Abstract
In order to compare gallstone disease data from India and Asian countries with Western countries, it is fundamental to follow a common gallstone classification. Gallstone disease has afflicted humans since the time of Egyptian kings, and gallstones have been found during autopsies on mummies. Gallstone prevalence in adult population ranges from 10 to 15 %. Gallstones in Western countries are distinguished into the following classes: cholesterol gallstones that contain more than 50 % of cholesterol (nearly 75 % of gallstones) and pigment gallstones that contain less than 30 % of cholesterol by weight, which can be subdivided into black pigment gallstones and brown pigment gallstones. It has been shown that ultrastructural analysis with scanning electron microscopy is useful in the classification and study of pigment gallstones. Moreover, x-ray diffractometry analysis and infrared spectroscopy of gallstones are of fundamental importance for an accurate stone analysis. An accurate study of gallstones is useful to understand gallstone pathogenesis. In fact, bacteria are not important in cholesterol gallstone nucleation and growth, but they are important in brown pigment gallstone formation. On the contrary, calcium bilirubinate is fundamental in black pigment gallstone formation and probably also plays an important role in cholesterol gallstone nucleation and growth.
Keywords: Gallstone classification, Cholesterol gallstones, Black pigment gallstones, Brown pigment gallstones
Introduction
In order to compare gallstone disease data from India and Asian countries with Western countries, it is fundamental to follow a common gallstone classification. Previously published papers report discordant data from chemical analysis of gallstones and classify gallstones as pure or mixed cholesterol and bilirubin gallstones [1]; in other articles, black pigment gallstones have not been considered [2]. Moreover, misinterpretations about cholesterol gallstone pathogenesis have also been reported [2, 3].
Gallstone disease has afflicted humans since the time of Egyptian kings, and gallstones have been found during autopsies on mummies [4]. Gallstone prevalence in adult population ranges from 10 to 15 % [5]. Gallstones in Western countries are distinguished into the following classes: cholesterol gallstones that contain more than 50 % of cholesterol (nearly 75–80 % of gallstones) and pigment gallstones that contain less than 30 % of cholesterol by weight, which can be subdivided into black pigment gallstones (10–15 %) and brown pigment gallstones (5–10 %) [6–11] (Fig. 1). Cholesterol gallstones are associated with risk factors such as dyslipidemia, obesity, type 2 diabetes, hyperinsulinemia (metabolic syndrome epidemic), gallbladder hypomotility, total parenteral nutrition, gender (female), and old age [1, 12]. Black pigment gallstones (Fig. 1) are associated with typical hyperbilirubinbilia factors such as hemolysis, ineffective erythropoiesis, pathologic enterohepatic cycling of unconjugated bilirubin, and liver cirrhosis and with parietal gallbladder mucosa factors such as Rokitansky–Aschoff sinuses of the gallbladder [12, 13]. Brown pigment gallstones are nearly 5 % of gallstones and are found mainly in the common bile duct; they are associated with biliary infection [8–10].
Fig. 1.
Gross visual classification of gallstones
Materials and Method
During the prospective study of 179 consecutive patients who underwent cholecystectomy, histologic examination of the gallbladder, and gallstone and bile analysis, 195 gallstones were found and classified. Sixteen patients had combination stones (i.e., the presence of two different populations of stones in the same gallbladder, usually in the patients with cholesterol composite or mixed gallstones). The present study was done in San Martino University Hospital, Genoa, Italy and in Policlinico Le Scotte Hospital, University of Siena, Siena, Italy. Patients’ data were collected with a particular interest for the typical risk factors of gallstone disease. Gallstones were analyzed with x-ray diffractometry (Siemens D500 diffractometer), infrared spectroscopy (PerkinElmer 357 infrared spectroscopy), and scanning electron microscopy (ISI SS40 scanning electron microscope) (Table 1). Identification of the various compounds was done by comparing the observed spectra and diffraction angles with those reported in the literature [11, 14–16]. In addition, in a subgroup of patients with gallbladder adenomyomatosis (increase in depth and number of Rokitansky–Aschoff sinuses), scanning electron microscopy of the gallbladder wall was done [17]. Gallstones obtained during surgery were washed, dried, and photographed. After cross section with scalpel, gallstones were classified according to morphologic criteria. In fact, cholesterol and pigment gallstones can be distinguished by gross inspection of the external surface and of the nucleus. Pigment gallstones can be distinguished as black and brown simply on the basis of their color. Brown gallstones are brownish yellow and are soft. Black pigment gallstones are small and irregular; in cross section, they have glass-like appearance. In the 41 patients who had both adenomyomatosis and intraparietal black pigment microstones, the gallbladder wall and gallstones were submitted for scanning electron microscopy analysis. The gallbladder was opened and washed with deionized water. Specimens were fixed with 2.5 % glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 24 h, postfixed in 1 % aqueous osmium tetroxide (OsO4) for 30 min, dehydrated by increasing concentrations of aqueous alcohol, and submitted to critical point drying. The various specimens of gallbladder walls were postfixed and affixed to an aluminum stub by means of double cellophane tape, and subsequently, they were coated with 20-nm-thick gold layer by sputter coating procedure. Dried gallstones were split in half, mounted on aluminum stub with double cellophane tape, and coated with gold sputtering. Entire cross-section surfaces were scanned with scanning electron microscopy.
