Abstract
Motivated by the conceptual framework of multi-scale biomechanics, this narrative review highlights recent major advances with a focus on gait and joint kinematics, then tissue-level mechanics, cell mechanics and mechanotransduction, matrix mechanics, and finally the nanoscale mechanics of matrix macromolecules. A literature review was conducted from January 2014 to April 2015 using PubMed to identify major developments in mechanics related to osteoarthritis (OA). Studies of knee adduction, flexion, rotation, and contact mechanics have extended our understanding of medial compartment loading. In turn, advances in measurement methodologies have shown how injuries to both the meniscus and ligaments, together, can alter joint kinematics. At the tissue scale, novel findings have emerged regarding the mechanics of the meniscus as well as cartilage superficial zone. Moving to the cell level, poroelastic and poroviscoelastic mechanisms underlying chondrocyte deformation have been reported, along with the response to osmotic stress. Further developments have emerged on the role of calcium signaling in chondrocyte mechanobiology, including exciting findings on the function of mechanically activated cation channels newly found to be expressed in chondrocytes. Finally, AFM-based nano-rheology systems have enabled studies of thin murine tissues and brush layers of matrix molecules over a wide range of loading rates including high rates corresponding to impact injury. With OA acknowledged to be a disease of the joint as an organ, understanding mechanical behavior at each length scale helps to elucidate the connections between cell biology, matrix biochemistry and tissue structure/function that may play a role in the pathomechanics of OA.
Keywords: gait, joint kinematics, tissue mechanics, cell mechanics, molecular mechanics, mechanotransduction, AFM-nanoindentation
Introduction
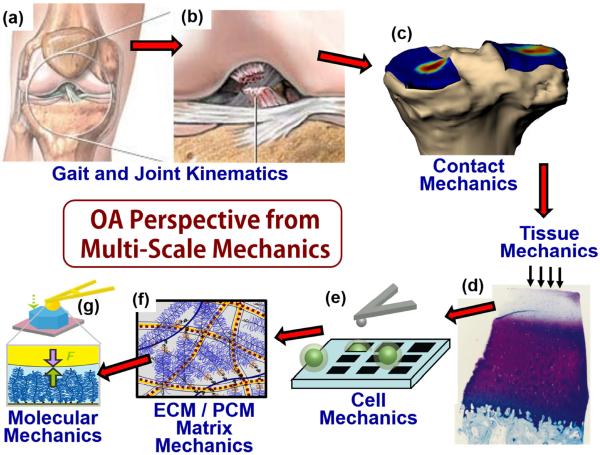
During the past decade, the concept of multiscale modeling has come to the forefront of many areas of engineering. Multiscale modeling in biomechanics focuses on describing the complex mechanical behavior of organisms at multiple length and time scales (e.g., organ, tissue, cells, molecules) and the resulting effects of mechanical forces on cell response1,2. This conceptual framework has gained increasing traction in musculoskeletal systems: while osteoarthritis (OA) is acknowledged to be a disease of the entire joint as an organ, macroscale mechanical forces associated with joint kinematics and aspects of gait are ultimately translated into nanoscale forces at the level of individual extracellular matrix (ECM) and cytoskeletal macromolecules3. Mechanical failure at the molecular level can eventually lead to joint-level pathology. At the cell-level microscale, both normal physiologic and pathologic overload forces are known to regulate cellular responses in essentially all musculoskeletal connective tissues. Thus, multiscale modeling approaches now aid in the estimation of forces that occur at the cell level by combining data from joint imaging and gait analyses with measurements of tissue biomechanical properties4,5. Motivated by this conceptual framework, this narrative review of the past year's research in mechanics has been structured with an overview, first, of gait and joint kinematics, then tissue-level mechanics, cell mechanics and mechanotransduction, matrix mechanics, and advances in molecular mechanics of the ECM (schematized in Fig. 1).
Fig 1.
Emphasis on OA-related mechanics has included the effects of traumatic joint injuries, such as rupture of the ACL (a,b), which leads to altered joint contact mechanics (c), initiation of degeneration of cartilage matrix (d) and accompanying changes to other joint hard and soft tissues, altered biomechanical properties of cells (e) and extracellular/pericellular matrices (f), and changes in specialized load bearing matrix molecules that can be quantified at the nanoscale (g). Images adapted from: (a,b) http://www.quincymedgroup.com/adam/dochtml/surgery; (c) Kindly provided by Dr. Guoan Li, Massachusetts General Hospital; (d) Johnson, DL, et al., Am. J. Sports Med. 26:409–414, 1998; (e) Ng, L, et al., J Biomechanics, 40:1011–1023, 2007; (f) Han, L, et al., Annu Rev Mater Res, 41:133–168, 2011; (g) Lee, H-Y, et al., Osteoarthritis Cartilage, 18:1477–1486, 2010.
Methods
PubMed was initially searched from approximately January 2014 through April 2015 with search terms including (“mechanics” OR “biomechanics” OR “mechano” OR “load”) AND (“osteoarthritis” OR “OA”), giving 176 results. Subtopic searches included cartilage mechanics and osteoarthritis; mechanobiology and osteoarthritis; subject-specific osteoarthritis; ACL (“osteoarthritis” or “OA”) biomechanics. Tables of contents from relevant journals during this time period were also reviewed. All of the abstracts from the articles in the search were reviewed, and 164 full texts were selected for consideration.
Gait and Joint Kinematics
Knee loading during gait is a critical factor associated with the progression of OA6–9. Decreasing the external knee adduction moment (KAM) has been the focus of interventions to reduce joint loading10–12. Recent studies suggest, however, that the external knee flexion moment (KFM) also plays a significant role in medial compartment loading13–15. This past year, Chehab et al.16 investigated both moments – KAM and KFM – in a 5-year prospective study of 16 patients with medial knee OA, testing the hypothesis that knee cartilage changes are associated with baseline KAM and KFM during early stance. As part of this study, cartilage thickness and changes in medial-to-lateral thickness ratio (MLTR) were measured using magnetic resonance imaging. Findings indicated that the medial compartment load is best approximated by a combination of KAM and KFM, rather than KAM alone. Specifically, the authors concluded that KAM is implicated more in femoral cartilage changes (as measured by changes in MLTR) and in subjects with more severe OA, while KFM has a greater influence on tibial cartilage thickness and subjects with less severe OA. These findings further indicated that interventions solely reducing KAM may be insufficient and, in fact, reducing KAM at the expense of an increased KFM may be detrimental to patient outcomes.
