Abstract
Senescence or normal physiologic aging portrays the expected age-related changes in the kidney as compared to a disease that occurs in some but not all individuals. The micro-anatomical structural changes of the kidney with older age include a decreased number of functional glomeruli from an increased prevalence of nephrosclerosis (arteriosclerosis, glomerulosclerosis, and tubular atrophy with interstitial fibrosis), and to some extent, compensatory hypertrophy of remaining nephrons. Among the macro-anatomical structural changes, older age associates with smaller cortical volume, larger medullary volume until middle age, and larger and more numerous renal cysts. Among carefully-screened healthy kidney donors, glomerular filtration rate declines at a rate of 6.3 ml/min/1.73m2 per decade. There is reason to be concerned that the elderly are being misdiagnosed with chronic kidney disease. Besides this expected kidney function decline, the lowest risk of mortality is at a glomerular filtration rate of ≥75 ml/min/1.73 m2 for age <55 years but at a lower glomerular filtration rate of 45-104 ml/min/1.73m2 for age ≥65 years. Changes with normal aging are still of clinical significance. The elderly have less renal functional reserve when they do actually develop chronic kidney disease and they are also at higher risk for acute kidney injury.
Keywords: Aging, Nephrosclerosis, Glomerulosclerosis, Kidney function, Glomerular filtration rate
Introduction
Aging is a natural, progressive and inevitable biological process characterized by a gradual decline of cellular function as well as progressive structural changes in many organ systems. These anatomic and physiological changes delineate the process of senescence, a term that portrays more predictable age-related alterations as opposed to those induced by diseases. In general, the rate of the physiologic decline is initially difficult to perceive, however, after certain age (late maturity) it undergoes acceleration. Like other organ systems, the kidneys also go through process of normal senescence, including both anatomical and physiological changes. These changes in a normal aging kidney are separate from kidney diseases that are relatively common in elderly such as diabetic nephropathy.1 It is difficult to distinguish the two distinct processes: inevitable organ-based senescence and disease-mediated structural and functional changes more common in the elderly. Nevertheless, it is important to emphasize that age-related diseases when superimposed on those of normal senescence, can significantly alter the rate of functional decline, exhaust renal functional reserve and predispose these patients to acute kidney injury.2
During the past 15 years there has been an increasing interest in the aging kidney. The likely reason is wide implementation of estimated glomerular filtration rate (eGFR) instead of serum creatinine for assessment of kidney function coupled to the adoption of an absolute (non-age-calibrated) threshold for defining CKD based on eGFR values alone (<60ml/min/1.73m2), which unsurprisingly led to a higher number of older adults diagnosed with chronic kidney disease (CKD). This diagnostic strategy also increased nephrology referrals, especially among individuals with mild to moderately reduced eGFR (30-59 mL/min/1.73m2).3 The mean eGFR among community living individuals over the age of 70 years is at or below the accepted threshold of <60 mL/min/1.73m2 used to define CKD.4 Older individuals represent a unique population in which the now “traditional” assumptions that an isolated and a persistent (over three months) eGFR<60 mL/min/1.73m2 defines CKD are not necessarily true. The psychological aspect of labeling older patients as having CKD is also of concern.4 Regardless of disease labeling, declining kidney function with normal aging is still of clinical relevance to medication dosing, selection of living kidney donors, and risk of CKD and acute kidney injury with loss of renal reserves.
Masked under the complex layers of estimating GFR and identifying CKD in the elderly, is the underlying structural pathology in the aged kidney. More and larger renal cysts,5,6 focal scars,7 increased cortical surface roughness,8 decreased cortical volume, increased medullary volume,9 and more renal artery atherosclerosis7 are evident on computed tomographic (CT) imaging of older kidneys. Likewise, global glomerulosclerosis, tubular atrophy, interstitial fibrosis, arteriosclerosis, arterial hyalinosis,10 tubular diverticuli,11 and to a lesser extent nephron hypertrophy (increased glomerular volume)12 become more evident on renal biopsies of older patient’s kidneys. How these structural changes relate to functional alterations of the kidney with aging is still being explored. In this review we will define the key structural and functional changes (with particular emphasis on GFR) that occur in the aging kidney and discuss their clinical significance.
Molecular Biology of Kidney Aging
Almost six decades ago, Dr. Harman was the first who proposed that free radical-induced accumulation of oxidative stress and damage at a cellular level was the primary cause of aging and a major determinant of lifespan.13 This simplified theory has been one of the most popular explanations of aging. More recently there has been an increased interest in mitochondrial theory of aging, including mitochondrial oxidative stress, mitochondrial damage and its subsequent influences on aging and health span.14
Regardless of an exact molecular mechanism of aging, decline at cellular level universally leads to gradual decay of many different tissues and organs and the kidneys are not spared. The universality of the process, present in all multi-cellular organisms makes it difficult to classify aging as a disease, per se. By affecting the basic structure and function of kidney cells, aging leads to GFR decline, changes in permeability of the capillary wall in glomeruli, increased susceptibility for podocyte injury, apoptosis, changes in tubular reabsorption and secretory capacities, changes in urinary concentration, and production of kidney-derived hormones and bioactive molecules.15-18 Podocytes, cells that have crucial function to maintain normal glomerular structure and capillary permeability, certainly undergo aging-related changes. It is hypothesized that progressive reduction in number of viable and normally functioning podocytes, along with decreased capacity for their regeneration and repair, ultimately lead to glomerular obsolescence and also subtle deterioration of the integrity of slit pore membrane in glomeruli, affecting both whole kidney GFR and albumin permeability at the single nephron level.17-19
Structural Changes of the Aging Kidney
It is established that aging is undoubtedly associated with structural changes in the kidney, including not only glomeruli, tubules and the interstitium, but also the vasculature. Four decades ago, in one of the earliest post-mortem studies, Darmady and others showed a decline in the number of non-sclerosed glomeruli (NSG), loss of tubules, vascular changes, and increased frequency of tubular diverticuli in apparently healthy aging individuals.20 Much later, studies of healthy living kidney transplant donors, with age spanning 6 decades, provided unique and ideal information on both structural and functional changes that occur with normal aging. Potential kidney donors undergo a battery of clinical evaluations, testing of kidney function, urinalyses and kidney computerized tomography (CT) angiograms to confirm health before donation. Finally, during the surgery, pre-implantational biopsy of the renal allograft can be performed, providing material for microscopic evaluation. Of note, similar evaluation of structural changes in kidneys with aging is possible in the “sudden death” autopsy cases (suicide or accidental death) of previously healthy individuals. However, the major drawback of these autopsy studies is lack of concurrent clinical information including kidney function tests.