Table 1.
Classification of gallstones according to gallstone morphology, ultrastructure, and composition (195 gallstones in 179 patients)
| Stones | Number 195 | Percent (%) | Composition cholesterol (%) | Composition bilirubinate (%) | Composition calcium carbonate (%) | Composition calcium palmitate (%) | Composition whitlockite (%) |
|---|---|---|---|---|---|---|---|
| Cholesterol pure | 70 (7 combi) | 36 ± 1 | 90 ± 5 | 0.8 ± 1.3 | 1 ± 0.5 | – | – |
| Cholesterol mixed | 64 (4 combi) | 33 ± 1 | 85 ± 10 | 2.5 ± 1 | 1.3 ± 0.8 | Traces | Traces |
| Black bilirubinate | 26 (16 + 10 combi) | 13 | 7.5 ± 5 | 62 ± 11 | 5.5 ± 5 | – | Traces |
| Black carbonate | 11 (6 + 5 combi) | 5 | 6 ± 4 | 60 ± 10 | 12 ± 3 | – | – |
| Black phosphate | 1 | 0.5 | 5 ± 4 | 50 ± 10 | 5 ± 4.5 | – | 40 ± 10 |
| Brown pigment | 8 | 4 | 10.5 ± 6 | 52 ± 16 | 0.5 ± 0.5 | 21 ± 9 | – |
| Composite | 12 (5 combi) | 6 ± 1 | 80 ± 10 | 5 ± 5 | 1 ± 0.5 | Traces | Traces |
| Rare stones | 3 | 1.5 | Various | Various | Various | Various | Various |
combi combination gallstones
Results
The composition of gallstones, according to x-ray diffractometry, infrared spectroscopy, and scanning electron microscopy, has been summarized in Table 1.
Sixty-four of the 179 patients had typical adenomyomatosis. Mean age was 50 years (range, 28–72 years). Thirty-eight of the 64 patients with adenomyomatosis had black gallstones, alone (n = 22) or in association with single pure (n = 7), single combination (n = 5), or multiple (n = 4) cholesterol gallstones in the same gallbladder.
Cholesterol gallstones can be subclassified as pure cholesterol gallstones, mixed (multiple) cholesterol gallstones, and combination or composite stones, usually on the basis of their gross aspect (Fig. 1). Reported data on gallstone composition are summarized in Table 1.
