Abstract
Split thickness skin graft (STSG) is a key method in the reconstructive ladder for covering skin defects used widely by surgeons from all specialties. The donor site is often a source of delayed healing, associated with considerable pain and discomfort even more than the recipient wound. The aim of this prospective randomized controlled study was to compare Helicoll® (EnColl Corp., Fremont, CA, USA), a type I pure collagen dressing, to OpSite® (Smith & Nephew, USA) dressing and to Scarlet Red® (Kendall HealthCare, USA) dressing in the treatment of standardized STSG donor sites. Thirty patients, over a 3-month period, underwent various reconstructive procedures, necessitating the use of STSGs. Following a simple randomized clinical protocol, the analysis of data included donor site pain, healing time of the donor site, initial absorption of the applied dressing and rate of infection with the three different dressings to form the basis of this paper. Patients in the Helicoll group reported significantly less pain, less infection rate and required no dressing change when compared with the OpSite (Johnson & Johnson, Langhorne, PA, USA) or the Scarlet Red groups. Healing time of the donor site in the Helicoll group was shorter than that in the Scarlet Red group; however, it was comparable to the OpSite group. This study indicates that Helicoll, as a donor site dressing, is successful in providing pain-free mobility with a measurable healing rate.
Keywords: Helicoll, Scarlet Red, OpSite, Split thickness skin graft (STSG), Donor site
Introduction
Although STSG donor sites heal when treated with various methods ranging from simple gauze dressings to complex cultured-cell dressings, many are very painful to patients [1, 2]. It is well documented that donor sites dressed with closed dressings have shorter healing time both clinically and histologically. They are associated with better patient comfort when compared to the open donor site dressing technique. This has been attributed to protection from dehydration, mechanical trauma and avoidance of exogenous contamination [3, 4]. We undertook a prospective randomized trial to examine the comparative comfort and ease of care of three different donor site dressings. The two standard dressings were used at our institution: the Scarlet Red, institutionally prepared by our pharmacy staff and the OpSite, an occlusive dressing. The two dressings were compared to a new occlusive dressing product, Helicoll, a type I pure collagen by. We randomized 30 patients requiring STSGs to receive one of the three dressings.
STSG donor sites cause painful wounds as the nerve endings in the dermis are left exposed once the STSG is harvested. Conventional donor site treatment with mesh gauze impregnated with various ointments causes pain even with the use of various medications [5]. To reduce pain, to provide better infection control and to hasten healing rate of the donor site of split skin graft, a number of closed donor site dressings have been developed in recent years as closed wounds heal faster than those left exposed [6, 7].
Healing of partial-thickness skin defects occurs in three steps: epithelial proliferation, epithelial migration, followed by dermal proliferation. Winter [8] and Lawrence and Blake [9] showed that the optimum condition for both epithelial proliferation and migration, and angiogenesis occurred under an occlusive dressing where a moist environment is maintained. Based on this improved understanding, a new class of synthetic adhesive moisture vapor permeable dressings (SAM) was introduced. These dressings are impermeable to both liquid and bacteria [10]. The best donor site dressing should be easy to apply, with minimal need for staff care. It should allow the donor site to heal with minimal bleeding, infection or pain. It should permit the patient full ambulation without disturbing the healing process and should be readily available and cost-effective [11]. At our institution, skin graft donor sites are mostly treated with the use of Scarlet Red mesh gauze or OpSite occlusive synthetic dressing.
Although Scarlet Red, 5% o-tollazo-o-tolyl azo-B-naphthol blend with lanolin, white petrolatum and olive oil, when applied to fine mesh gauze, has good results as the azo compound, it was shown to promote epithelization. It is, however, associated with pain [12]. On the other hand, OpSite dressing decreased pain but fluid collection, leakage and frequent dressing change requirement remain drawbacks to its use. Also, its application is limited to small donor areas [13, 14].
This trial consisted of using a new dressing, Helicoll, a bovine skin derivative that has not been cross-linked, but it is processed to obtain high-quality and type I pure collagen. It interacts with wound exudate to form a moist non-adherent gel. It provides homeostasis and accelerates tissue remodeling without causing irritation. In a limited study, Helicoll was found to accelerate the wound healing rate and reduce scar formation by depositing oriented and organized collagen fiber [15, 16].
Other types of collagen have been used for centuries as temporary biological wound dressings; however, they have not been used as a permanent skin transplant [17]. Helicoll is US Food and Drug Administration (FDA) approved as a type I pure collagen product that can be used as a permanent replacement and as a cellular dermal component of the skin.
Materials and Methods
This study was approved by the institutional review board of the University of Texas Medical Branch (IRB number 08-143) along with the institutional IRB approval at Christian Medical College, Vellore, TN, India. At hospital admission, the research team obtained informed medical consent from the parents and consent from children older than 15 years. Patients were randomized to receive OpSite, Scarlet Red or Helicoll as a donor site dressing. The patients stayed in the hospital for 24 h after their surgery, where dressings were changed if necessary, and all patients received a standard post-operative oral analgesic and discharged home to be seen in the clinic on the fifth post-operative day.
Thirty donor sites, one each from 30 patients, were studied. The age range was from 6 to19 years old. Data and photographs of the procedure and subsequent evolution were collected from all patients. Age, gender, size of donor area, post-operative donor site pain, time of healing of the donor site, frequency of dressing change and infection rate were compared between the three groups. Digital images of wounds were assessed for rate of healing, infection status and incorporation of the skin substitute into the deeper structures.