The structural changes in aging kidney can be divided in the two broad categories, micro-anatomical based on renal biopsy findings and macro-anatomical based on imaging studies such as CT scans.
A) Micro-anatomical changes
The major aging-related changes observed on microscopic evaluation include nephrosclerosis and nephron hypertrophy.
Nephrosclerosis
The main features of nephrosclerosis that can be found on a kidney biopsy include glomerulosclerosis (focal and global, but not segmental), tubular atrophy, interstitial fibrosis and arteriosclerosis (fibrointimal thickening) (Supplemental Figure 1). Arteriosclerosis of small arteries in the kidneys is thought to cause an ischemic injury to nephrons, which over time progresses into global glomerulosclerosis and tubular atrophy. The main features of ischemic-related changes in the glomerulus are pericapsular fibrosis, wrinkling of capillary tufts, and progressively thicker basement membrane. In addition, Bowman’s space gradually fills with a matrix-like hyaline material, most likely due to disrupted balance between formation and breakdown of the extracellular matrix in the glomerulus.21 Finally, glomerular tufts collapse, leading to development of globally sclerotic glomeruli (GSG). These GSG may eventually be completely re-absorbed, or atrophy to a size that is too small to be clearly identified on standard renal biopsy sections.22,23 In addition to the GSG, the corresponding tubule atrophies with fibrosis accumulating in the surrounding interstitium.24,25
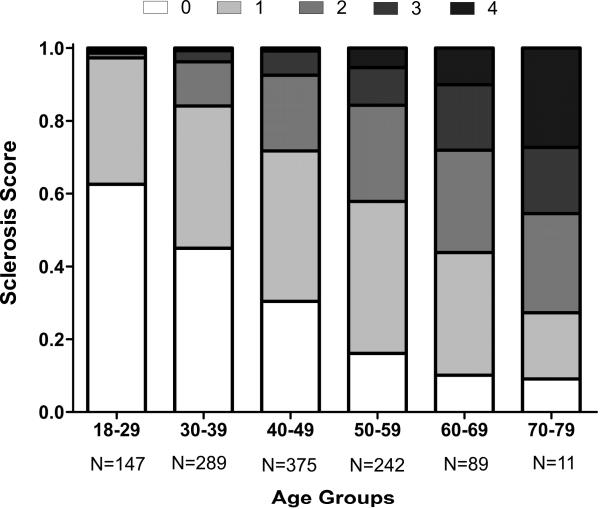
This increased prevalence of GSG with aging has been consistently replicated in several studies, including both autopsy-based studies and living kidney donors.10,22,24,26,27 In a sample of 1203 healthy living kidney donors there was a rising prevalence of GSG with increased age.24 For example, prevalence of GSG in the youngest group of kidney donors (18-29 years) was 19%, whereas it was 82% in the oldest age group (70-77 years). It can be assumed that with extreme elderly age, GSG would be universally found. Using a multi-center sample of 2052 living kidney donors, reference limits (95th percentiles) have now been defined for the expected number of GSG on a biopsy section as predicted by age and the total number of glomeruli present.28 In addition to GSG, tubular atrophy, interstitial fibrosis and arteriosclerosis also increase with older age and can be combined to define a sclerosis score (Figure 1). If nephrosclerosis is considered as the presence of two or more of the four main aforementioned abnormalities, prevalence among the youngest living donors was only 2.7% but increased to 73% among the oldest living donors.24 Interestingly, this increase in prevalence of nephrosclerosis with age appears to be independent of the age-related decline in whole kidney GFR.24
Fig 1. Sclerosis score by age group among 1203 living kidney donors.
Sclerosis score is defined as the total number of chronic histological abnormalities between any global glomerulosclerosis, any tubular atrophy, interstitial fibrosis >5% and any arteriosclerosis. In the figure, a score of 0 (absence of any abnormality) is white, a score of 4 (presence of all four pathological abnormalities) is black, and intermediate scores are on a gray scale.
Nephron Hypertrophy
Nephrons are generally larger with diabetes and obesity, due to both glomerular and tubular hypertrophy.29-31 It is reasonable to expect that with the aging-related increase in GSG that compensatory hypertrophy occurs in the remaining functional nephrons.32,33 Some studies have shown a decrease of average glomerular size with age20,23 whereas others show an increase with age.34-36 However, it is unknown whether authors from these studies excluded sclerosed glomeruli in calculating overall average glomerular size. A study assessing nephron hypertrophy in kidney donors found no difference in NSG volume with age, however the profile mean tubular area was higher with age and the glomerular density was lower with age.12 Glomerular density inversely correlates with average NSG volume and profile tubular area.12,32,37 As nephrons hypertrophy, they disperse glomeruli further apart and thereby decrease their profile (cross-sectional) density. Glomerular density is lower in biopsies where <10% of the total glomeruli are sclerotic suggesting the nephrons hypertrophy with age can be detected in regions without significant nephrosclerosis. Glomerular density is higher in regions that have >10% of sclerotic glomeruli, suggesting that in regions of significant nephrosclerosis, atrophy of nephrons with age brings glomeruli closer together.32
Nephron hypertrophy (higher NSG volume, larger profile tubular area, and lower glomerular density) appears to associate relatively weakly with older age alone but does associate more strongly with certain comorbidities that become more common with aging such as obesity and hyperuricemia.12 Importantly, older age (independent of comorbidities) is much more strongly associated with nephrosclerosis than with nephron hypertrophy.12
Number of functional nephrons declines in the aging kidney
Nephron endowment is thought to be an important determinant of renal health. There are approximately 700,000 to 1.8 million functional nephrons per kidney; however, this number progressively decreases due to aging with increased nephrosclerosis.36,38,39 The extent to which nephron endowment at birth is related to age-related structural or functional changes in the kidney is unclear. However, individuals with low birth weight likely have lower nephron endowment that accelerates their age-related decline in kidney function leading to earlier hypertension, CKD, and end-stage renal disease.40-42 Thus, nephron endowment at birth may contribute to risk of kidney disease when elderly.