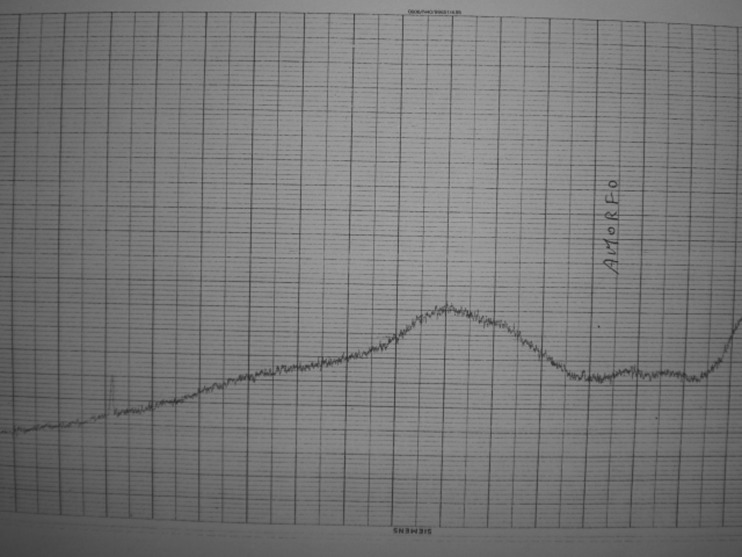
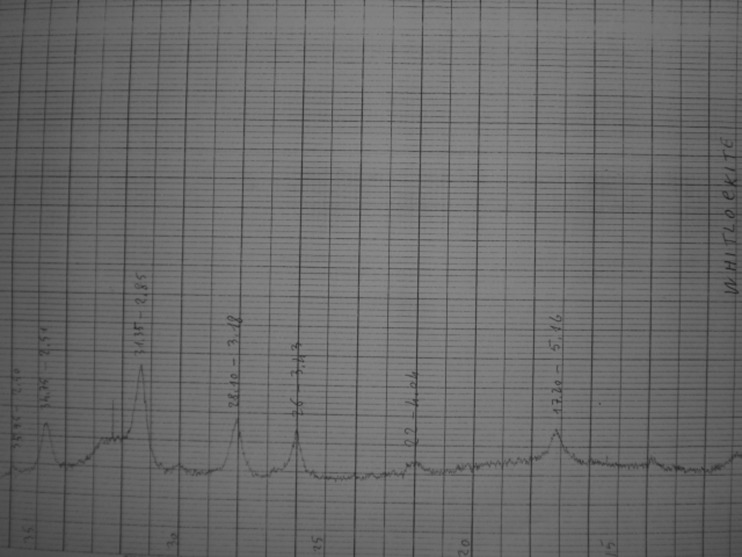
Two different populations of gallstones in the same gallbladder are referred to as combination stones [17]. Black pigment gallstone (Fig. 1) (11 % of gallstones; 19 % if we consider the “combination stones”) composition is as follows: cholesterol (7–8 %), calcium bilirubinate (73.5–50.5 %), calcium carbonate (5.8–7.2 %), and calcium phosphate (0–20 %) [17]. At x-ray diffractometry, they are usually amorphous (Fig. 2), but the irregular subtypes contain whitlockite (Fig. 3). Brown pigment gallstone (Fig. 1) composition is as follows: cholesterol (9.9–11.1 %), calcium bilirubinate (36–68 %), calcium carbonate (traces), and calcium palmitate (18–30 %) [17].
Fig. 2.
X-ray diffraction of smooth black pigment gallstones performed with a Siemens D500 diffractometer. Black pigment gallstones: calcium bilirubinate is amorphous at x-ray diffractometry
Fig. 3.
X-ray diffraction of “spicular or blackberry” black pigment gallstones performed with a Siemens D500 diffractometer. Black pigment gallstone subtype with irregular surface contains great amount of whitlockite at x-ray diffractometry with the typical diffraction angles
Discussion
The chemical analysis by semiquantitative titrimetric and colorimetric methods [1] is insufficient to analyze all gallstone populations because these methods do not cover all the types of pigment gallstones. The ultrastructural analysis with scanning electron microscopy has proved to be useful in the classification and study of pigment gallstones [9, 10, 16–19]. Moreover, the classification of gallstones based on thin-section petrographic microscopy does not consider brown pigment gallstones and should be enriched by the ultrastructural analysis of gallstones [18, 19]. In addition, x-ray diffractometry analysis, as well as infrared spectroscopy of gallstones, is of fundamental importance for an accurate stone analysis [9, 10, 14, 16, 17].
An accurate study of gallstones is very useful to understand gallstone pathogenesis. In fact, bacteria are not important in cholesterol gallstone formation [3], but they are important in brown pigment gallstone formation [19]. A correct understanding of brown pigment stone pathogenesis can be of great interest in the prevention of biliary stent occlusion [20]. Parietal or gallbladder mucosa factors play an import role in cholesterol and black pigment gallstone formation; in fact, ultrastructural analysis of the gallbladder wall demonstrated the formation of black calcium bilirubinate microstones within Rokitansky–Aschoff sinuses of the gallbladder [21]. In addition, calcium bilirubinate has been demonstrated to act as cement between cholesterol crystals during cholesterol gallstone formation [12, 13].