All skin grafts were harvested with an electric Padgett dermatome from the thigh approximately 16–80 cm2 in dimension and 0.015 in. in thickness. Dressings were applied larger than the donor defect to ensure good adherence. To provide additional comfort and better immobilization, an elastic bandage was applied. Dressings were assessed by interview and questionnaire at 24-, 48-, 120-h (5 days), and 1-week intervals. The wound was assessed on a daily basis for pain severity, amount and type of exudates underneath the dressing, adherence of the dressing and the rate of infection. Self-assessment of pain was quantified on a scale of zero (no pain) to five (the most severe pain). Donor site pain intensities were assessed by a visual analogue scale. The data were statistically evaluated with the Mann–Whitney U test. In the immediate post-operative period the patient was asked to assess the pain or discomfort when touch or pressure was applied to the donor site by a blinded investigator. In the late post-operative period, the patients were requested to record the severity of pain felt while walking; similarly, the requirement of analgesic for donor site pain was evaluated in all cases. Re-epithelialization was assessed by a single observer from the first post-operative day. However, it is beyond the scope of this study to delineate the exact rate of re-epithelialization in each group, but it is an area of interest for future study. Digital images of the wounds were viewed by independent observers.
Study Subject Inclusion Criteria
Age limit for study: 6–19 years.
Gender eligibility: Both sexes in general good health.
Signed or agreeing to the informed consent provided
Subject with a donor site or sites generally comparable in size and wound characteristics
No other injury close to the donor site area
The subject requires hospitalization for initial treatment
Subject agrees to participate in follow-up evaluations
Study Subject Exclusion Criteria
Major illnesses that could affect wound healing such as the following:
Peripheral vascular disease
Insulin-dependent diabetes
Blood clotting disorder
Pregnancy
Illness requiring ventilator support or having a systemic infection or hemodynamic instability, defined as a mean arterial pressure less than 60
Subjects receiving treatment with medications that inhibit or compromise wound healing, except the use of aspirin or anticoagulants for deep vein thrombosis prophylaxis
Possible systemic infection or significant local infection
Patient is receiving, or has received within 1 month prior to visit 1, any treatment known to impair wound healing, including, but not limited to, corticosteroids, immunosuppressive drugs, cytotoxic agents, radiation therapy and chemotherapy
Participation in another clinical trial within 30 days prior to the screening visit or during this study
Methods of Application
Helicoll is removed from its package and soaked in sterile saline for 5 min. It is then peeled from the packaging sheet and applied to the donor site while ensuring that all entrapped air is removed. A non-adherent dressing (Adaptic, Johnson & Johnson USA with Bacitracin ointment, G&W Labs, USA) was applied over the Helicoll and wrapped with Kerlix® (Kendall, USA) and ace bandage. The material is applied only once to the donor site wound. OpSite required Mastisol application to 2 cm of normal skin surrounding the donor site for good adherence. Additional compression is achieved with Kerlix and Ace bandage. Pressure dressing is needed to reduce the amount of fluid collection. The pressure dressing remained in situ for 24 h. The following day the dressing was inspected to check for the presence of hematoma and, if the dressing was dry, it was simply reapplied. Wet dressings were changed, as needed. Serosanguineous fluid collection and leakage from under the edge of the OpSite required aspiration using sterile technique with reinforcement of the OpSite dressing.
Scarlet Red was applied directly over the donor site and was dressed with Adaptic non-adherent gauze between the Scarlet Red and the gauze bandage followed by Kerlix and surgical bandage. Eight hours after the procedure, both Kerlix and Adaptic were removed and the Scarlet Red was allowed to dry.
Statistical Analysis
Our initial power analysis of the study was as follows: We were interested in detecting pain score differences of 1.5 or greater as our primary objective of the study and anticipated a SD (standard deviation) of one on the pain score. Because we were comparing Helicoll to two other methodologies, we reduced the alpha to 0.025. We are able to detect differences of this magnitude with ten patients per group with these assumptions. Descriptive analyses were conducted to compare demographics and medical characteristics. Pain scores were assessed using two-way ANOVA with factors, treatment and time. Post-hoc correction was done using Tukey’s test.
Follow-up Visits
During the follow-up visits, patients were examined and digital photographs taken. Of the 30 patients, 28 patients returned for a complete set of follow-up visits.
Results
Between May 2008 and August 2008, a total of 30 patients were enrolled in this trial. Their ages ranged from 6 to 19 years. Detailed demographics are shown in (Table 1). No significant difference was seen in patients’ age and gender in each group.
Table 1.
Demographics
| Scarlet Red | OpSite | Helicoll | |
|---|---|---|---|
| Number of patients | 10 | 10 | 10 |
| Sex | 6M/4F | 8M/2F | 5M/5F |
| Age (years); mean ± SD | 6–19; 9.1 ± 3.5 | 6–19; 9.3 ± 3.6 | 6–19; 9.1 ± 3.6 |
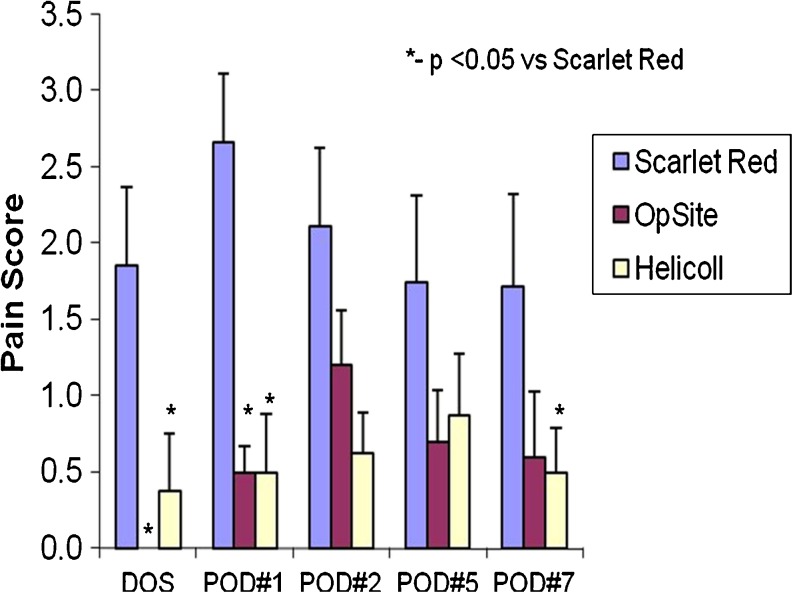
All patients tolerated the dressings and no allergic reactions were observed. The wounds were assessed for characteristics that include comfort, pain level, ability to ambulate and an overall acceptance of the dressing. Relief from pain was the most frequently identified patient benefit. All patients in the Scarlet Red group (100%) had pain, only 25% in the OpSite group reported pain and none of the patients in the Helicoll group experienced donor site pain (P < 0.05).