Interestingly, Adouni et al.17 used a musculoskeletal model of the lower extremity based on kinematics at gait midstance and suggested that the partitioning of contact loads in the tibiofemoral compartment is strongly influenced by knee adduction angle. Specifically, they showed that a 1.5° drop in adduction angle reduced the medical contact force by 12% and the medial/lateral load ratio by 80%, while a 50% decrease in the KAM decreased those same measures by only 4% and 13%, respectively, thus emphasizing the role of joint adduction rotation. Adouni et al.18 examined how OA-associated alterations in rotations and moments at the lower extremity joints influenced muscle activation using a validated knee finite element (FE) model. Their investigation revealed that muscle forces decreased at nearly all stance periods of gait in severe OA patients compared to normal controls. Increases in tissue strains observed in severe OA patients were partly associated with kinematics of gait and partly due to deterioration of biomechanical properties of cartilages and menisci.
The availability of new computational algorithms for complex 3-D joint contact mechanics was recently summarized by Ateshian et al.19 In this regard, numerical FE methods have relaxed the need for modeling assumptions such as small strain, linear behavior of tissues. Such contact analysis approaches can also predict variations in strain within cartilage layers based on multiphasic material representations. These methodologies could hopefully lead to patient-specific analysis and treatment from readily obtainable medical imaging data19. As an example, Henak et al.20 used subject-specific FE modeling to evaluate the chondrolabral contact mechanics of human hips with acetabular dysplasia (a major predisposition of hip OA). Findings showed that the labrum plays a much larger role in dysplastic hips than in normal hips. Specifically, the labrum in patients with dysplasia experiences loads 2.8–4 times greater than normal hips. The study strongly supported the conclusion that the labrum should be preserved during surgery in patients with acetabular dysplasia.
Tissue Injuries Alter Joint Kinematics
Moore and Burris21 studied the spatial and location-dependent tribological and material properties of the bovine stifle cartilage. Their results showed significant differences in cartilage properties by region, which supports the notion that injuries (such as ACL or meniscus tears) that alter joint loading patterns can lead to OA progression by loading of underprepared cartilage. With an eye towards the effects of injured menisci on contact stress patterns in the joint, Wang et al.22 measured regional contact stresses experienced by articular cartilage and menisci in 12 human cadaveric knees subjected to multi-directional loads mimicking gait. To accurately simulate gate, the investigators developed a normalized cross-correlation algorithm applied to contact stresses on the tibial plateaus. Their results demonstrated that the medial and lateral menisci carry loads at different points in the gait cycle: the posterior aspect of the medial meniscus distributed load only during the early phase of stance, while the posterior aspect of the lateral meniscus distributed load during both the early and late phases of stance. In a complementary study of the function of the medial meniscus in force transmission and stability, Walker et al.23 studied 10 normal knees subjected to compression alone, compression and anterior shear, or compression and posterior shear. Depending on the type of load, they found that, on average, 58% of the load was transmitted through the meniscus and the remaining 42% through the uncovered cartilage. These insights concerning contact mechanics of uninjured knees prove very helpful in identifying abnormalities that can exist in injured knees, with the hope of better understanding the pathomechanics of post-traumatic osteoarthritis (PTOA).
Anterior cruciate ligament (ACL) tears are often accompanied by meniscus tears, though many studies have examined isolated meniscus and isolated ACL injuries separately. To test hypotheses relating such joint injuries to joint kinematics in vivo, investigators continue to use state of the art dual (biplanar) fluoroscopic imaging in conjunction with MRI. Hosseini et al.24 examined knee kinematics during stair climbing of 21 patients prior to undergoing ACL reconstruction. Five patients had isolated ACL injuries, eight patients had combined ACL and medial meniscus injuries, and eight patients had combined ACL and lateral meniscus injuries. The authors concluded that combined ACL and meniscus injuries altered the kinematics of the knee joint in a different way than that caused by ACL injury alone, and that future research focus on interventions for patients with combined ACL and meniscus injuries to protect the knee from abnormal kinematics that could lead to cartilage degeneration. Carter et al.25 investigated in vivo tibiofemoral contact strain patterns during quasi-static lunges and biomarkers in the synovial fluid of patients with isolated medial meniscus tears. Using biplanar fluoroscopy, they found that cartilage strains increased at maximum flexion angle in the injured knee as compared to the contralateral control. Furthermore, matrix metalloproteinase (MMP) activity was correlated with cartilage strain at maximum flexion angle. These findings suggested a possible mechanism by which injurious strain, when combined with upregulated MMP activity, could progress to PTOA.
Animal models of combined meniscus and ligament injuries have also been pursued. Fischenich et al.26 examined changes in meniscal mechanics and proteoglycan content after combined ACL and meniscus injury in a 12-week rabbit model. In this study, the combined injury resulted in significant decreases in meniscus elastic modulus and GAG coverage over time. The authors noted that there were no similar ACL transection-alone studies that measured the mechanical properties of the meniscus, but they hypothesized that the effects of the combined injury likely amplify the development of OA. Thus, future research was suggested on the effects of such combined joint tissue injuries.
Tissue Mechanics
This past year, particular attention focused on regional variations in the biomechanical properties of various musculoskeletal tissues. For example, it is well known that the equilibrium and dynamic compressive moduli of articular cartilage increases with depth from the superficial zone (SZ) towards the deep zone of the tissue, in direct correlation with the depth dependent composition and ultrastructure of the tissue's ECM. Given the importance of SZ in articulation, lubrication and the initiation of OA-like pathologies, particular attention has been paid to this region of cartilage27 regarding development and function. Gannon et al.28 hypothesized that immature cartilage lacked a functional SZ and, therefore, removal of the SZ would cause a decrease in the dynamic modulus only in mature cartilage. Using osteochondral cores from the medial and lateral ridges of the femoropatellar grooves of different-aged porcine knees, they found that cartilage SZ dramatically adapted with age, thereby playing a key role in determining the tissue's dynamic compressive stiffness.