In the light of these studies, primary prevention of cholesterol gallstone formation and growth could act not only on hypercholesterolemia factors (metabolic epidemic syndrome) but also on hyperbilirubinbilia factors and gallbladder mucosa factors. In addition, the medical treatment of gallstones by oral drugs as ursodeoxycholic acid or the preventive effects of statins or ezetimibe are effective only in pure cholesterol or some mixed cholesterol stones; patient selection is of fundamental importance because the administration of these drugs among patients with calcified cholesterol gallstones or among brown or black pigment gallstones is completely ineffective.
Another crucial question arises from the study of gallbladder carcinoma: Is gallbladder carcinoma a unique entity or comprises different subgroups with different risk factors? Gallbladder carcinoma comprises two subgroups: one subgroup comprises squamous cell carcinoma, adenosquamous cell carcinoma, and some adenocarcinomas associated with the appearance of gallstones and the other subgroup comprises papillary adenocarcinoma and some types of adenocarcinomas not associated with gallstone formations but with other conditions such as pancreatobiliary reflux, pancreatobiliary maljunction, and gallbladder adenoma [22]. The different histological subtypes of gallbladder cancers have different responses to different chemotherapies [23, 24]. The accurate study of gallstones associated with gallbladder cancer revealed that only large cholesterol or combination gallstones or long-standing gallstones are associated with an increased risk of developing gallbladder carcinoma but not brown or black pigment gallstones or gallbladder infection [22, 25–27].
Prevention and treatment of these risk factors among high-risk groups of patients could probably reduce gallbladder carcinoma incidence, but further studies are necessary in order to evaluate these questions. Finally, ultrastructural studies on gallstones among uremic patients have demonstrated that chronic kidney insufficiency is associated with an increased risk of developing symptomatic cholesterol, black pigment, and blackberry gallstone disease [28]. In conclusion, a correct classification of gallstones is useful to find all the conditions associated with the different types of gallstones in order to plan primary and secondary gallstone prevention and treatment.
Acknowledgments
Conflict of Interest
The author states that there is no conflict of interest and that no funds were received for this study.
References
- 1.Jarrar BM, Al-Rowaili MA. Chemical composition of gallstones from Al-Jouf Province of Saudi Arabia. Malays J Med Sci. 2011;18(2):47–52. [PMC free article] [PubMed] [Google Scholar]
- 2.Kawai M, Iwahashi M, Uchiyama K, Ochiai M, Tanimura H, Yamaue H. Gram-positive cocci are associated with the formation of completely pure cholesterol stones. Am J Gastroenterol. 2002;97(1):83–88. doi: 10.1111/j.1572-0241.2002.05425.x. [DOI] [PubMed] [Google Scholar]
- 3.Cariati A, Cetta F, Re-Kawai, et al. Bacteria are not important in the formation of pure cholesterol stones. Am J Gastroenterol. 2002;97(11):2921–2922. doi: 10.1111/j.1572-0241.2002.07072.x. [DOI] [PubMed] [Google Scholar]
- 4.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 5.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368(9531):230–239. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 6.Diehl AK. Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am. 1991;20(1):1–19. [PubMed] [Google Scholar]
- 7.Ostrow JD. The etiology of pigment gallstones. Hepatology. 1984;4:215S–222S. doi: 10.1002/hep.1840040840. [DOI] [PubMed] [Google Scholar]
- 8.Maki T. Pathogenesis of calcium bilirubinate gallstone: role of E. coli, beta-glucuronidase, and coagulation by inorganic ions, polyelectrolytes, and agitation. Ann Surg. 1966;164:90–100. doi: 10.1097/00000658-196607000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cetta F. Bile infection documented as initial event in the pathogenesis of brown pigment biliary stones. Hepatology. 1986;6:482–489. doi: 10.1002/hep.1840060327. [DOI] [PubMed] [Google Scholar]