Other Changes
Autopsy studies reveal a significant association between older age and the proportion of nephrons displaying tubular diverticuli.20 This is consistent with the finding that simple cysts (arising from these diverticuli) also show an increase in prevalence with aging.6
B) Macro-anatomical changes
The major aging-related changes found on CT scans include smaller cortical volume and presence of cysts and tumors, often benign.
Kidney Volume
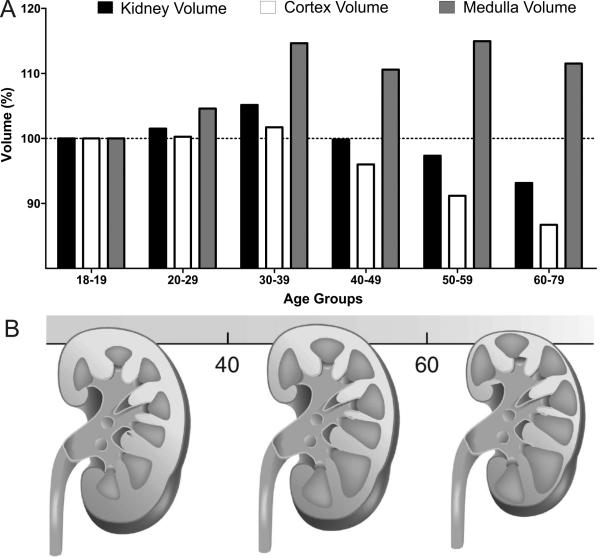
Ultrasound and especially computerized tomography are the tools that allow fairly accurate assessment of the kidney size with aging. Two decades ago Emamian and colleagues used ultrasound in a sample of more than 600 adult volunteers, and showed that larger kidney volumes correlated with younger age, height, weight and total body surface area.43 Another contemporary study using CT scan in 360 patients with no kidney disease, authors found that in both genders, parenchymal thickness of a kidney declined 10% per older decade of age.44 A decline in kidney dimensions with age was replicated in a more recent study with a large sample size of 1040 asymptomatic patients.45 Furthermore, in a cohort of more than 1000 patients, Bax and others demonstrated that concomitant atherosclerosis accelerated the age-related decline of kidney size.46 Conversely, a recent study that used 224 healthy potential transplant donors <65 years of age, showed that kidney parenchymal volume correlated directly with body size and male gender, but not with age.47 In a larger cohort of 1344 potential kidney donors that included healthy individuals aged 18-75 years, kidney volume was relatively stable through age 50 years, and then started to decrease after this age.9 This study also found that the reason for a relatively constant kidney volume with aging through age 50 years was the cancelling effects of a declining cortical volume while medullary volumes increased with age (Figure 2). This trend changed after the age of 50 years, when cortical volume continued to decline in both men and women, whereas medullary volume declined only in women and remained relatively stable in men. Finally, it is possible that age-related increase in renal sinus fat may also be the reason for obscured decline in total kidney volume with aging in studies that do not adequately distinguish the kidney parenchyma from non-parenchymal regions when assessing kidney dimensions.43,44
Fig 2. Effect of age on total kidney, cortical and medullar volumes.
(A) Among 1281 living kidney donors cortical volume declines, whereas medullary volume increases, making total kidney volume relatively stable until about 50 years of age. After which, medullary volume does not increase anymore and total kidney volume begins to decline. Results were normalized to the total kidney, cortical or medullar volumes in 18-19-year age group. (B) Schematic illustration of cortical and medullary volume changes with aging (Modified with permission from Kidney International).78
The volume-losing lesions of GSG with atrophy of corresponding tubules (nephrosclerosis) would explain this age-related decline in kidney cortical volume. Likewise compensatory hypertrophy of non-sclerosed glomeruli and their attached tubules may help maintain kidney parenchymal volume with aging, at least until beyond middle age.24,32,34,35 In particular, hypertrophy of tubules that make up the medullary volume and attach to juxtamedullary glomeruli may explain the initial rise in medullary volume to compensate for sclerosis and atrophy of superficial nephrons in the cortex.48 The more accelerated loss of total kidney volume after about age 50 years9,49,50 is consistent with the hypertrophy of remaining functional nephrons no longer adequately compensating for volume loss from nephrosclerosis.32
Kidney Cysts and Tumors
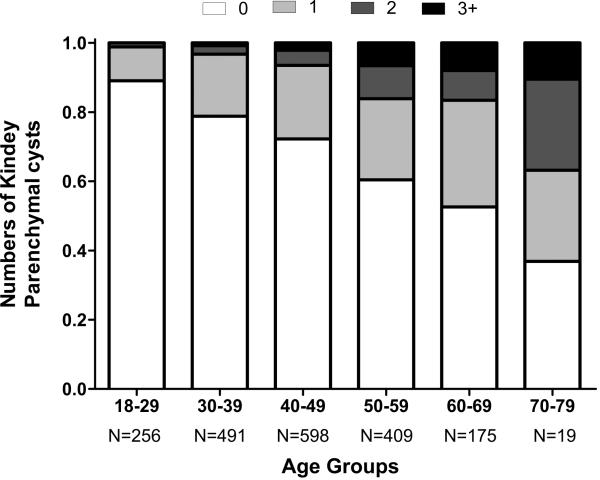
Kidney parenchymal cysts are common and usually designated as “benign” when not due to malignancy or autosomal dominant polycystic kidney disease (ADPKD). These cysts become more frequent, larger and abundant with older age (Figure 3).6,51 Even among healthy adults, there is an association of these cortical and medullary cysts with larger body size, male gender, higher blood pressure, and albuminuria.6 Similarly to cortical and medullary parenchymal cysts, parapelvic, hyperdense cysts and angiomyolipomas are also more common with older age.6 Unlike parenchymal cysts, parapelvic cysts do not associate with hypertension and albuminuria, consistent with their lymphatic origin52 rather than the tubular origin of parenchymal cysts.11
Fig 3. Number of cortical and medullary simple cysts ≥5mm by age among 1948 potential living kidney donors.