Hosseini et al.29 used a previously validated fibril-reinforced cartilage swelling model accounting for the depth-dependence of collagen structure and composition to simulate data from channel-indentation tests that highlighted the role of SZ in cartilage load distribution. They found that the tangential fibers of the SZ served to transfer compressive loads from directly loaded regions to adjacent regions of the tissue. Thus, SZ tissue is able to recruit a larger area of deep zone cartilage to carry compressive loads. This finding is particularly relevant to explain, in part, why fibrillation of the SZ can accelerate cartilage degradation. Removal of SZ during arthroscopic debridement should therefore be approached with caution due to biomechanical consequences on surrounding cartilage. To further explore the role of SZ collagen fibrils on the compressive properties of cartilage, Grenier et al.30 used collagenase to degrade collagen (with associated removal of proteoglycans) at the surface of cartilage explants to yield an early-stage OA-like phenotype. Surface fibrillation and accompanying proteoglycan removal increased the apparent hydraulic permeability and decreased both the instantaneous and equilibrium confined compression moduli of cartilage SZ and middle zone. In a related sets of experiments, Griffin et al.31 examined the effects of collagenase and trypsin treatments on microscale changes in the shear modulus of cartilage plugs. Confocal reflectance micrographs revealed increasing collagen degradation caused by collagenase, but not by trypsin, but both treatments caused GAG loss, decreases in the dynamic (viscoelastic) shear modulus, and increased energy dissipation confined to the upper 400 μm of SZ tissue, as assessed by confocal strain mapping. These findings are consistent with previous reports32,33 that loss of aggrecan-GAGs causes a decrease in the shear modulus of cartilage, even when the collagen network is undisturbed. The authors noted the importance of measuring cartilage properties at a local microscale level, given the inhomogeneous nature of the tissue. In a complementary study, Mansfield et al.34 used multiphoton microscopy to visualize microscale strains in cartilage SZ, and second harmonic generation to visualize the collagen fibril network. They observed heterogeneous microscale deformations, with shearing between different regions of collagen under tensile strain, corrugations in the articular surface, and a nonuniform distribution of strain in the axial direction. Further exploring depth-dependent collagen orientation on cartilage shear behavior, Silverberg et al.35 used confocal elastography, polarized light microscopy and Fourier-transform infrared imaging to relate tissue shear properties to collagen fiber orientation and concentration. They reported a strong correlation between the magnitude of the complex shear modulus and collagen concentration, and proposed that the collagen network in cartilage is near a percolation threshold: very small changes in collagen density correlated with large changes in shear modulus.
Others investigators have worked to model OA-related processes computationally36–38. This is a challenging process, since loading events occur on the time scale of milliseconds-to-minutes while cartilage degradation takes place over years (hence, the need for multi-scale modeling). Woodhouse et al.38, for example, modeled mechanically induced degeneration of cartilage using a two-component poroelastic model with a flow restricting boundary condition which accounts for tissues, e.g., the meniscus, which can provide resistance to fluid flow at the superficial surface, unlike a traditional free-flow condition. They modeled short-term consolidation including depth-dependent properties associated with the depth-dependent increase in aggrecan concentration. In parallel with experimental studies, several poroelastic modeling approaches have focused on cyclic loading, incorporating aggrecan concentration-dependent hydraulic permeability39 and fibril-reinforced anisotropy37,40, with applications to normal and OA-like degeneration. Taken together, these modeling studies demonstrate the importance of understanding cartilage consolidation and intra-tissue pressure distributions that occur during cyclic joint loading, with relevance to walking, other joint movements, and the types of cyclic motions may exacerbate degradation of OA cartilage.
The biomechanics of meniscus has received great attention this past year: a recent special issue of the Journal of Biomechanics was devoted to the Biomechanics and Mechanobiology of the Meniscus. Of particular interest, Wheatley et al.41 examined the stress relaxation properties of menisci 12 weeks after traumatic knee injury in a rabbit PTOA model using macroscopic displacement-controlled indentation. They used a transversely isotropic, hyper-poro-viscoelastic constitutive formulation incorporated into a FE model to fit their data. Injury caused a decrease in tissue stiffness, an increase in the rate of relaxation, but no change in the total extent of relaxation. Interestingly, this study was the first to examine differences in relaxation rate between healthy and injured rabbit menisci, incorporating both poro- and viscoelastic properties of the meniscus.
Nanomechanical atomic force microscopy (AFM)-based indentation has also been applied to characterize properties of the meniscus. Kwok et al.42 used 5 μm diameter spherical AFM probe tips and estimated the Young's modulus of the outer, middle and inner regions of normal, aged and degenerated human menisci using force-displacement indentation curves. Their results showed distinct regional nanomechanical profiles underlying age-dependent tissue degeneration and OA. Li et al.43 reported the first use of AFM-based nanoindentation to characterize the properties of murine menisci. Using 10 μm diameter spherical AFM probe tips and 6- to 24-week old male C57BL/6 mice, they observed a linear (non-Hertzian) force-depth behavior attributed to the tensile resistance of the collagen fibril bundles of the meniscus surface. In addition, the effective stiffness was dependent upon the rate of indentation, likely due to poroviscoelastic behavior. The ability to perform such tests on exceedingly small murine samples may open the door to studies associated with murine developmental biology and OA models.
Cell Mechanics
During the past decade, experimental measurements of equilibrium and time-varying moduli of single cells have utilized several techniques including micropipette aspiration and AFM-based nanoindentation. Time-dependent stress relaxation data have been modeled in terms of flow independent viscoelasticity44 and, more recently, poroelastic models emphasizing the importance of intracellular fluid-solid frictional interactions45. This past year, the strain rate dependence of the stress relaxation behavior of primary chondrocytes46 and the frequency dependence of the dynamic oscillatory stiffness of individual bone marrow stromal cells47 have both been attributed to intracellular poroelastic and/or poroviscoelastic mechanisms. These recent results present interesting hypotheses relating deformations of fluid-filled intracellular cytoskeletal network to deformation of the fluid-filled ECM in tissues that can sustain high strain under dynamic joint loading conditions. The pericellular matrix surrounding chondrocytes provides a distinct biomechanical and biochemical link between the cell and the ECM. Wilusz et al.48 have nicely reviewed current progress on the measurement of PCM mechanical properties to further increase our understanding of PCM function.