- 10.Malet PF, Takabajashi A, Trotman BW, et al. Black and brown pigment gallstones differ in microstructure and microcomposition. Hepatology. 1984;4:227–234. doi: 10.1002/hep.1840040210. [DOI] [PubMed] [Google Scholar]
- 11.Soloway RD, Trotman BW, Maddrey WC, Nakayama F. Pigment gallstone composition in patients with hemolysis or infection/stasis. Dig Dis Sci. 1986;31:454–460. doi: 10.1007/BF01320307. [DOI] [PubMed] [Google Scholar]
- 12.Cariati A, Piromalli E. Limits and perspective of oral therapy with statins and aspirin for the prevention of symptomatic cholesterol gallstone disease. Expert Opin Pharmacother. 2012;13(9):1223–1227. doi: 10.1517/14656566.2012.685161. [DOI] [PubMed] [Google Scholar]
- 13.Cariati A, Piromalli E. Ultrastructural basis of the failure of oral dissolution therapy with bile salts and /or statin for cholesterol gallstone. Expert Opin Pharmacother. 2012;13(9):1387–1388. doi: 10.1517/14656566.2012.663550. [DOI] [PubMed] [Google Scholar]
- 14.Malet PF, Dabazies MA, Huang G, et al. Quantitative infrared spectroscopy of common bile duct gallstones. Gastroenterology. 1988;94:1217–1221. doi: 10.1016/0016-5085(88)90015-7. [DOI] [PubMed] [Google Scholar]
- 15.Sutor DJ, Wooley SE. The nature and incidence of gallstones containing calcium. Gut. 1973;14:215–220. doi: 10.1136/gut.14.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cetta F. The role of bacteria in pigment gallstone disease. Ann Surg. 1991;213(4):315–326. doi: 10.1097/00000658-199104000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cariati A, Cetta F. Rokitansky–Aschoff sinuses of the gallbladder are associated with black pigment gallstone formation: a scanning electron microscopy study. Ultrastruct Pathol. 2003;27:265–270. doi: 10.1080/01913120309913. [DOI] [PubMed] [Google Scholar]
- 18.Vitek L, Carey MC. New pathophysiological concepts underlying pathogenesis of pigment gallstones. Clin Res Hepatol Gastroenterol. 2012;36:122–129. doi: 10.1016/j.clinre.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cariati A, Traverso E. Ultrastructural analysis and classification of pigment gallstones. J Lab Clin Med. 2003;142(6):431–432. doi: 10.1016/S0022-2143(03)00155-0. [DOI] [PubMed] [Google Scholar]
- 20.Cariati A, Piromalli E. Prevention of biliary stent occlusion. Dig Dis Sci. 2012;57(7):1971–1972. doi: 10.1007/s10620-012-2109-4. [DOI] [PubMed] [Google Scholar]
- 21.Cariati A, Piromalli E. Role of parietal (gallbladder mucosa) factors in the formation of black pigment gallstones. Clin Res Hepatol Gastroenterol. 2012;36:e50–e51. doi: 10.1016/j.clinre.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Cariati A, Cetta F. Squamous cell and nonsquamous cell carcinomas of the gallbladder have different risk factors. Lancet Oncol. 2003;4:393–394. doi: 10.1016/s1470-2045(03)01134-3. [DOI] [PubMed] [Google Scholar]
- 23.Cariati A, Piromalli E. Chemotherapy and histological stratification of biliary tract cancers. Oncology. 2012;82:352–353. doi: 10.1159/000338660. [DOI] [PubMed] [Google Scholar]
- 24.Cariati A. Cisplatin plus gemcitabine for biliary tract cancer. New Engl J Med. 2010;363(2):192. doi: 10.1056/NEJMc1005317. [DOI] [PubMed] [Google Scholar]
- 25.Cariati A, Puglisi R, Zaffarano R, Accarpio FT, Cetta F. Helicobacter pylori and the risk of benign and malignant biliary tract disease. Cancer. 2003;98:656–657. doi: 10.1002/cncr.11549. [DOI] [PubMed] [Google Scholar]
- 26.Behari A, Kapoor VK. Asymptomatic gallstones—to treat or not to? Indian J Surg. 2012;74:4–12. doi: 10.1007/s12262-011-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cariati A, Piromalli E (2012) Is preventive cholecystectomy for gallbladder carcinoma in Northern India cost effective? Indian J Surg. doi:10.1007/s12262-012-0601-x [DOI] [PMC free article] [PubMed]
- 28.Cariati A (2012) Blackberry pigment (whitlockite) gallstones in uremic patients. Clin Res Hepatol Gastroenterol. doi:10.1016/j.clinre.2012.08.004 [DOI] [PubMed]