In the figure fraction with no cysts is shown in white, whereas black represents the fraction of 3 or more cysts. Intermediate fractions on a gray scale represent presence of 1 or 2 cysts.
Other Structural Changes
Other parenchymal changes that also become more frequent with normal aging include parenchymal calcifications, cortical focal scars, fibromuscular dysplasia (FMD) and renal artery atherosclerosis without stenosis.7 Of these changes, atherosclerosis of renal arteries is the most prominent, with a prevalence of 0.4% for age <30 years that increases to 25% for age 60-75 years.7
Functional Changes of the Aging Kidney
The primary measurement used to assess functional status of the kidney is total glomerular filtration rate (GFR). At present there is no way to directly assess the single nephron GFR in human subjects. Overall, single nephron GFR on average should be the whole kidney GFR divided by the number of functioning glomeruli. However, there can be considerable heterogeneity in single nephron GFR even between subjects with the same total GFR. It is likely that with aging, nephrosclerosis increases the heterogeneity of single nephron GFR. Understanding the extent total GFR detects underlying age-related parenchymal changes in the kidney is of critical importance since kidney CT angiograms and biopsies are not routinely performed in most patients.
Age-related Decline in GFR
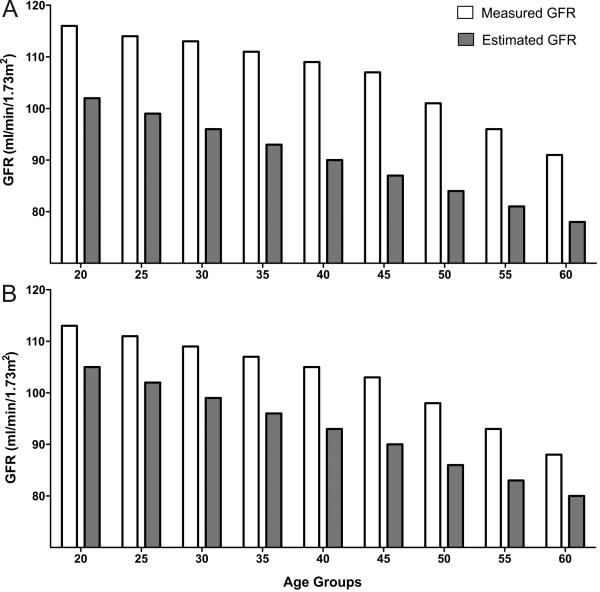
One of the pioneers of kidney physiology, Dr. Homer Smith introduced the concept of clearance, and described methods to measure GFR (inulin clearance), renal blood flow and secretion/reabsorption rates.53 In the same book, Dr. Smith described age-related decline of urea clearance. Contemporary work by Davies and Shock also confirmed the concept of age-related decline in GFR. Among apparently healthy adults there was a linear decline of GFR beyond the age of 30 years, such as that by age 90 years, GFR was reduced by an average of 46% from that found in youth.54 A subsequent longitudinal study several decades later showed that in 254 relatively healthy individuals (some of whom had diabetes) followed for up to 14 years there was average annual decline in urinary creatinine clearance (unadjusted for body surface area) of 0.75ml/min.55 About one third of these 254 individuals had an increase rather than a decrease in creatinine clearance with aging. But this finding might have been the consequence of the imprecision of calculation of the slopes of creatinine clearance over time as well as transient hyperfiltration that can be associated with comorbidities of aging, in particular, obesity, diabetes, and subclinical cardiovascular disease.56 The rate of decline in GFR of −7.5ml/min/decade is quite similar to −6.3ml/min/decade observed in a cohort of potential kidney donors (Figure 4).24 As with any physiological parameter, there is normal variation with GFR and age-specific reference ranges for GFR are available.57,58 It is worthwhile noting that the upper reference limit (not just the lower reference limit) for GFR decreases with normal aging, and the distribution of creatinine clearance slopes with aging is Gaussian, being consistent with a physiological rather than a pathological process.57,58
Fig 4.