Cellular structures such as primary cilia can serve as mechanotransducers of signals that lead to changes in the mechanical properties of the tissue's extracellular matrix. It was previously reported that transgenic mice whose chondrocytes lacked primary cilia experienced an upregulation of OA markers and developed OA-like cartilage49; however, an analysis of the cartilage's mechanical properties had not been performed. Irianto et al.50 reanalyzed the data from microindentation of tibial plateau cartilage of transgenic and wild type control mice49 (~180 μm diameter impermeable plane ended indenter) and found a decrease in both the instantaneous and equilibrium moduli of cartilage in transgenic animals, especially in deep zone tissue, suggesting the potential importance of primary cilia to development. At the same time, cartilage tissue lesions can change the microenvironment of cells and thereby induce catabolic signaling51. Moo et al.51 applied displacement controlled compression stress relaxation tests to bovine osteochondral cores and measured cell deformation using dual-photon microscopy. Combining FE modeling with experimental data, they found that chondrocytes near lesions (cut surfaces) deformed less axially than cells in intact ECM in equilibrium. However, cells near lesions experienced large tensile strains in the principal height direction which, taken together, were hypothesized to elicit catabolic responses near the lesion. In a related study, Guo et al.52 used a hyperelastic biphasic model to predict changes in cell microenvironment within cartilage disks subjected to unconfined compression. They found changes dependent on both depth and radial location: chondrocytes in the mid-radial location had increased volume during the early stage of loading.
Changes in cell volume have also been hypothesized to play a significant role in glycosaminoglycan (GAG) synthesis53,54. Gao et al.55 proposed a model to describe this volume-dependent GAG synthesis, and their study showed the possibility of identifying the effects of both osmotic and mechanical loading conditions for isolated cells as well as cells within a matrix. Chondrocytes are known to increase their water content in response to hypo-osmotic conditions56. Cell volume then decreases by extrusion of water and solutes, and this adaptive mechanism is called regulatory volume decrease (RVD). It has been reported that hypo-osmotic challenge results in significant decreases in chondrocyte stiffness57. Wang et al.58 presented novel findings that hypo-osmotic challenge causes changes in the cells' mechanical properties only when RVD is activated, and that RVD is only activated by sudden hypo-osmotic challenge. A gradual decrease in osmolality, as is the case with the development of OA (i.e., loss of GAG), is unlikely to activate these mechanisms and subsequent mechanical changes. Thus, these findings have direct implications to interpreting chondrocyte response to hypo-osmotic conditions as they may relate to OA.
Mechanotransduction
Calcium signaling has been a focus of several chondrocyte mechanotransduction studies this past year. Lee et al.59 reported a number of exciting findings related to the Piezo1 and Piezo2 cation-permeable mechanically activated ion channels which had been identified previously in several cell types60,61. First, they found robust expression of both Piezo1/2 in primary porcine chondrocytes. In addition, they observed that co-expression of Piezo1/2 could sustain mechanically induced Ca2+ influx. Then, using tipless AFM cantilevers, high compressive strains applied to chondrocytes evoked Ca2+ transients that could be inhibited by GsMTx4, a piezo-blocking peptide from tarantula venom, and by Piezo1- or Piezo2-specific siRNA. Pre-incubating porcine cartilage explants with GsMTx4 also inhibited cell death around a biopsy wound induced by injurious cutting into the cartilage. Based on these results, Lee et al. hypothesized that this approach could attenuate piezo-mediated cartilage mechanotransduction caused by injurious strains, leading to a potential therapeutic target for PTOA59.
Madden et al.62 suggested that calcium signaling is different in intact cartilage compared to isolated chondrocytes. They dynamically loaded rabbit femoral condyle and patellar bone-cartilage samples that were incubated in calcium-sensitive dyes and imaged continuously under 10–40% compression in situ. Calcium signaling played a larger role in dynamic, as opposed to static, loading of intact cartilage, particularly above a threshold tissue strain of 10%. Hdud et al.63 examined the contribution of ERK1/2 and p38 MAPK signaling to the regulation of two osmolyte channels (transient receptor potential vanilloid 4 (TRPV4) and large conductance Ca2+ activated K+ channel (BKCa)) in response to osmotic pressure changes, motivated by the known changes in osmotic stress caused by mechanical loading. Their results showed that the TRPV4 channel contributes to early stages of hypo-osmotic stress and the BKCa channel responds to elevated intracellular calcium, as regulated in part by activation of ERK and p38. In a complementary study, Zhou et al.64 compared the effects of treadmill exercise loading on normal rats versus rats that were orally administered with monosodium iodoacetate (MIA) to induce OA-like cartilage changes. Using immunohistochemical analyses, they found that treadmill loading decreased protein expression of TRPV5 cation-selective channels in chondrocytes isolated from normal cartilage, channels that mediate Ca2+ transmembrane transport. A similar trend was found in chondrocytes from MIA-OA cartilage. We note, however, that this latter finding resulted from orally administered MIA rather than the commonly used, less toxic approach via intra-articular (i.a.) injection; repeating with i.a. injection would thus be informative.
While the majority of studies on mechanotransduction summarized here highlight the effects of compressive or osmotic stress, complex 3-D loading of cartilage in vivo results in significant tensile strains as well. Bell et al.65 devised new techniques based on nonlinear microscopy combined with tensile loading to detect the strain distribution in strips of adult equine metacarpophalangeal cartilage at the scale of individual cells. They found that anisotropies in the collagen network at the cellular scale gave rise to significant variations in the distribution of strain. A systematic review of the effects of cyclic strain on chondrocyte metabolism was recently published by Bleuel et al,66 accounting for the range of values of strain and frequency used in various studies. They reported that short term (2hr), low frequency (0.17 Hz), low strain amplitude (<3%) elicited little or no cellular response, while moderate (3–10%) strain amplitudes at frequencies up to 0.5Hz for 2-to-12hr typically resulted in anabolic responses. However, above a threshold strain of 10% at 0.5Hz, for 12hr of loading, catabolic events predominated. Interestingly, for compression, Li et al.67 previously found that moderate dynamic compression of cartilage explants inhibited the pro-catabolic response of cartilage to compressive mechanical injury in the presence of TNF-α and IL-6, but dynamic strains above a threshold (in this study, 30% strain amplitude) accentuated cartilage degradation in the same inflammatory environment. Thus, cyclic tensile and compressive deformations both appear to be palliative at low amplitude, but degradative above a strain amplitude threshold.