There were seven outpatient visits in the OpSite group versus two visits in the Helicoll group and none in the Scarlet Red group. The outpatient visits were more frequent with the OpSite group due to leakage from the edges of the OpSite dressing requiring dressing change. OpSite had to be reapplied in five cases. All ten patients in the OpSite group had variable degrees of fluid collection, and four patients treated with OpSite had donor site infections (P < 0.05). Infectious complications were not encountered in patients in the other two groups.
OpSite
Pain started on the fourth or fifth post-operative day in eight patients due to maceration of the wound attributed to fluid collection beneath the OpSite. Fluid collection was seen in all ten OpSite cases. Five patients required a small fenestration of the OpSite to drain the collecting fluid and a new film was used to reinforce the remaining parts of the original dressing followed by a secondary absorbent dressing to prevent constant leaking. Three patients had yellow discharge under the wrinkled OpSite and required removal and daily dressing change with Adaptic and Bacitracin. Four patients had purulent exudates associated with redness. Patients were diagnosed clinically to have donor site wound infection, but no cultures were obtained. One patient developed a rash and two patients had dry crusts on the donor site. One patient had clots that were removed by making an incision in the OpSite. Six wounds healed in 7 days and four healed in 12 days. The transparent dressing appears to offer many advantages over opaque ones and gives a better follow-up of the donor site. OpSite promotes more rapid and less painful healing; however, it tends to be labor intensive, especially if associated with large fluid collection. This problem requires frequent dressing changes. It is our observation that fluid collections are best untreated and that wrinkled or dislodged OpSite should be removed to prevent infection (Figs. 1, 2, 3, and 4).
Fig. 1.
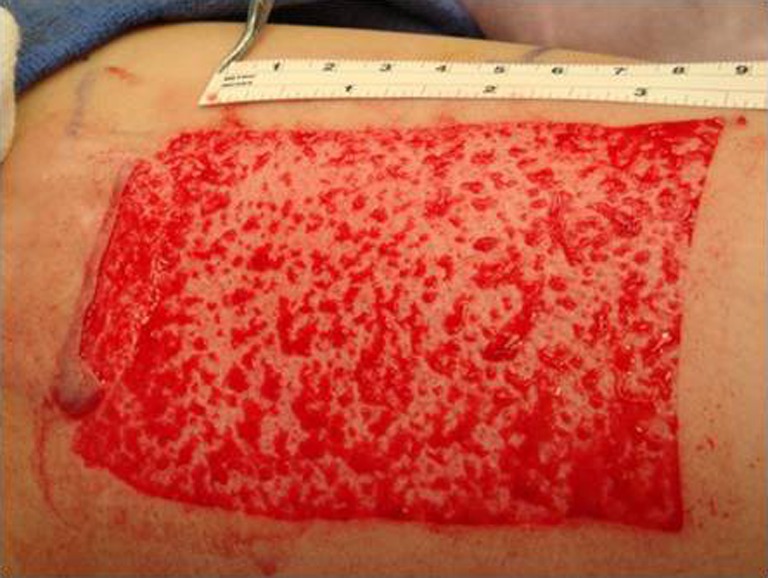
Intra-operative pictures of donor site
Fig. 2.
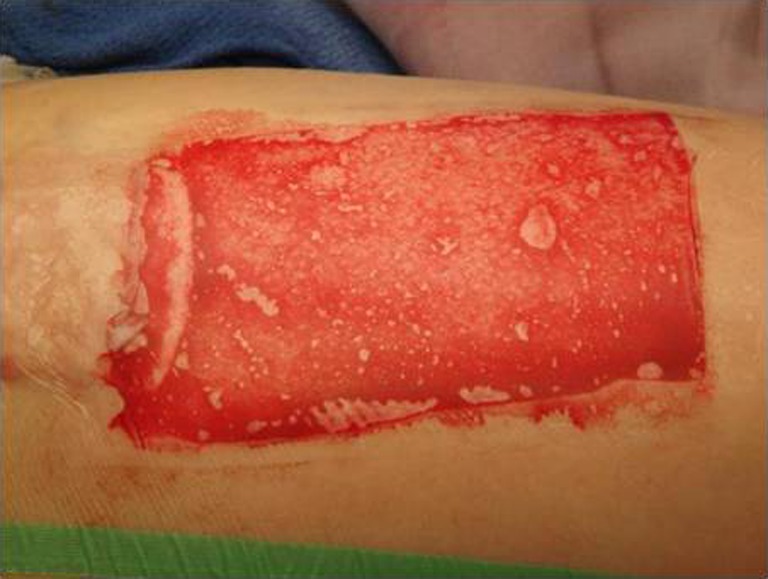
Application of OpSite to donor site wound
Fig. 3.