GFR declines with normal aging whether measured or estimated (MDRD study equation) among 1057 potential kidney donors in both women (A) and men (B).24
A series of studies by Fliser et al. proposed that age-related decline in GFR is largely driven by a vascular (arterial) process, since the filtration fraction does not start to increase until 60 years of age, whereas the GFR begins to decline at 30-40 years of age.59-61 It was tempting to speculate that nephrosclerosis and GFR decline are linked. However, as noted earlier, increased prevalence of nephrosclerosis with aging does not clearly explain the GFR decline with aging.24 In fact, in multivariable models adjusting for age and sex, nephrosclerosis on renal biopsy associates with urinary albumin excretion, nocturnal blood pressure, and hypertension but not with GFR.24 There is evidence that cortical atrophy with aging is linked to the same process that causes GFR decline, though there still are factors that contribute to GFR decline independent of cortical volume decline.9 Lew et al. suggested that some of the age-related GFR decline could be secondary to diminished protein intake, since decreased protein intake is common among older individuals and leads to a lower GFR.62
“CKD” due to age-related GFR decline
According to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines of 2012,63 CKD is present if the patient has a GFR less than 60 ml/min/1.73m2 that persists for 3 months or longer, even in the absence of any confirmatory findings of kidney damage (e.g. abnormal albuminuria).A recent study performed among 610 individuals older than 70 years, demonstrated that half had a measured GFR less than 60 mL/min/1.73m2 and would therefore meet criteria for the diagnosis of CKD, by KDIGO criteria.4 In clinical practice, estimated GFR (eGFR) based on serum creatinine is more widely used. However, the equations used for eGFR were derived using all CKD patients (MDRD study equation) or mostly CKD patients (CKD-EPI equation) and both underestimate measured GFR in healthy adults, thus inflating the prevalence of CKD. This occurs because healthy individuals have a higher GFR at the same serum creatinine level as CKD patients due to higher muscle mass.64 For healthy patients with an eGFR of 45-59 ml/min/1.73 m2, the CKD-EPI equation underestimated GFR by 16% and the MDRD study equation underestimates GFR by 25%.65 Thus, both the normal age-related decline in GFR and the underestimation of GFR by equations in healthy older patients lead to an over-diagnosis of CKD in the elderly. It has been suggested by the KDIGO 2012 guidelines that eGFR based on serum cystatin C measurements can be used in elderly patients to confirm whether eGFR based on creatinine of 45-59ml/min/1.73m2 without albuminuria is indeed reflective of true CKD. This is not an adequate solution to the problem as the normal age-related decline in GFR is not addressed. Further, while eGFR based on cystatin C is less biased by muscle mass, there is also misclassification of CKD due to the non-GFR biology of cystatin C that reflects obesity, inflammation, and atherosclerosis.66
The central argument to using eGFR, despite its inaccuracy, is that eGFR is an independent predictor of adverse events (prognosis). All eGFR equations include age as a predictor variable. Thus, age itself is a confounding factor in interpreting eGFR in the elderly since age is a strong predictor of cardiovascular diseases and overall mortality. Direct assessment of muscle mass for calculating eGFR rather than indirectly inferring muscle mass from age for eGFR is not practical but highlights the problem with eGFR inflating CKD in the elderly.67 Further, among elderly over age of 70 years without proteinuria, those who have eGFR between 45-59 ml/min/1.73m2 may not have significantly increased risk of mortality in comparison to those with eGFR ≥60 ml/min/1.73m2.68 Another study found that an increase in CV mortality starts to occur in elderly over 65 years of age when eGFR is less than 45 ml/min/1.73m2.69
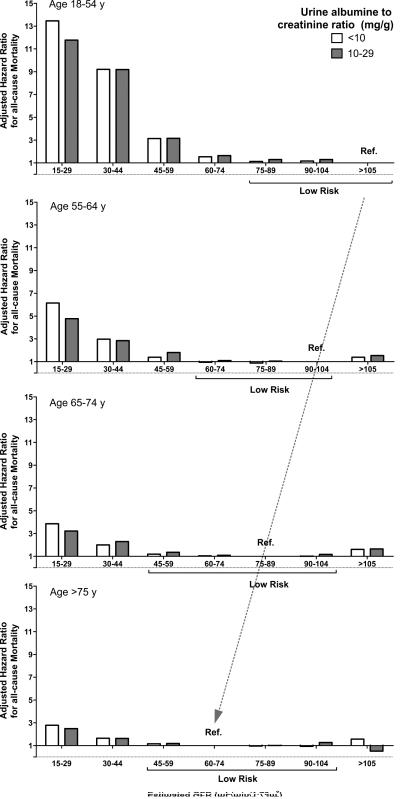
The largest studies on outcomes with eGFR across different age groups have been conducted by the CKD Prognosis Consortium that analyzed 46 cohorts with more than 2 million individuals.70 A conclusion drawn from these data was that eGFR less than 60 mL/min/1.73m2 is a threshold that should be used across the entire age spectrum to define CKD (i.e., no age-calibration of eGFR-thresholds is needed to define CKD).70 There are important objections to this conclusion. First, an emphasized justification for the <60 mL/min/1.73m2 threshold in the elderly has been the high absolute risk of mortality even though the relative risk is quite low. Comparing absolute risk across age groups is problematic because humans have a limited life expectancy. A 20-year dying in the next 10 years is very different than a 80-year old dying in the next 10 years. Relative risk rather than absolute risk better accounts for age differences in expected survival. Second, there is an increased risk of kidney failure in elderly patients with eGFR levels <60 mL/min/1.73m2, but this is still a relatively rare event in elderly patients with eGFR 45 to 59 ml/min/1.73m2 and better viewed as a normal age-related loss of renal reserves rather than an active kidney disease. Most elderly patients with these modest reductions in GFR will die before they ever develop end-stage renal disease.71 Third, very few elderly have a “normal” eGFR (>90 ml/min/1.73m2), thus some studies have used a mildly reduced eGFR of 75-89 ml/min/1.73m2 as the reference group.70 However the optimal reference group that defines low risk of mortality depends on age. The eGFR range associated with low mortality risk for age <55 years is ≥75 ml/min/1.73m2 but this decreased to 45-104 ml/min/1.73m2 for age ≥65 years (Figure 5). 70 This decline in the GFR range that corresponds to the lowest mortality risk with older age is consistent with a physiological rather than a pathological process.
Fig 5. Adjusted Hazard Ratios for all-cause mortality by categories of eGFR and age in patients with little or no albuminuria.
Associations of eGFR with all-cause mortality are presented across 4 different age groups (18-54, 55-64, 65-74 and >75 years) for <10 and 10-29 mg/g urine albumin to creatinine ratios. Reference group for individuals 18-54 years old is eGFR >105 ml/min/1.73m2, for 55-64-year-old is eGFR 90-104 ml/min/1.73m2, for 65-74-year-old is 75-90 ml/min/1.73m2, and for older than 75 years, 60-74 ml/min/1.73m2. Adjusted hazard ratios were based on pooled estimates from 33 population cohort studies. Note the shift of low risk eGFR groups to lower ranges of eGFR with older age (dashed arrow). Figure calculated from published tables.70
Clinical Significance of the Aging Kidney
Normal senescence of kidneys with resultant decreased function is of clinical relevance when managing elderly patients. Dose adjustment of water-soluble medications that are cleared through kidneys may be needed and caution with non-steroidal anti-inflammatory drugs and contrast agents is warranted. Age-related nephrosclerosis with loss of functional nephron reserve undoubtedly makes even elderly patients more susceptible to acute kidney injury and a more severe initial presentation of a truly progressive chronic kidney disease.72 Age-related kidney functional decline has very little effect on life expectancy, and alone, should not exclude motivated older individuals from kidney donation. In a recent study, Berger et al found that 219 healthy living donors older than 70 years actually had lower mortality rate as compared to healthy age-matched controls.73 In contrast, a Norwegian study reported that kidney donors had increased long-term risk for ESRD, cardiovascular and overall mortality compared to a control group.74 However, this study did not present whether the risks were different between younger and older donors.