Above-threshold impact injury is also known to cause apoptotic cell death within intact cartilage. Jang et al.68 found that mechanically induced cell death required integrin activation and signaling. They concluded that inhibition of focal adhesions could ameliorate cell death by disrupting mechanisms associated with transmission of extracellular strains to the intracellular cytoskeleton. Waterset al.69 examined the effect of cartilage impact injury on the release of biomarkers, with particular attention to strain and strain rate. They found that cell viability, tissue thickness, and GAG and PGE2 release after impact were primarily peak strain dependent, while a prolonged release of PGE2 was dependent upon the strain rate.
Extracellular Matrix and Molecular Mechanics
While mouse models continue to reveal critically important biological disease mechanisms, correlative studies of murine tissue biomechanics have been hampered by the difficulty of measuring the properties of such small, thin tissues. However, progress was made this past year in adapting AFM-based nanoindentation methods to characterize the broad-spectrum dynamic as well as quasi-equilibrium biomechanical properties of murine knee tissues43,70,71. In one study, Batista et al.70 used nanoindentation to quantify the biomechanical properties of femoral condyle cartilage in mice with deletion of chondroadherin (CHAD), a small leucine rich protein in the ECM. Deletion of CHAD previously revealed no observable OA-like phenotype72. However, nanoindentation revealed a ~70–80% reduction in the effective indentation modulus of CHAD−/− superficial zone cartilage compared to wild type (WT) controls at ages 11 weeks, 4 months and 1 year. The presence of significant rate dependence of the indentation stiffness in both WT and CHAD−/− knee cartilage suggested the importance of both fluid flow induced poroelasticity and intrinsic viscoelasticity in murine cartilage biomechanical properties. While CHAD is not a load-bearing ECM macromolecule, these results were consistent with the long-standing hypothesis of Dick Heinegård and his group that CHAD (and other “minor” ECM components) can play major roles in the assembly and function of cartilage ECM. While an OA-phenotype had not yet been explicitly identified in these animals, these results suggest that AFM-based nanoindentation is a very sensitive tool that can show the earliest changes in cartilage mechanical properties that may be a precursor to progression of OA.
Nia et al.71 adapted a recently developed AFM-based nano-rheology system to probe the dynamic nanomechanical properties of murine cartilage over the wide frequency range of 1Hz to 10kHz relevant to running, jumping and high impact loading rates73. Using a fibril-reinforced poroelastic model, they found that fluid–solid interactions were more sensitive indicators of loss of mechanical function, caused by chondroitinase-ABC-induced GAG depletion, compared to the quasi-equilibrium Young's modulus more commonly assessed via force-displacement AFM-based nanoindentation tests. The fluid-flow-dependent hydraulic permeability increased by ~16-fold and the high frequency modulus was substantially reduced by GAG depletion, while the equilibrium Young's modulus was only slightly (but not significantly) decreased. This measurement methodology could prove useful for future studies utilizing genetically engineered and OA-related murine models.
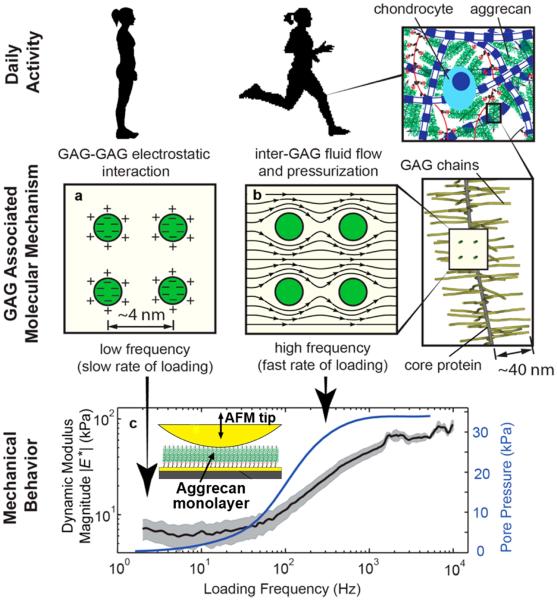
AFM-based technologies have now enabled studies of the molecular nanomechanics of ECM macromolecules. Nia et al.74 measured the magnitude and phase of the complex compressive modulus of an aggrecan-only monolayer (with G1-domains end-grafted onto a gold-coated chip) at the same aggrecan density as that in normal articular cartilage. Using an applied displacement amplitude of only ~2nm over the 1Hz to 10kHz frequency range, the results yielded the low-frequency-limit equilibrium stiffness, an increase in stiffness with increasing loading frequency (Fig. 2), and a peak in the phase angle that was used to compute the nanoscale hydraulic permeability of the aggrecan assembly via FE modeling. The results confirmed that aggrecan solid-fluid interactions are the dominant mechanism underlying cartilage's poroelastic behavior even at the nanometer displacement scale, and the hydraulic permeability of aggrecan layers at the molecular level closely matches the permeability of intact cartilage at the macroscale. Thus, while aggrecan GAG-GAG electrostatic interactions are known to be a critical determinant of cartilage's equilibrium modulus, the self-stiffening behavior of cartilage at higher loading rates derives mainly from the ability of densely packed GAGs to resist fluid flow at the nanoscale74, leading to the known pressurization of the tissue at the macroscale and now equally demonstrated at the nanoscale (Fig. 2).
Fig 2.
Adapted from Nia, HT, et al., ACS Nano, 9:2614–2625, 2015 (ref. #72). Molecular mechanisms associated with the loading rate dependence of aggrecan, manifested in the dynamic mechanical stiffening of a dense aggrecan layer with increasing loading rate. (a) Aggrecan molecular mechanics during quasi-static loading is dominated by electrostatic and steric interactions between GAG chains that resist compression; (b) During dynamic loading, fluid-solid interactions (associated with viscous drag within GAG-GAG spaces and concomitant fluid pressurization) dominate molecular mechanics; (c) Magnitude (± 95% confidence interval) of the dynamic complex modulus versus frequency for a dense aggrecan brush layer (~45 mg/mL, end-grafted onto a gold-coated silicon chip (insert) immersed in 0.1M NaCl) subjected to displacement amplitudes of only 2 nm over the 1 Hz to 10 kHz frequency range via an AFM-based high bandwidth rheology system. Solid blue line: fluid pressurization within the aggrecan layer due to solid-fluid viscous interactions (estimated using a fibril reinforced poroelastic model) results in self-stiffening, i.e., an increase in dynamic modulus with increasing loading frequency. The nanoscale hydraulic permeability computed for this dense aggrecan network is similar to the macroscale permeability of the intact parent cartilage tissue (from which the aggrecan was extracted) having the same aggrecan density.