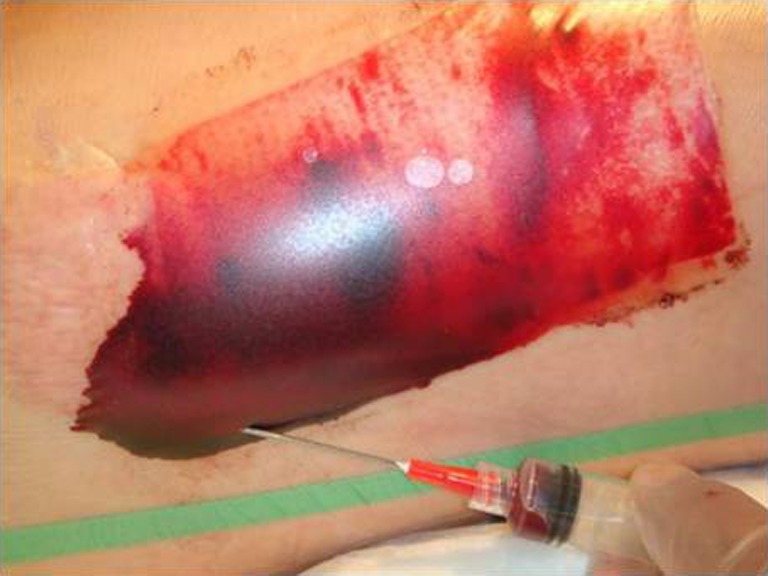
Post-operative serosanguineous collection and attempt at its aspiration under sterile technique
Fig. 4.
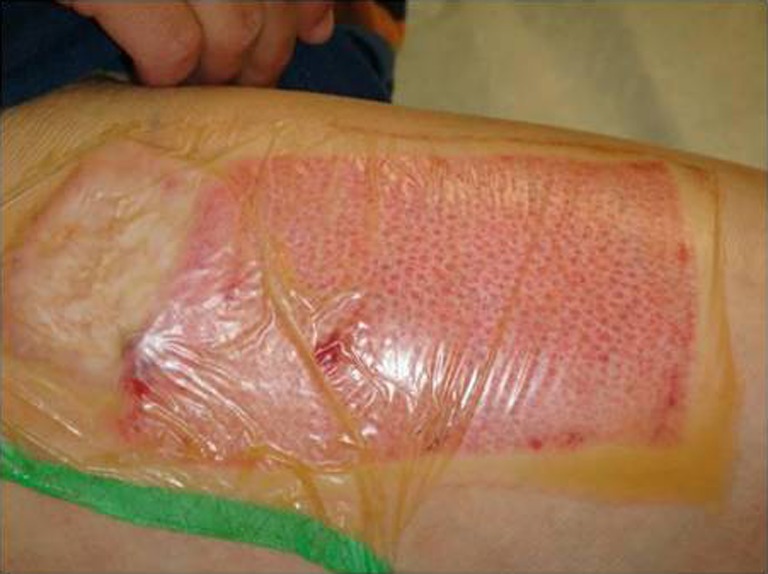
Donor site wound on post-operative day 8
Scarlet Red
Scarlet Red used to be manufactured by Tyco and Kendall, Mansfield, MA. Once its supply was discontinued, it was formulated by our pharmacy staff as a 2% Scarlet Red ointment dressing.
Patients with Scarlet Red experienced pain on the day of surgery, which limited their movement. Outer dressings were removed on the first post-operative day and it was allowed to dry. None of the patients with Scarlet Red had infections. As Scarlet Red dries, it shrinks and becomes tight over the donor area and this tightness makes ambulation and movement difficult and painful. Although absorption was good in this patients group, three Scarlet Red wounds required unplanned dressing changes because the dressings were soggy and lifted off the donor site. Delayed wound healing was seen in three cases. Wounds healed in 10–12 days. The Scarlet Red dressing is best for scalp donor site grafts. The open technique of leaving the wound uncovered is the least expensive but is very painful and associated with prolonged healing times. Patients seemed to complain most when the rolled gauze fluff was removed on the first post-operative day. The coagulum caused the Scarlet Red to stick to the gauze and removal was quite painful (Figs. 5, 6, 7, and 8).
Fig. 5.
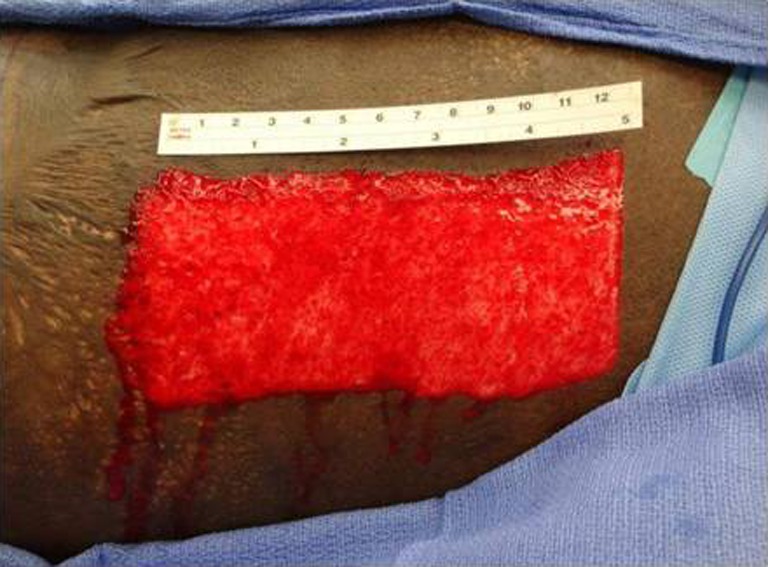
Donor site at harvest
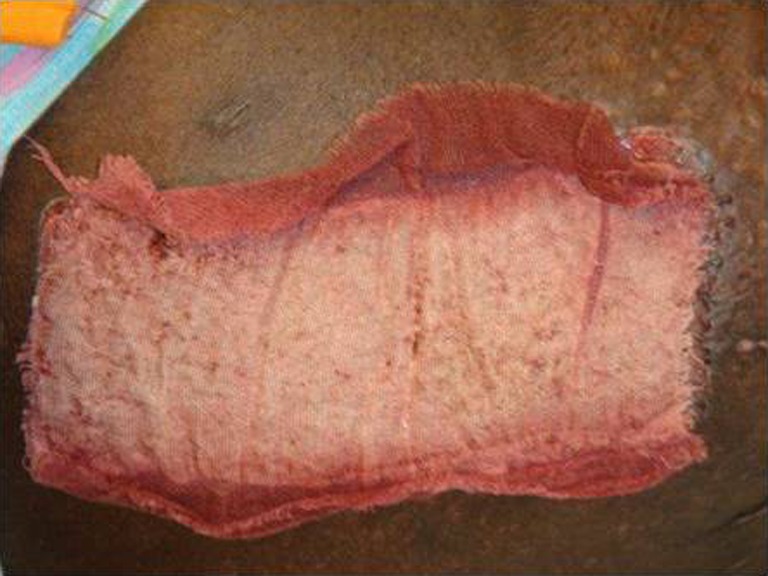
Fig. 6.