Conclusions
Senescence or normal healthy aging of the kidney is characterized by progressively increasing nephrosclerosis with loss of functional glomeruli and decreased overall kidney function as determined by GFR. To some extent the decline in kidney cortical volume from nephrosclerosis is compensated by nephron hypertrophy of the remaining nephrons most notable in the medulla. As the patients ages beyond about 50 years this compensation is less adequate and total kidney volume begin to decline. Nephrosclerosis and cortical volume loss do not fully explain the age-related decline in GFR and there may be less metabolic demand for GFR in the elderly.75-77 Fixed GFR thresholds for diagnosing CKD are not reasonable because they fail to account for the decline in GFR with normal aging. Both studies of GFR in normal healthy populations and those of mortality outcome by level of GFR in the general population support the use of a lower GFR reference range to define CKD in the absence of other signs of kidney damage, probably in the vicinity of <45ml/min/1.73m2 among elderly adults.
Supplementary Material
Supplemental Fig 1. Representative kidney biopsy images with the main features of nephrosclerosis. A) Two globally sclerotic glomeruli (GSG) are labeled with black arrowheads. Non-sclerotic glomerulus (NSG) is labeled with a black star. GSG are surrounded by tubular atrophy. B) Thickened intima of a small to medium size artery (the area between red and yellow boundaries). C) Two foci of tubular atrophy and interstitial fibrosis are magnified.
Clinical Summary.
There is a rising prevalence of nephrosclerosis with aging, from 2.7% for healthy individuals younger than 29 years up to 73% for healthy individuals over age 70 years.
Total kidney volume remains stable through about age 50 years due to declining cortical volume and a compensatory medullary volume increase, but decreases with aging beyond 50 years.
Glomerular filtration rate declines with normal aging and mortality data supports the use of a lower range of glomerular filtration rate to define normal in the elderly compared to younger adults.
There are substantial reasons to be concerned that a fixed glomerular filtration rate threshold of < 60 ml/min/1.73m2 to define chronic kidney disease leads to over-diagnosis in the elderly and under-diagnosis in younger adults.
Acknowledgments
Financial Disclosure:
This study was supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358).
Contributor Information
Aleksandar Denic, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN.
Richard J. Glassock, Department of Medicine, Geffen School of Medicine at UCLA, Los Angeles, CA.
Andrew D. Rule, Division of Nephrology and Hypertension, Division of Epidemiology, Mayo Clinic, Rochester, MN
References
- 1.Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 2012 Aug;82(3):270–277. doi: 10.1038/ki.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Castro EM, Cordova HR. Aging and the kidney. Bol Asoc Med P R. 2011 Jul-Sep;103(3):57–62. [PubMed] [Google Scholar]
- 3.Hemmelgarn BR, Zhang J, Manns BJ, et al. Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. Jama. 2010 Mar 24;303(12):1151–1158. doi: 10.1001/jama.2010.303. [DOI] [PubMed] [Google Scholar]
- 4.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012 Oct 2;157(7):471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 5.Al-Said J, Brumback MA, Moghazi S, Baumgarten DA, O'Neill WC. Reduced renal function in patients with simple renal cysts. Kidney Int. 2004 Jun;65(6):2303–2308. doi: 10.1111/j.1523-1755.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 6.Rule AD, Sasiwimonphan K, Lieske JC, Keddis MT, Torres VE, Vrtiska TJ. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am J Kidney Dis. 2012 May;59(5):611–618. doi: 10.1053/j.ajkd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenz EC, Vrtiska TJ, Lieske JC, et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol. 2010 Mar;5(3):431–438. doi: 10.2215/CJN.07641009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan X, Rule AD, Elsherbiny H, et al. Automated Assessment of Renal Cortical Surface Roughness From Computerized Tomography Images and Its Association with Age. Acad Radiol. 2014 Jul 30; doi: 10.1016/j.acra.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Vrtiska TJ, Avula RT, et al. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014 Mar;85(3):677–685. doi: 10.1038/ki.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo M, Kiyohara Y, Kato I, et al. Risk factors for renal glomerular and vascular changes in an autopsy-based population survey: the Hisayama study. Kidney Int. 2003 Apr;63(4):1508–1515. doi: 10.1046/j.1523-1755.2003.00886.x. [DOI] [PubMed] [Google Scholar]
- 11.Baert L, Steg A. Is the diverticulum of the distal and collecting tubules a preliminary stage of the simple cyst in the adult? J Urol. 1977 Nov;118(5):707–710. doi: 10.1016/s0022-5347(17)58167-7. [DOI] [PubMed] [Google Scholar]
- 12.Elsherbiny HE, Alexander MP, Kremers WK, et al. Nephron Hypertrophy and Glomerulosclerosis and Their Association with Kidney Function and Risk Factors among Living Kidney Donors. Clinical Journal of the American Society of Nephrology. 2014;9 doi: 10.2215/CJN.02560314. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956 Jul;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 14.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiggins JE, Goyal M, Sanden SK, et al. Podocyte hypertrophy, "adaptation," and "decompensation" associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005 Oct;16(10):2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 16.Esposito C, Dal Canton A. Functional changes in the aging kidney. J Nephrol. 2010 Sep-Oct;(Suppl 15):S41–45. [PubMed] [Google Scholar]