In summary, this past year has brought forward dramatic advances in the relation between initiation/progression of OA and the mechanical behavior of joints and their constituent tissues, cells and ECM molecules. Discoveries regarding the effects of mechanical loading on cellular responses have provided new insights into the catabolic and anabolic pathways that link mechanics to tissue degradation, remodeling and repair. And importantly, new engineering technologies to assess and interpret measurements at scales from whole joint kinematics all the way down to molecular mechanics have greatly enhanced our understanding of the linkages between joint function and the molecular cell biology and matrix biochemistry of joint tissues in health and disease.
Acknowledgments
Role of funding source: NIH Grant AR060331. The funding source had no involvement in the design, collection, analysis and interpretation of data, nor in the writing and submission of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: All authors searched the literature, selected the articles, summarized the results, and wrote the manuscript.
Conflict of interest: The authors have no conflict of interest.
References
- 1.De S, Kuhl E, Hwang W, editors. Multiscale Modeling in Biomechanics and Mechanobiology. Springer; London: 2015. [Google Scholar]
- 2.Viceconti M, Humphrey JD, Erdemir A, Tawhai M. Multiscale modelling in biomechanics. Interface Focus. 2015;5:20140077–20140077. [Google Scholar]
- 3.Erdemir A, Bennetts C, Davis S, Reddy A, Sibole S. Multiscale cartilage biomechanics: technical challenges in realizing a high-throughput modelling and simulation workflow. Interface Focus. 2015;5:20140081. doi: 10.1098/rsfs.2014.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanska P, Mononen ME, Korhonen RK. A multi-scale finite element model for investigation of chondrocyte mechanics in normal and medial meniscectomy human knee joint during walking. J Biomech. 2015;48:1397–1406. doi: 10.1016/j.jbiomech.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 5.Gao J, Roan E, Williams JL. Regional Variations in Growth Plate Chondrocyte Deformation as Predicted By Three-Dimensional Multi-Scale Simulations. 2015. pp. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baliunas AJ, Hurwitz DE, Ryals AB, Karrar A, Case JP, Block JA, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 7.Astephen JL, Deluzio KJ. Changes in frontal plane dynamics and the loading response phase of the gait cycle are characteristic of severe knee osteoarthritis application of a multidimensional analysis technique. Clin Biomech (Bristol, Avon) 2005;20:209–217. doi: 10.1016/j.clinbiomech.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman KR, Hughes C, Morrey BF, Morrey M, An KN. Gait characteristics of patients with knee osteoarthritis. J Biomech. 2001;34:907–915. doi: 10.1016/s0021-9290(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 9.Hunt MA, Birmingham TB, Giffin JR, Jenkyn TR. Associations among knee adduction moment, frontal plane ground reaction force, and lever arm during walking in patients with knee osteoarthritis. J Biomech. 2006;39:2213–2220. doi: 10.1016/j.jbiomech.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Fantini Pagani CH, Potthast W, Brüggemann G-P. The effect of valgus bracing on the knee adduction moment during gait and running in male subjects with varus alignment. Clin Biomech (Bristol, Avon) 2010;25:70–76. doi: 10.1016/j.clinbiomech.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Erhart JC, Mündermann A, Elspas B, Giori NJ, Andriacchi TP. Changes in knee adduction moment, pain, and functionality with a variable-stiffness walking shoe after 6 months. J Orthop Res. 2010;28:873–879. doi: 10.1002/jor.21077. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler JW, Shull PB, Besier TF. Real-time knee adduction moment feedback for gait retraining through visual and tactile displays. J Biomech Eng. 2011;133:041007. doi: 10.1115/1.4003621. [DOI] [PubMed] [Google Scholar]
- 13.Walter JP, D'Lima DD, Colwell CW, Fregly BJ. Decreased knee adduction moment does not guarantee decreased medial contact force during gait. J Orthop Res. 2010;28:1348–1354. doi: 10.1002/jor.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manal K, Buchanan TS. An electromyogram-driven musculoskeletal model of the knee to predict in vivo joint contact forces during normal and novel gait patterns. J Biomech Eng. 2013;135:021014. doi: 10.1115/1.4023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manal K, Gardinier E, Snyder-Mackler L, Buchanan T. An alternate predictor of peak medial compartment loading: the product of the peak knee extensor and abductor moments. Am Soc Biomech. 2013:0–1. [Google Scholar]
- 16.Chehab EF, Favre J, Erhart-Hledik JC, Andriacchi TP. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:1833–1839. doi: 10.1016/j.joca.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adouni M, Shirazi-Adl a. Partitioning of knee joint internal forces in gait is dictated by the knee adduction angle and not by the knee adduction moment. J Biomech. 2014;47:1696–1703. doi: 10.1016/j.jbiomech.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Adouni M, Shirazi-Adl a. Evaluation of knee joint muscle forces and tissue stresses-strains during gait in severe OA versus normal subjects. J Orthop Res. 2014;32:69–78. doi: 10.1002/jor.22472. [DOI] [PubMed] [Google Scholar]
- 19.Ateshian GA, Henak CR, Weiss JA. Toward patient-specific articular contact mechanics. J Biomech. 2015;48:779–786. doi: 10.1016/j.jbiomech.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henak CR, Abraham CL, Anderson AE, Maas SA, Ellis BJ, Peters CL, et al. Patient-specific analysis of cartilage and labrum mechanics in human hips with acetabular dysplasia. Osteoarthritis Cartilage. 2014;22:210–217. doi: 10.1016/j.joca.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore AC, Burris DL. Tribological and material properties for cartilage of and throughout the bovine stifle: support for the altered joint kinematics hypothesis of osteoarthritis. Osteoarthritis Cartilage. 2015;23:161–169. doi: 10.1016/j.joca.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Chen T, Torzilli P, Warren R, Maher S. Dynamic contact stress patterns on the tibial plateaus during simulated gait: A novel application of normalized cross correlation. J Biomech. 