Application of scarlet red to donor site wound
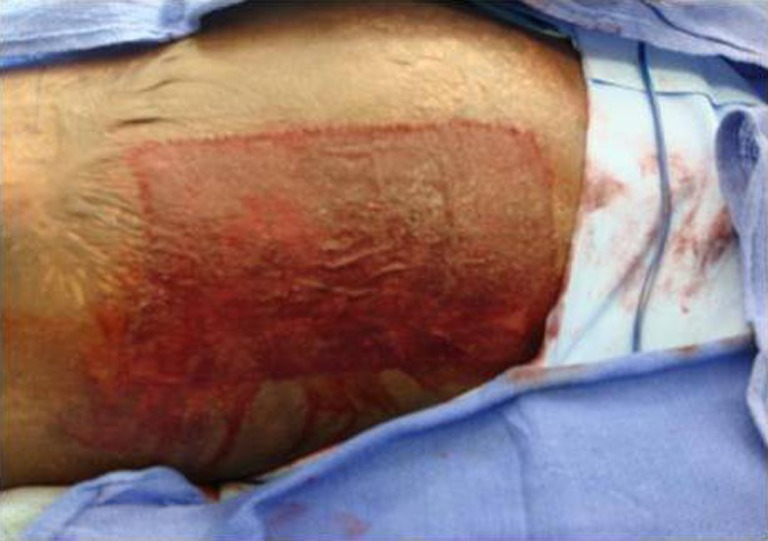
Fig. 7.
Donor site at post-operative day 5
Fig. 8.
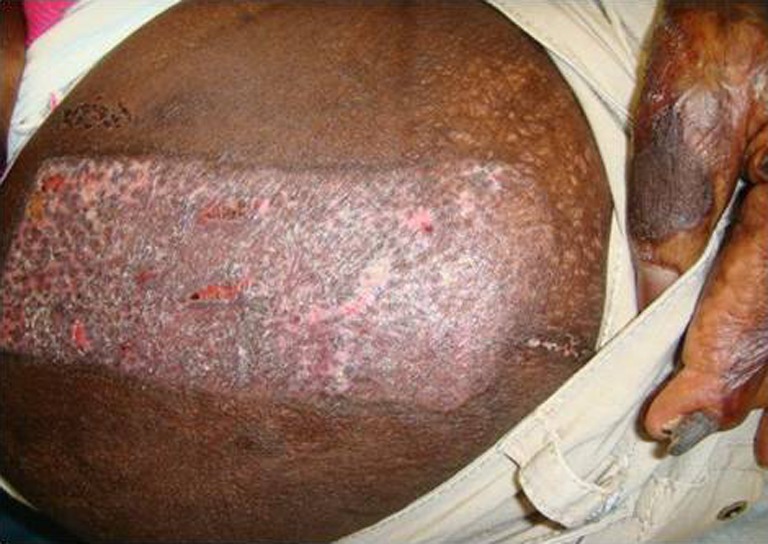
Donor site at post-operative day 22
Helicoll
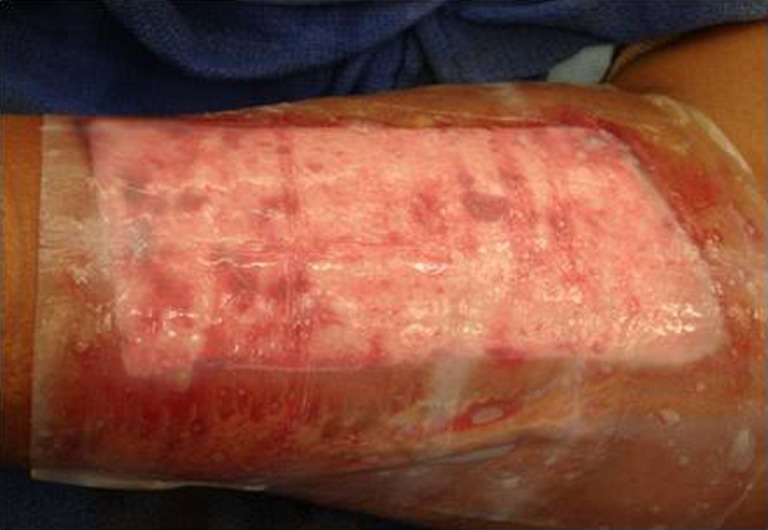
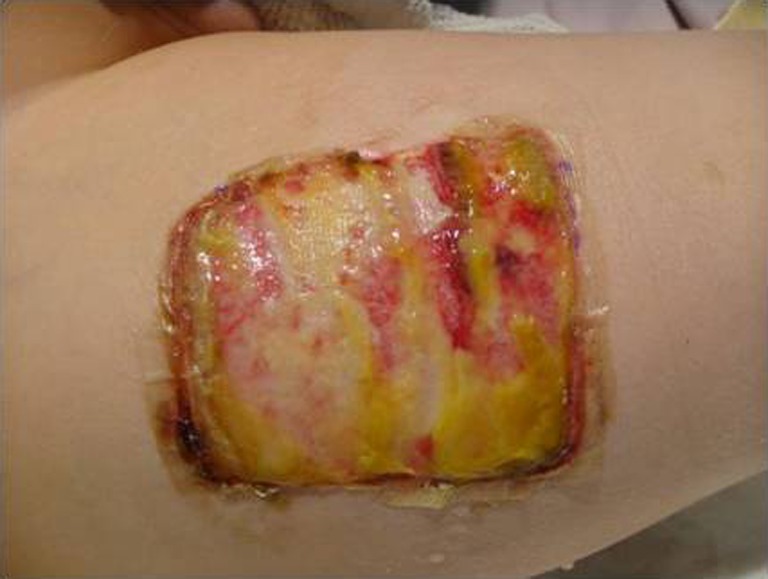
Helicoll, on the other hand, was well tolerated by patients because of the absence of pain in the donor site and the resulting freedom of movement. Pain was reported on the fourth post-operative day, by eight of our patients, probably because of the degradation of the product and its incorporation into the wound leaving behind a closed wound. The first two cases initially had daily inspection of the wound bed to evaluate the wound healing progress, but this disturbed the rate of wound healing. None of the Helicoll patients had infections. A yellow gel observed on the fourth post-operative day is due to the degradation of the product. Helicoll wounds healed in 7–10 days. The most valuable aspects of Helicoll include much greater patient comfort on the day of surgery and easier removal of dressings later on. This indicates indirectly the wound healing was significantly increased in the group treated with Helicoll (Figs. 9, 10, 11, 12, and 13).