- 17.Huber TB, Edelstein CL, Hartleben B, et al. Emerging role of autophagy in kidney function, diseases and aging. Autophagy. 2012 Jul 1;8(7):1009–1031. doi: 10.4161/auto.19821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiggins JE. Aging in the glomerulus. J Gerontol A Biol Sci Med Sci. 2012 Dec;67(12):1358–1364. doi: 10.1093/gerona/gls157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Hansen KM, Pippin JW, et al. De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol. 2012 Mar 1;302(5):F571–580. doi: 10.1152/ajprenal.00516.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darmady EM, Offer J, Woodhouse MA. The parameters of the ageing kidney. J Pathol. 1973 Mar;109(3):195–207. doi: 10.1002/path.1711090304. [DOI] [PubMed] [Google Scholar]
- 21.Martin JE, Sheaff MT. Renal ageing. J Pathol. 2007 Jan;211(2):198–205. doi: 10.1002/path.2111. [DOI] [PubMed] [Google Scholar]
- 22.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl. 2003 Feb;83:S31–37. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 23.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992 Feb;232(2):194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 24.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010 May 4;152(9):561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancilla E, Avila-Casado C, Uribe-Uribe N, et al. Time-zero renal biopsy in living kidney transplantation: a valuable opportunity to correlate predonation clinical data with histological abnormalities. Transplantation. 2008 Dec 27;86(12):1684–1688. doi: 10.1097/TP.0b013e3181906150. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan C, Pasternack B, Shah H, Gallo G. Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol. 1975 Aug;80(2):227–234. [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez Martul E, Veiga Barreiro A. Importance of kidney biopsy in graft selection. Transplant Proc. 2003 Aug;35(5):1658–1660. doi: 10.1016/s0041-1345(03)00573-6. [DOI] [PubMed] [Google Scholar]
- 28.Kremers WK, Denic A, Lieske JC, et al. Distinguishing age-related from disease-related glomerulosclerosis on kidney biopsy: the Aging Kidney Anatomy study. Nephrol Dial Transplant. 2015 Apr 16; doi: 10.1093/ndt/gfv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayslett JP, Kashgarian M, Epstein FH. Functional correlates of compensatory renal hypertrophy. J Clin Invest. 1968 Apr;47(4):774–799. doi: 10.1172/JCI105772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol. 2004 Jan;286(1):F8–15. doi: 10.1152/ajprenal.00208.2003. [DOI] [PubMed] [Google Scholar]
- 31.Luyckx VA, Shukha K, Brenner BM. Low nephron number and its clinical consequences. Rambam Maimonides Med J. 2011 Oct;2(4):e0061. doi: 10.5041/RMMJ.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rule AD, Semret MH, Amer H, et al. Association of kidney function and metabolic risk factors with density of glomeruli on renal biopsy samples from living donors. Mayo Clin Proc. 2011 Apr;86(4):282–290. doi: 10.4065/mcp.2010.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grantham JJ. Solitary renal cysts: worth a second look? Am J Kidney Dis. 2012 May;59(5):593–594. doi: 10.1053/j.ajkd.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Goyal VK. Changes with age in the human kidney. Exp Gerontol. 1982;17(5):321–331. doi: 10.1016/0531-5565(82)90032-8. [DOI] [PubMed] [Google Scholar]
- 35.Abdi R, Slakey D, Kittur D, Racusen LC. Heterogeneity of glomerular size in normal donor kidneys: impact of race. Am J Kidney Dis. 1998 Jul;32(1):43–46. doi: 10.1053/ajkd.1998.v32.pm9669422. [DOI] [PubMed] [Google Scholar]
- 36.Fulladosa X, Moreso F, Narvaez JA, Grinyo JM, Seron D. Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol. 2003 Oct;14(10):2662–2668. doi: 10.1097/01.asn.0000088025.33462.b0. [DOI] [PubMed] [Google Scholar]
- 37.Tsuboi N, Kawamura T, Koike K, et al. Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin J Am Soc Nephrol. 2010 Jan;5(1):39–44. doi: 10.2215/CJN.04680709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan JC, Workeneh B, Busque S, Blouch K, Derby G, Myers BD. Glomerular function, structure, and number in renal allografts from older deceased donors. J Am Soc Nephrol. 2009 Jan;20(1):181–188. doi: 10.1681/ASN.2008030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan JC, Busque S, Workeneh B, et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010 Oct;78(7):686–692. doi: 10.1038/ki.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008 Jan;19(1):151–157. doi: 10.1681/ASN.2007020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White SL, Perkovic V, Cass A, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009 Aug;54(2):248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 42.Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013 Jul 20;382(9888):273–283. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 43.Emamian SA, Nielsen MB, Pedersen JF, Ytte L. Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR Am J Roentgenol. 1993 Jan;160(1):83–86. doi: 10.2214/ajr.160.1.8416654. [DOI] [PubMed] [Google Scholar]
- 44.Gourtsoyiannis N, Prassopoulos P, Cavouras D, Pantelidis N. The thickness of the renal parenchyma decreases with age: a CT study of 360 patients. AJR Am J Roentgenol. 1990 Sep;155(3):541–544. doi: 10.2214/ajr.155.3.2117353. [DOI] [PubMed] [Google Scholar]
- 45.Glodny B, Unterholzner V, Taferner B, et al. Normal kidney size and its influencing factors - a 64-slice MDCT study of 1.040 asymptomatic patients. BMC Urol. 2009;9:19. doi: 10.1186/1471-2490-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bax L, van der Graaf Y, Rabelink AJ, Algra A, Beutler JJ, Mali WP. Influence of atherosclerosis on age-related changes in renal size and function. Eur J Clin Invest. 2003 Jan;33(1):34–40. doi: 10.1046/j.1365-2362.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 47.Johnson S, Rishi R, Andone A, et al. Determinants and functional significance of renal parenchymal volume in adults. Clin J Am Soc Nephrol. 2011 Jan;6(1):70–76. doi: 10.2215/CJN.00030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuel T, Hoy WE, Douglas-Denton R, Hughson MD, Bertram JF. Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol. 2005 Oct;16(10):3102–3109. doi: 10.1681/ASN.2005010123. [DOI] [PubMed] [Google Scholar]
- 49.Rao UV, Wagner HN., Jr Normal weights of human organs. Radiology. 1972 Feb;102(2):337–339. doi: 10.1148/102.2.337. [DOI] [PubMed] [Google Scholar]