2014;47:568–574. doi: 10.1016/j.jbiomech.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker PS, Arno S, Bell C, Salvadore G, Borukhov I, Oh C. Function of the medial meniscus in force transmission and stability. J Biomech. 2015;48:1383–1388. doi: 10.1016/j.jbiomech.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 24.Hosseini A, Li J-S, Gill TJ, Li G. Meniscus Injuries Alter the Kinematics of Knees With Anterior Cruciate Ligament Deficiency. Orthop J Sport Med. 2014;2 doi: 10.1177/2325967114547346. 2325967114547346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter TE, Taylor K a, Spritzer CE, Utturkar GM, Taylor DC, Moorman CT, et al. In vivo cartilage strain increases following medial meniscal tear and correlates with synovial fluid matrix metalloproteinase activity. J Biomech. 2015:1–8. doi: 10.1016/j.jbiomech.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischenich KM, Coatney G a, Haverkamp JH, Button KD, DeCamp C, Haut RC, et al. Evaluation of meniscal mechanics and proteoglycan content in a modified anterior cruciate ligament transection model. J Biomech Eng. 2014;136:1–8. doi: 10.1115/1.4027468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gannon AR, Nagel T, Kelly DJ. The role of the superficial region in determining the dynamic properties of articular cartilage. Osteoarthritis Cartilage. 2012;20:1417–1425. doi: 10.1016/j.joca.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Gannon AR, Nagel T, Bell AP, Avery NC, Kelly DJ. The changing role of the superficial region in determining the dynamic compressive properties of articular cartilage during postnatal development. Osteoarthritis Cartilage. 2015;23:975–984. doi: 10.1016/j.joca.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Hosseini SM, Wu Y, Ito K, Van Donkelaar CC. The importance of superficial collagen fibrils for the function of articular cartilage. Biomech Model Mechanobiol. 2014;13:41–51. doi: 10.1007/s10237-013-0485-0. [DOI] [PubMed] [Google Scholar]
- 30.Grenier S, Bhargava MM, Torzilli P a. An in vitro model for the pathological degradation of articular cartilage in osteoarthritis. J Biomech. 2014;47:645–652. doi: 10.1016/j.jbiomech.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin DJ, Vicari J, Buckley MR, Silverberg JL, Cohen I, Bonassar LJ. Effects of enzymatic treatments on the depth-dependent viscoelastic shear properties of articular cartilage. J Orthop Res. 2014;32:1652–1657. doi: 10.1002/jor.22713. [DOI] [PubMed] [Google Scholar]
- 32.Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res. 1993;11:771–781. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- 33.Jin M, Grodzinsky AJ. Effect of Electrostatic Interactions between Glycosaminoglycans on the Shear Stiffness of Cartilage: A Molecular Model and Experiments. Macromolecules. 2001;34:8330–8339. [Google Scholar]
- 34.Mansfield JC, Bell JS, Winlove CP. The Micromechanics of the Superficial Zone of Articular Cartilage. Osteoarthr Cartil. 2015:1–11. doi: 10.1016/j.joca.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Silverberg JL, Barrett AR, Das M, Petersen PB, Bonassar LJ, Cohen I. Structure-function relations and rigidity percolation in the shear properties of articular cartilage. Biophys J. 2014;107:1721–1730. doi: 10.1016/j.bpj.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattei L, Campioni E, Accardi MA, Dini D. Finite element analysis of the meniscectomised tibio-femoral joint: implementation of advanced articular cartilage models. Comput Methods Biomech Biomed Engin. 2014;17:1553–1571. doi: 10.1080/10255842.2012.758253. [DOI] [PubMed] [Google Scholar]
- 37.Mononen ME, Jurvelin JS, Korhonen RK. Implementation of a gait cycle loading into healthy and meniscectomised knee joint models with fibril-reinforced articular cartilage. Comput Methods Biomech Biomed Engin. 2015;18:141–152. doi: 10.1080/10255842.2013.783575. [DOI] [PubMed] [Google Scholar]
- 38.Woodhouse FG, Gardiner BS, Smith DW. Short-term consolidation of articular cartilage in the long-term context of osteoarthritis. J Theor Biol. 2015;368:102–112. doi: 10.1016/j.jtbi.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Miramini S, Smith DW, Gardiner BS, Grodzinsky AJ. Time evolution of deformation in a human cartilage under cyclic loading. Ann Biomed Eng. 2015;43:1166–1177. doi: 10.1007/s10439-014-1164-8. [DOI] [PubMed] [Google Scholar]
- 40.Speirs AD, Beaulé PE, Ferguson SJ, Frei H. Stress distribution and consolidation in cartilage constituents is influenced by cyclic loading and osteoarthritic degeneration. J Biomech. 2014;47:2348–2353. doi: 10.1016/j.jbiomech.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 41.Wheatley BB, Fischenich KM, Button KD, Haut RC, Haut Donahue TL. An optimized transversely isotropic, hyper-poro-viscoelastic finite element model of the meniscus to evaluate mechanical degradation following traumatic loading. J Biomech. 2015;48:1454–1460. doi: 10.1016/j.jbiomech.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwok J, Grogan S, Meckes B, Arce F, Lal R, D'Lima D. Atomic force microscopy reveals age-dependent changes in nanomechanical properties of the extracellular matrix of native human menisci: Implications for joint degeneration and osteoarthritis. Nanomedicine Nanotechnology, Biol Med. 2014;10:1777–1785. doi: 10.1016/j.nano.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Doyran B, Gamer LW, Lu XL, Qin L, Ortiz C, et al. Biomechanical Properties of murine meniscus surface via AFM-based nanoindentation. J Biomech. 2015;48:1364–1370. doi: 10.1016/j.jbiomech.2015.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darling EM, Zauscher S, Guilak F. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthritis Cartilage. 2006;14:571–579. doi: 10.1016/j.joca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Moeendarbary E, Valon L, Fritzsche M, Harris AR, Moulding DA, Thrasher AJ, et al. The cytoplasm of living cells behaves as a poroelastic material. Nat Mater. 2013;12:253–261. doi: 10.1038/nmat3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen TD, Oloyede A, Singh S, Gu Y. Microscale consolidation analysis of relaxation behavior of single living chondrocytes subjected to varying strain-rates. J Mech Behav Biomed Mater. 2015;49:343–354. doi: 10.1016/j.jmbbm.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Lee B, Han L, Frank EH, Grodzinsky AJ, Ortiz C. Dynamic nanomechanics of individual bone marrow stromal cells and cell-matrix composites during chondrogenic differentiation. J Biomech. 