Fig. 9.
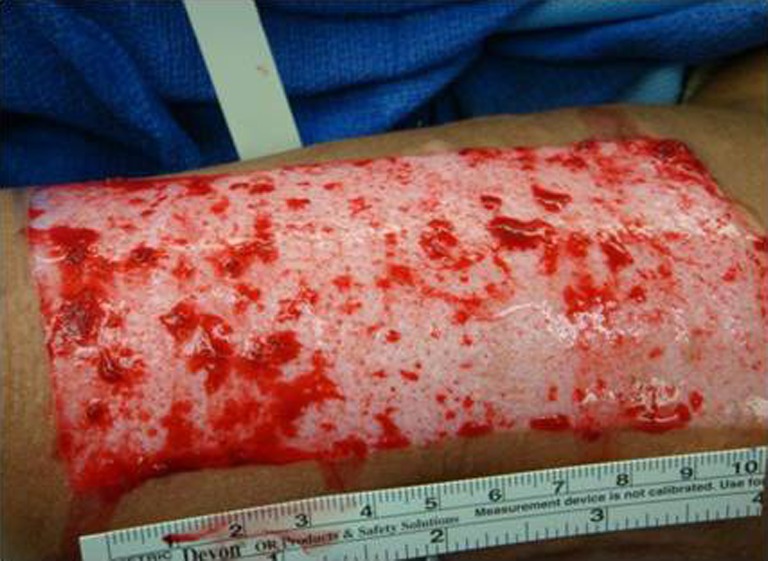
Intra-operative picture of donor site
Fig. 10.
Helicoll application to it followed by Adaptic and Bacitracin dressings
Fig. 11.
Donor site on post-operative day 5; notice the incorporation of the product
Fig. 12.
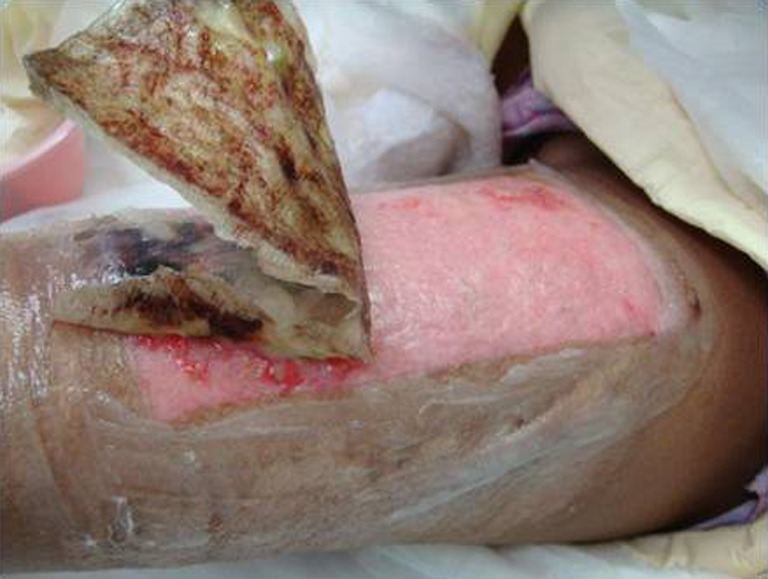
Donor site dressing removal on post-operative day 8
Fig. 13.
Illustration of pain score in different patient groups on day of surgery and post-operative days 1–7. Statistical significances as shown for pain scores were assessed using two-way ANOVA with factors, treatment and time
Discussion
STSG donor sites often can be more painful and uncomfortable for patients than the recipient wound. In 1962, Winter [8] and Chang et al. [18–20] demonstrated that moisture enhances wound re-epithelialization and angiogenesis thus accelerating the healing rate. Transparent semi-occlusive dressing with a hydrocolloid base, such as Biobrane, allows fast, yet stable healing with reduced donor site pain but was associated with high infection rates secondary to exudate accumulation [21–23]. Another standard donor site management is to use an alginate dressing. These dressings desiccate and adhere tightly to the wound bed, making it difficult and painful to remove [24–26]. Helicoll is a reconstituted type I pure collagen sheet, derived from a bovine source and free of contaminants such as lipids, elastin or other immunogenic proteins. It is a semi-occlusive, self-adhesive collagen membrane with unique advantages of biocompatibility and flexibility. Because it maintains a physiologically moist environment at the wound surface and is biodegradable, it is associated with minimal post-operative wound pain. The traditionally used dressing for donor sites at our institution has been mesh gauze impregnated with Scarlet Red and a synthetic polymer, OpSite. These forms of dressings continue to be cost-effective compared with other types of dressings. Scarlet Red dressings are simple and the most commonly used dressing due to its easy availability, ease of application and low cost. It, however, is associated with delayed healing rates [24] when compared to other dressings. Problems include pain between and during dressing changes. There is even more pain if the dressing has to be removed after being incorporated into the healing site, as it does not always falls off easily. The dressing is adherent and associated with trivial trauma liable to damage the new epithelium [27, 28].