- 50.McLachlan M, Wasserman P. Changes in sizes and distensibility of the aging kidney. Br J Radiol. 1981 Jun;54(642):488–491. doi: 10.1259/0007-1285-54-642-488. [DOI] [PubMed] [Google Scholar]
- 51.Eknoyan G. A clinical view of simple and complex renal cysts. J Am Soc Nephrol. 2009 Sep;20(9):1874–1876. doi: 10.1681/ASN.2008040441. [DOI] [PubMed] [Google Scholar]
- 52.Schwarz A, Lenz T, Klaen R, Offermann G, Fiedler U, Nussberger J. Hygroma renale: pararenal lymphatic cysts associated with renin-dependent hypertension (Page kidney). Case report on bilateral cysts and successful therapy by marsupialization. J Urol. 1993 Sep;150(3):953–957. doi: 10.1016/s0022-5347(17)35660-4. [DOI] [PubMed] [Google Scholar]
- 53.Smith HW. The Kidney: The Structure and Function in Health and Disease. Oxford University Press; New York: 1951. [Google Scholar]
- 54.Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950 May;29(5):496–507. doi: 10.1172/JCI102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985 Apr;33(4):278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 56.Eriksen BO, Lochen ML, Arntzen KA, et al. Subclinical cardiovascular disease is associated with a high glomerular filtration rate in the nondiabetic general population. Kidney Int. 2014 Jul;86(1):146–153. doi: 10.1038/ki.2013.470. [DOI] [PubMed] [Google Scholar]
- 57.Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M. Age-and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007 Sep;72(5):632–637. doi: 10.1038/sj.ki.5002374. [DOI] [PubMed] [Google Scholar]
- 58.Poggio ED, Rule AD, Tanchanco R, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009 May;75(10):1079–1087. doi: 10.1038/ki.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fliser D, Ritz E. Renal haemodynamics in the elderly. Nephrol Dial Transplant. 1996;11(Suppl 9):2–8. doi: 10.1093/ndt/11.supp9.2. [DOI] [PubMed] [Google Scholar]
- 60.Fliser D, Franek E, Joest M, Block S, Mutschler E, Ritz E. Renal function in the elderly: impact of hypertension and cardiac function. Kidney Int. 1997 Apr;51(4):1196–1204. doi: 10.1038/ki.1997.163. [DOI] [PubMed] [Google Scholar]
- 61.Fliser D, Ritz E. Relationship between hypertension and renal function and its therapeutic implications in the elderly. Gerontology. 1998;44(3):123–131. doi: 10.1159/000021995. [DOI] [PubMed] [Google Scholar]
- 62.Lew SW, Bosch JP. Effect of diet on creatinine clearance and excretion in young and elderly healthy subjects and in patients with renal disease. J Am Soc Nephrol. 1991 Oct;2(4):856–865. doi: 10.1681/ASN.V24856. [DOI] [PubMed] [Google Scholar]
- 63.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013 Jun 4;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 64.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004 Dec 21;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 65.Murata K, Baumann NA, Saenger AK, Larson TS, Rule AD, Lieske JC. Relative performance of the MDRD and CKD-EPI equations for estimating glomerular filtration rate among patients with varied clinical presentations. Clin J Am Soc Nephrol. 2011 Aug;6(8):1963–1972. doi: 10.2215/CJN.02300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rule AD, Glassock RJ. GFR estimating equations: getting closer to the truth? Clin J Am Soc Nephrol. 2013 Aug;8(8):1414–1420. doi: 10.2215/CJN.01240213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, Melton LJ., 3rd For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 2009 May;75(10):1071–1078. doi: 10.1038/ki.2008.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muntner P, Bowling CB, Gao L, et al. Age-specific association of reduced estimated glomerular filtration rate and albuminuria with all-cause mortality. Clin J Am Soc Nephrol. 2011 Sep;6(9):2200–2207. doi: 10.2215/CJN.02030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stengel B, Metzger M, Froissart M, et al. Epidemiology and prognostic significance of chronic kidney disease in the elderly--the Three-City prospective cohort study. Nephrol Dial Transplant. 2011 Oct;26(10):3286–3295. doi: 10.1093/ndt/gfr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. Jama. 2012 Dec 12;308(22):2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007 Oct;18(10):2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 72.James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010 Dec 18;376(9758):2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 73.Berger JC, Muzaale AD, James N, et al. Living kidney donors ages 70 and older: recipient and donor outcomes. Clin J Am Soc Nephrol. 2011 Dec;6(12):2887–2893. doi: 10.2215/CJN.04160511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014 Jul;86(1):162–167. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 75.Keys A, Taylor HL, Grande F. Basal metabolism and age of adult man. Metabolism. 1973 Apr;22(4):579–587. doi: 10.1016/0026-0495(73)90071-1. [DOI] [PubMed] [Google Scholar]
- 76.Lew SW, Bosch JP. Effect of diet on creatinine clearance and excretion in young and elderly healthy subjects and in patients with renal disease. J Am Soc Nephrol. 1991 Oct;2(4):856–865. doi: 10.1681/ASN.V24856. [DOI] [PubMed] [Google Scholar]
- 77.Daugirdas JT, Meyer K, Greene T, Butler RS, Poggio ED. Scaling of measured glomerular filtration rate in kidney donor candidates by anthropometric estimates of body surface area, body water, metabolic rate, or liver size. Clin J Am Soc Nephrol. 2009 Oct;4(10):1575–1583. doi: 10.2215/CJN.05581008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Neill WC. Structure, not just function. Kidney Int. 2014 Mar;85(3):503–505. doi: 10.1038/ki.2013.426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig 1. Representative kidney biopsy images with the main features of nephrosclerosis. A) Two globally sclerotic glomeruli (GSG) are labeled with black arrowheads. Non-sclerotic glomerulus (NSG) is labeled with a black star. GSG are surrounded by tubular atrophy. B) Thickened intima of a small to medium size artery (the area between red and yellow boundaries). C) Two foci of tubular atrophy and interstitial fibrosis are magnified.