic dilation of the lumen seen in mucoceles. In our patient, the appendix had an extreme amount of edema and its chubby appearance was noteworthy. He had been referred to pediatric oncology 1 week ago.
Akbulut et al. found unusual pathological findings in adult appendectomy series as 1 % [28]. This percentage (1.91 %) was significantly higher in our series, which consisted of only pediatric patients. Some studies point out that fibrosis development after parasitosis may cause appendicitis [29, 30]. Parasitosis is also a common phenomenon in our country, and we believe that this condition may be responsible for fibrous obliteration. Nonspecific changes including fibrous obliteration were observed in 11 of the 1,306 (0.84 %) appendices. These were not declared to be the part of unusual manifestations according to the data in the PubMed.
Conclusion
The most common factor in our series was E. vermicularis. GA, eosinophilic appendicitis, and appendiceal tumors had the same frequency in children. The appropriate antiparasitic treatment after the appendectomy must be utilized to prevent reinfection and chronic inflammation. One should be careful about GA, since it can set ground for Crohn’s disease and carcinoma. Further tests, more careful clinical attention, years of long follow-up, and a multidisciplinary approach are essential for patients with unusual pathological findings.
Unusual pathological findings are more commonly observed in children than in adults. We emphasize and strongly recommend that all appendectomy specimens be examined histologically, even if macroscopically normal.
References
- 1.Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910–925. doi: 10.1093/oxfordjournals.aje.a115734. [DOI] [PubMed] [Google Scholar]
- 2.Agarwala N, Liu CY. Laparoscopic appendectomy. J Am Assoc Gynecol Laparosc. 2003;10:166–168. doi: 10.1016/S1074-3804(05)60292-7. [DOI] [PubMed] [Google Scholar]
- 3.da Silva DF, da Silva RJ, da Silva MG, Sartorelli AC, Rodrigues MA. Parasitic infection of the appendix as a cause of acute appendicitis. Parasitol Res. 2007;102:99–102. doi: 10.1007/s00436-007-0735-0. [DOI] [PubMed] [Google Scholar]
- 4.Aydin O. Incidental parasitic infestations in surgically removed appendices: a retrospective analysis. Diagn Pathol. 2007;2:16. doi: 10.1186/1746-1596-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma KW, Chia NH, Yeung HW, Cheung MT. If not appendicitis, then what else can it be? A retrospective review of 1492 appendectomies. Hong Kong Med J. 2010;16:12–17. [PubMed] [Google Scholar]
- 6.AbdullGaffar B. Granulomatous diseases and granulomas of the appendix. Int J Surg Pathol. 2010;18:14–20. doi: 10.1177/1066896909349246. [DOI] [PubMed] [Google Scholar]
- 7.Cruz DB, Friedrisch BK, Fontanive Junior V, da Rocha VW. Eosinophilic acute appendicitis caused by Strongyloides stercoralis and Enterobius vermicularis in an HIV-positive patient. BMJ Case Rep. 2012 doi: 10.1136/bcr.01.2012.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchana-Sato V, Detry O, Polus M, et al. Carcinoid tumor of the appendix: a consecutive series from 1237 appendectomies. World J Gastroenterol. 2006;12:6699–6701. doi: 10.3748/wjg.v12.i41.6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coşkun H, Bostanci O, Dilege ME, et al. Carcinoid tumors of appendix: treatment and outcome. Ulus Travma Acil Cerrahi Derg. 2006;12:150–154. [PubMed] [Google Scholar]
- 10.Hatzipantelis E, Panagopoulou P, Sidi-Fragandrea V, Fragandrea I, Koliouskas DE. Carcinoid tumors of the appendix in children: experience from a tertiary center in northern Greece. J Pediatr Gastroenterol Nutr. 2010;51:622–625. doi: 10.1097/MPG.0b013e3181e05358. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro R, Eldar S, Sadot E, Venturero M, Papa MZ, Zippel DB. The significance of occult carcinoids in the era of laparoscopic appendectomies. Surg Endosc. 2010;24:2197–2199. doi: 10.1007/s00464-010-0926-0. [DOI] [PubMed] [Google Scholar]