OpSite is a transparent polyurethane dressing that significantly reduces pain relative to open dressings, but the wound exudates get trapped under it and the dressings have to be changed several times to allow for fluid removal. The accumulated fluid tends to be thicker in consistency over time rendering dressing change more laborious. Even though the fluid is usually sterile [29, 30], clinical infection has been reported and was observed in four cases in this group. In some cases, hematoma formed under the OpSite dressing after ambulation and could not be aspirated further leading to the increased infection rate in this group [31].
The comparison of Helicoll with mesh gauze and OpSite on donor sites demonstrated a reduction in pain when compared to the traditional and time-tested approach. Other collagen dressings are available and are composed of types I and III bovine collagen. The difference between these collagen sheets and Helicoll dressing is that the collagen [32] provides a scaffolding for epithelial re-growth and prevents exudation from the raw area. After 48 h the film is transformed into a stiff sheet that is stable enough to withstand pressure and shearing from clothes. Thus, it protects the donor site from mechanical trauma and infection. When re-epithelialization is completed, the overlying film and coagulated blood separate spontaneously. Thus, removal of the dressing is easy and pain-free. If a donor site infection occurs, it would result in complete degradation of the film and is associated with significant donor site pain [4]. In contrast Helicoll, a type I pure collagen, has the advantage of being a permanent skin transplant that replaces a cellular dermal component. It undergoes degradation on the fourth post-operative day and gets incorporated into the wound. Thus, Helicoll dressings appear to have a greater advantage over other dressing materials in providing a pain-free donor site, early mobilization of the patient and a decreased morbidity. An average donor site healing time of 8.2 days with Helicoll is comparable to the results with the regular OpSite dressings [33, 34]; however, it is beyond the scope of this study to delineate the exact rate of re-epithelialization in each group.
Our results compare favorably with other donor site dressings. About patient comfort, Helicoll had the best results and an overall mean healing time was of 7.8 days for both Helicoll and OpSite compared with 10.5 days with Scarlet Red dressing. An overall healing correlates with a complete removal of the dressing as the wound is mature enough to withstand minor trauma without breakdown and bleeding. The accelerated healing, early mobilization and the reasonable cost make Helicoll a good dressing.
Conclusion
Based on the results of this prospective study of STSG donor sites in 30 patients, we conclude that Helicoll is a reliable donor site dressing. Its ease of application, documented safety, reasonable cost, and evident capacity to promote measurable healing place it in line with other components of our armamentarium of dressings for STSG donor sites.
Acknowledgements
This study has been conducted solely for research purposes and this study was not sponsored by any product distributor or manufacturer. Accordingly, there have been no conflicts of interest in this research study.
References
- 1.Davies JW. Synthetic materials for covering burn wounds: progress towards perfection. Part I. Short term dressing materials. Burns Incl Therm Inj. 1983;10(2):94–103. doi: 10.1016/0305-4179(83)90005-0. [DOI] [PubMed] [Google Scholar]
- 2.Hickerson WL, Kealey GP, Smith DJ, Jr, Thomson PD. A prospective comparison of a new synthetic donor site dressing versus an impregnated gauze dressing. J Burn Care Rehabil. 1994;15(4):359–363. doi: 10.1097/00004630-199407000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Feldman DL, Rogers A, Karpinski RH. A prospective trial comparing Biobrane, Duoderm and xeroform for skin graft donor sites. Surg Gynecol Obstet. 1991;173(1):1–5. [PubMed] [Google Scholar]
- 4.Kilinc H, Sensoz O, Ozdemir R, Unlu RE, Baran C. Which dressing for split-thickness skin graft donor sites? Ann Plast Surg. 2001;46(4):409–414. doi: 10.1097/00000637-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Friedman GD, Capozzi A, Pennisi VR. Care of the split-thickness skin graft donor site. J Trauma. 1974;14(2):163–167. doi: 10.1097/00005373-197402000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Person K, Salemark L. How to dress donor sites of split thickness skin grafts: a prospective, randomised study of four dressings. Scand J Plast Reconstr Surg Hand Surg. 2000;34(1):55–59. doi: 10.1080/02844310050160178. [DOI] [PubMed] [Google Scholar]
- 7.Brady SC, Snelling CF, Chow G. Comparison of donor site dressings. Ann Plast Surg. 1980;5(3):238–243. doi: 10.1097/00000637-198009000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. J Wound Care. 1962;4(8):366–367. [PubMed] [Google Scholar]
- 9.Lawrence JE, Blake GB. A comparison of calcium alginate and scarlet red dressings in the formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig healing of split thickness skin graft donor sites. Br J Plast Surg. 1991;44(4):247–249. doi: 10.1016/0007-1226(91)90065-R. [DOI] [PubMed] [Google Scholar]
- 10.Barnett A, Berkowitz RL, Mills R, Vistnes LM. Comparison of synthetic adhesive moisture vapor permeable and fine mesh gauze dressings for split-thickness skin graft donor sites. Am J Surg. 1983;145(3):379–381. doi: 10.1016/0002-9610(83)90206-4. [DOI] [PubMed] [Google Scholar]
- 11.Ponten B, Nordgaard JO. The use of collagen film (Cutycol) as a dressing for donor areas in split skin grafting. Scand J Plast Reconstr Surg. 1976;10(3):237–240. doi: 10.3109/02844317609012975. [DOI] [PubMed] [Google Scholar]
- 12.Prasad JK, Feller I, Thomson PD. A prospective controlled trial of Biobrane versus scarlet red on skin graft donor areas. J Burn Care Rehabil. 1987;8(5):384–386. doi: 10.1097/00004630-198709000-00009. [DOI] [PubMed] [Google Scholar]
- 13.James JH, Watson AC. The use of OpSite, a vapour permeable dressing, on skin graft donor sites. Br J Plast Surg. 1975;28(2):107–110. [PubMed] [Google Scholar]
- 14.Weber RS, Hankins P, Limit one E, et al. Split-thickness skin graft donor site management. A randomized prospective trial comparing a hydrophilic polyurethane absorbent foam dressing with a petrolatum gauze dressing. Arch Otolaryngol Head Neck Surg. 1995;121(10):1145–1149. doi: 10.1001/archotol.1995.01890100055009. [DOI] [PubMed] [Google Scholar]
- 15.Gunasekaran S, Kwolek M, Dhanikachalam A (2006) A comparative second-degree burn treatment trial collagen dressing vs. silver sulphadiazine alone. Presented at the 31st Annual Meeting of Society of Biomaterials, Pittsburgh, PA, USA.