2015;48:171–175. doi: 10.1016/j.jbiomech.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilusz RE, Sanchez-adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang C-F, Ramaswamy G, Serra R. Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthritis Cartilage. 2012;20:152–161. doi: 10.1016/j.joca.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Irianto J, Ramaswamy G, Serra R, Knight MM. Depletion of chondrocyte primary cilia reduces the compressive modulus of articular cartilage. J Biomech. 2014;47:579–582. doi: 10.1016/j.jbiomech.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moo EK, Han SK, Federico S, Sibole SC, Jinha A, Abu Osman NA, et al. Extracellular matrix integrity affects the mechanical behaviour of in-situ chondrocytes under compression. J Biomech. 2014;47:1004–1013. doi: 10.1016/j.jbiomech.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Guo H, Maher S a, Torzilli P a. A biphasic multiscale study of the mechanical microenvironment of chondrocytes within articular cartilage under unconfined compression. J Biomech. 2014;47:2721–2729. doi: 10.1016/j.jbiomech.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- 54.Wong M, Wuethrich P, Buschmann MD, Eggli P, Hunziker E. Chondrocyte biosynthesis correlates with local tissue strain in statically compressed adult articular cartilage. J Orthop Res. 1997;15:189–196. doi: 10.1002/jor.1100150206. [DOI] [PubMed] [Google Scholar]
- 55.Gao X, Zhu Q, Gu W. Analyzing the effects of mechanical and osmotic loading on glycosaminoglycan synthesis rate in cartilaginous tissues. J Biomech. 2015;48:573–577. doi: 10.1016/j.jbiomech.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bush PG, Hall AC. Regulatory volume decrease (RVD) by isolated and in situ bovine articular chondrocytes. J Cell Physiol. 2001;187:304–314. doi: 10.1002/jcp.1077. [DOI] [PubMed] [Google Scholar]
- 57.Guilak F, Erickson GR, Ting-Beall HP. The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophys J. 2002;82:720–727. doi: 10.1016/S0006-3495(02)75434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z, Irianto J, Kazun S, Wang W, Knight MM. The rate of hypo-osmotic challenge influences regulatory volume decrease (RVD) and mechanical properties of articular chondrocytes. Osteoarthr Cartil. 2015;23:289–299. doi: 10.1016/j.joca.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Lee W, Leddy H a, Chen Y, Lee SH, Zelenski N a, McNulty AL, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci. 2014;111:E5114–E5122. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao R, Xu XZS. Mechanosensitive channels: in touch with Piezo. Curr Biol. 2010;20:R936–R938. doi: 10.1016/j.cub.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madden RMJ, Han SK, Herzog W. The effect of compressive loading magnitude on in situ chondrocyte calcium signaling. Biomech Model Mechanobiol. 2014:135–142. doi: 10.1007/s10237-014-0594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hdud IM, Mobasheri A, Loughna PT. Effect of osmotic stress on the expression of TRPV4 and BKCa channels and possible interaction with ERK1/2 and p38 in cultured equine chondrocytes. AJP Cell Physiol. 2014:1050–1057. doi: 10.1152/ajpcell.00287.2013. [DOI] [PubMed] [Google Scholar]
- 64.Zhou X, Wang W, Miao J, Bai L. Expression and significance of transient receptor potential cation channel V5 in articular cartilage cells under exercise loads. Biomed Reports. 2014:813–817. doi: 10.3892/br.2014.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bell JS, Christmas J, Mansfield JC, Everson RM, Winlove CP. Micromechanical response of articular cartilage to tensile load measured using nonlinear microscopy. Acta Biomater. 2014;10:2574–2581. doi: 10.1016/j.actbio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Bleuel J, Zaucke F, Brüggemann G-P, Niehoff A. Effects of Cyclic Tensile Strain on Chondrocyte Metabolism: A Systematic Review. PLoS One. 2015;10:e0119816. doi: 10.1371/journal.pone.0119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Frank EH, Wang Y, Chubinskaya S, Huang H-H, Grodzinsky AJ. Moderate dynamic compression inhibits pro-catabolic response of cartilage to mechanical injury, tumor necrosis factor-α and interleukin-6, but accentuates degradation above a strain threshold. Osteoarthritis Cartilage. 2013;21:1933–1941. doi: 10.1016/j.joca.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jang KW, Buckwalter J a, Martin J a. Inhibition of cell-matrix adhesions prevents cartilage chondrocyte death following impact injury. J Orthop Res. 2014;32:448–454. doi: 10.1002/jor.22523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waters NP, Stoker AM, Carson WL, Pfeiffer FM, Cook JL. Biomarkers affected by impact velocity and maximum strain of cartilage during injury. J Biomech. 2014;47:3185–3195. doi: 10.1016/j.jbiomech.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 70.Batista M a, Nia HT, Önnerfjord P, Cox K a, Ortiz C, Grodzinsky AJ, et al. Nanomechanical phenotype of chondroadherin-null murine articular cartilage. Matrix Biol. 2014;38:84–90. doi: 10.1016/j.matbio.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nia HT, Gauci SJ, Azadi M, Hung H-H, Frank E, Fosang AJ, et al. High-bandwidth AFM-based rheology is a sensitive indicator of early cartilage aggrecan degradation relevant to mouse models of osteoarthritis. J Biomech. 2015;48:162–165. doi: 10.1016/j.jbiomech.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hessle L, Stordalen G a, Wenglén C, Petzold C, Tanner EK, Brorson SH, et al. The Skeletal Phenotype of Chondroadherin Deficient Mice. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0063080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nia HT, Bozchalooi IS, Li Y, Han L, Hung H-H, Frank E, et al. High-bandwidth AFM-based rheology reveals that cartilage is most sensitive to high loading rates at early stages of impairment. Biophys J. 2013;104:1529–1537. doi: 10.1016/j.bpj.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nia HT, Han L, Bozchalooi IS, Roughley P, Youcef-Toumi K, Grodzinsky AJ, et al. Aggrecan nanoscale solid-fluid interactions are a primary determinant of cartilage dynamic mechanical properties. ACS Nano. 2015;9:2614–2625. doi: 10.1021/nn5062707. [DOI] [PMC free article] [PubMed] [Google Scholar]