- 12.Collins DC. 71,000 human appendix specimens. A final report summarizing forty years’ study. Am J Proctol. 1963;14:265–281. [PubMed] [Google Scholar]
- 13.Jones AE, Phillips AW, Jarvis JR, Sargen K. The value of routine histopathological examination of appendicectomy specimens. BMC Surg. 2007;7:17. doi: 10.1186/1471-2482-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamisa I. A clinicopathological review of 324 appendices removed for acute appendicitis in Durban, South Africa: a retrospective analysis. Ann R Coll Surg Engl. 2009;91:688–692. doi: 10.1308/003588409X12486167521677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ariyarathenam AV, Nachimuthu S, Tang TY, Courtney ED, Harris SA, Harris AM. Enterobius vermicularis infestation of the appendix and management at the time of laparoscopic appendectomy: case series and literature review. Int J Surg. 2010;8:466–469. doi: 10.1016/j.ijsu.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Listorto G, Ferranti F, Mancini G, et al. The role of Enterobius vermicularis in etiopathogenesis of appendicitis. Minerva Chir. 1996;51:293–296. [PubMed] [Google Scholar]
- 17.Dahlstrom JE, Macarthur EB. Enterobius vermicularis: a possible cause of symptoms resembling appendicitis. Aust N Z J Surg. 1994;64:692–694. doi: 10.1111/j.1445-2197.1994.tb02059.x. [DOI] [PubMed] [Google Scholar]
- 18.Shivakumar P, Shanmugam RP, Mani CS. Idiopathic granulomatous appendicitis: a rare appendicular pseudo tumor. Trop Gastroenterol. 2010;31:130–131. [PubMed] [Google Scholar]
- 19.Yayla D, Alpman BN, Dolek Y. Granulomatous appendicitis in a 12-year-old boy. J Pediatr Surg. 2010;45:e27–e29. doi: 10.1016/j.jpedsurg.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Gu J, Allan C. Idiopathic granulomatous appendicitis: a report of three consecutive cases. ANZ J Surg. 2010;80:201. doi: 10.1111/j.1445-2197.2010.05237.x. [DOI] [PubMed] [Google Scholar]
- 21.Stephenson J, Snoddy WT. Appendiceal lesions. Observation in 4,000 appendectomies. Arch Surg. 1961;83:661–666. doi: 10.1001/archsurg.1961.01300170017005. [DOI] [PubMed] [Google Scholar]
- 22.Jona JZ, Belin RP, Burke JA. Eosinophilic infiltration of the gastrointestinal tract in children. Am J Dis Child. 1976;130:1136–1139. doi: 10.1001/archpedi.1976.02120110098015. [DOI] [PubMed] [Google Scholar]
- 23.Aravindan KP. Eosinophils in acute appendicitis: possible significance. Indian J Pathol Microbiol. 1997;40:491–498. [PubMed] [Google Scholar]
- 24.Crabbe MM, Norwood SH, Robertson HD, Silva JS. Recurrent and chronic appendicitis. Surg Gynecol Obstet. 1986;163:11–13. [PubMed] [Google Scholar]
- 25.Radha S, Afroz T, Satyanarayana G. Primary marginal zone B-cell lymphoma of appendix. Indian J Pathol Microbiol. 2008;51:392–394. doi: 10.4103/0377-4929.42523. [DOI] [PubMed] [Google Scholar]
- 26.Stewart RJ, Mirakhur M. Primary malignant lymphoma of the appendix. Ulster Med J. 1986;55:187–189. [PMC free article] [PubMed] [Google Scholar]
- 27.Pickhardt PJ, Levy AD, Rohrmann CA, Kende A. Primary neoplasms of the appendix: radiologic spectrum of disease with pathologic correlation. Radiographics. 2003;23:645–662. doi: 10.1148/rg.233025134. [DOI] [PubMed] [Google Scholar]
- 28.Akbulut S, Tas M, Sogutcu N. Unusual histopathological findings in appendectomy specimens: a retrospective analysis and literature review. World J Gastroenterol. 2011;17:1961–1970. doi: 10.3748/wjg.v17.i15.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elazary R, Maly A, Khalaileh A, et al. Schistosomiasis and acute appendicitis. Isr Med Assoc J. 2005;7:533–534. [PubMed] [Google Scholar]
- 30.Karatepe O, Adas G, Tukenmez M, Battal M, Altiok M, Karahan S. Parasitic infestation as cause of acute appendicitis. G Chir. 2009;30:426–428. [PubMed] [Google Scholar]