- 16.Gunasekaran S, Rajendran MK, Swaminathan T, Dhawan S (2003) Modified collagen for burns enhances healing rate possibly by cell signaling. Presented at the American Society for Dermatologic Surgery Annual Meeting.
- 17.Salisbury RE, Wilmore DW, Silverstein P, Pruitt BA., Jr Biological dressings for skin graft donor sites. Arch Surg. 1973;106(5):705–706. doi: 10.1001/archsurg.1973.01350170069017. [DOI] [PubMed] [Google Scholar]
- 18.Leicht P, Siim E, Sorensen B. Treatment of donor sites—Duoderm or Omiderm? Burns Incl Therm Inj. 1989;15(1):7–10. doi: 10.1016/0305-4179(89)90060-0. [DOI] [PubMed] [Google Scholar]
- 19.Vanstraelen P. Comparison of calcium sodium alginate (KALTOSTAT) and porcine xenograft (E-Z DERM) in the healing of split-thickness skin graft donor sites. Burns. 1992;18(2):145–148. doi: 10.1016/0305-4179(92)90014-L. [DOI] [PubMed] [Google Scholar]
- 20.Chang H, Wind S, Kerstein MD. Moist wound healing. Dermatol Nurs. 1996;8(3):174–176. [PubMed] [Google Scholar]
- 21.Innes ME, Umraw N, Fish JS, Gomez M, Cartotto RC. The use of silver coated dressings on donor site wounds: a prospective, controlled matched pair study. Burns. 2001;27(6):621–627. doi: 10.1016/S0305-4179(01)00015-8. [DOI] [PubMed] [Google Scholar]
- 22.Varma SK, Henderson HP, Hankins CL. Calcium alginate as a dressing for mini skin grafts (skin soup) Br J Plast Surg. 1991;44(1):55–56. doi: 10.1016/0007-1226(91)90180-R. [DOI] [PubMed] [Google Scholar]
- 23.Feldman DL. Which dressing for split-thickness skin graft donor sites? Ann Plast Surg. 1991;27(3):288–291. doi: 10.1097/00000637-199109000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Attwood AI. Calcium alginate dressing accelerates split skin graft donor site healing. Br J Plast Surg. 1989;42(4):373–379. doi: 10.1016/0007-1226(89)90001-5. [DOI] [PubMed] [Google Scholar]
- 25.Bettinger D, Gore D, Humphries Y. Evaluation of calcium alginate for skin graft donor sites. J Burn Care Rehabil. 1995;16(1):59–61. doi: 10.1097/00004630-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Disa JJ, Alizadeh K, Smith JW, Hu Q, Cordeiro PG. Evaluation of a combined calcium sodium alginate and bio-occlusive membrane dressing in the management of split-thickness skin graft donor sites. Ann Plast Surg. 2001;46(4):405–408. doi: 10.1097/00000637-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Freshwater MF, Su CT, Hopes JE. A comparison of polyurethane foam dressing and fine mesh gauze in the healing of donor sites. Plast Reconstr Surg. 1978;61(2):275–276. doi: 10.1097/00006534-197802000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Tan ST, Roberts RH, Blake GB. Comparing Duo DERM E with scarlet red in the treatment of split skin graft donor sites. Br J Plast Surg. 1993;46(1):79–81. doi: 10.1016/0007-1226(93)90071-I. [DOI] [PubMed] [Google Scholar]
- 29.Dinner MI, Peters CR, Sherer J. Use of a semipermeable polyurethane membrane as a dressing for split-skin graft donor sites. Plast Reconstr Surg. 1979;64(1):112–114. doi: 10.1097/00006534-197907000-00031. [DOI] [PubMed] [Google Scholar]
- 30.Klein RL, Rothmann BF, Marshall R. Biobrane—a useful adjunct in the therapy of outpatient burns. J Pediatr Surg. 1984;19(6):846–847. doi: 10.1016/S0022-3468(84)80382-6. [DOI] [PubMed] [Google Scholar]
- 31.Zapata-Sirvent R, Hansbrough JF, Carroll W, Johnson R, Wakimoto A. Comparison of Biobrane and Scarlet Red dressings for treatment of donor site wounds. Arch Surg. 1985;120(6):743–745. doi: 10.1001/archsurg.1985.01390300081014. [DOI] [PubMed] [Google Scholar]
- 32.Horch RE, Stark GB. Comparison of the effect of a collagen dressing and a polyurethane dressing on the healing of split thickness skin graft (STSG) donor sites. Scand J Plast Reconstruct Surg Hand Surg. 1998;32(4):407–413. doi: 10.1080/02844319850158499. [DOI] [PubMed] [Google Scholar]
- 33.Dyson M, Young SR, Hart J, Lynch JA, Lang S. Comparison of the effects of moist and dry conditions on the process of angiogenesis during dermal repair. J Invest Dermatol. 1992;99(6):729–733. doi: 10.1111/1523-1747.ep12614460. [DOI] [PubMed] [Google Scholar]
- 34.Young T, Fowler A. Nursing management of skin grafts and donor sites. Br J Nurs. 1998;7(6):324–326. doi: 10.12968/bjon.1998.7.6.5731. [DOI] [PubMed] [Google Scholar]