Abstract
Tafazzin is a transacylase that affects cardiolipin fatty acid composition and mitochondrial function. Mutations in human tafazzin cause Barth syndrome yet the enzyme has mostly been characterized in yeast. To study tafazzin in higher organisms, we isolated mitochondria from Drosophila and mammalian cell cultures. Our data indicate that tafazzin binds to multiple protein complexes in these organisms, and that the interactions of tafazzin lack strong specificity. Very large tafazzin complexes could only be detected in the presence of cardiolipin, but smaller complexes remained intact even upon treatment with phospholipase A2. In mammalian cells, tafazzin had a half-life of only 3–6 h, which was much shorter than the half-life of other mitochondrial proteins. The data suggest that tafazzin is a transient resident of multiple protein complexes.
Keywords: Barth syndrome, Cardiolipin, Membrane assembly, Mitochondria, Protein complex, Tafazzin
1. Introduction
Tafazzin is a ubiquitous mitochondrial enzyme that catalyzes acyl transfer from a phospholipid to a lysophospholipid (Xu et al., 2006a). Tafazzin was first discovered as the genetic origin of Barth syndrome (Bione et al., 1996), a mitochondrial disease affecting multiple organ systems but in particular heart and skeletal muscles (Barth et al., 1983; Kelley et al., 1991; Spencer et al., 2006). When tafazzin is absent or dysfunctional, alterations occur in the molecular species pattern of cardiolipin (Vreken et al., 2000; Schlame et al., 2002) and monolyso-cardiolipin accumulates at the expense of cardiolipin (Valianpour et al., 2005). These effects were first observed in patients and subsequently reproduced in cell and animal models of the disease such as yeast (Gu et al., 2004), flies (Xu et al., 2006b), and mice (Acehan et al., 2011a; Phoon et al., 2012; Soustek et al, 2011). Tafazzin reacts indiscriminately with all phospholipid and lysophospholipid species but requires substrates to be presented in a non-bilayer state (Schlame et al., 2012). It is therefore the propensity of cardiolipin to form non-bilayer structures what makes it the primary substrate of tafazzin.
The import into mitochondria and the assembly of tafazzin have only been studied in yeast. Initially, it was reported that tafazzin is bound to the outer membrane (Brandner et al., 2005) but subsequent studies have established that tafazzin is localized both at the inner face of the outer membrane and at the outer face of the inner membrane (Claypool et al., 2006; Gebert, 2009). Tafazzin is anchored to the membrane by an internal peptide segment; this segment does not cross the membrane but protrudes into its hydrophobic interior (Claypool at al., 2006). Blue Native PAGE analysis has demonstrated that tafazzin is assembled into protein complexes of variable sizes (Claypool at al., 2006; 2008). Most of these complexes have an apparent mass of 400 kDa or less, but some tafazzin seems to be associated with larger supercomplexes that also contain the ADP-ATP-carrier and the ATP synthase (Claypool et al., 2008). Such supercomplexes only form if cardiolipin is present in the membrane. In light of the paucity of such data in organisms besides yeast, we characterized the assembly of tafazzin in mitochondria from Drosophila and mammalian cell cultures. Specifically, we examined the association of tafazzin with protein complexes and measured its half-life time.
2. Materials and Methods
2.1. Drosophila strains
Flies were grown in 7.5 cm culture vials at 22°C on standard cornmeal-sucrose yeast medium. All fly strains were characterized in previous reports. Specifically, we created a mutant of Drosophila melanogaster, in which the major form of tafazzin (isoform A) was deleted by imprecise excision of a transposable element inserted into the tafazzin gene CG8766 (ΔTAZ) (Xu et al., 2006b). In addition, we obtained from the Bloomington Drosophila Stock Center a strain, in which the cardiolipin synthase gene CG4774 was disrupted by insertion of a transposable element (ΔCLS) (Acehan et al., 2011b). Finally, we created a transgenic fly strain that expressed the tafazzin isoform B (TAZ-B) in the ΔTAZ background (Xu et al., 2009).
2.2. Cell cultures
Human embryonic kidney 293 cells (HEK 293), human lymphoblasts, and rat H9c2 cardiomyoblast cells (H9c2) were grown under standard culture conditions. The HEK 293 and H9c2 cells were cultured in 10% FBS-DMEM medium containing 100 IU/mL penicillin and 100 µg/mL streptomycin. The lymphoblast cell lines were previously established by Epstein-Barr virus transformation of leukocytes isolated from whole blood samples of normal subjects and patients with Barth syndrome (Xu et al., 2005). Lymphoblasts were cultured in 10% FBS-RPMI 1640-PS medium.
2.3. Manipulation of tafazzin expression
Expression of various forms of tafazzin was described in detail (Xu et al., 2009). Expression of full-length human taffazin in H9c2 rat cardiomyoblast cells was accomplished by stable transfection with pCDNA3-FL-tafazzin DNA using FuGene® 6 (Roche). For tafazzin knock-down, pre-designed BLOCK-iT™ miR RNAi hairpin sequences, specifically targeting individual isoforms of human tafazzin, were subcloned into the pcDNA™6.2-GW/EmGFP-miR vector (BLOCK-iT™ Pol II miR RNAi expression vectors, Invitrogen) followed by transfections into HEK 293 cells with FuGene® 6. The stably transfected HEK 293 cells were maintained with a selective antibiotic (10 µg/mL blasticidin). Knockdown efficiency was >80% as determined by RT-PCR and Western Blot. Expression of the A-isoform of Drosophila tafazzin in Sf9 insect cells, was described by Xu et al. (2006a).
2.4. Isolation of crude mitochondria and microsomes
Homogenates of whole flies or cells were prepared in ice-cold isolation buffer containing 0.28 M sucrose, 0.01 M Tris, and 0.25 mM EDTA (pH 7.3 at 4 °C) with Roche protease inhibitor cocktail, using a tight-fitting Teflon-glass homogenizer. The homogenates were filtered through gauze, spun at 1,000 g for 5 minutes to remove debris and nuclei, and then filtered again. This filtration/centrifugation/filtration step was repeated once. Mitochondria were finally collected by centrifugation at 8,000 g for 10 minutes. The pellets were washed and re-suspended in a small volume of isolation buffer. Microsomes were isolated from the post-mitochondrial supernatant by centrifugation at 100,000 g for 1 hour at 4 °C. The concentration of protein was determined by the method of Lowry et al. (1951).
2.5. 2D-Blue Native/SDS-PAGE
The standard procedures described by Wittig et al. (2006) were followed. Aliquots of isolated mitochondria, corresponding to 200–400 µg protein, were solubilized by digitonin (6 g/g protein) in a buffer containing 50 mM NaCl, 50 mM imidazole, 2 mM aminohexanoic acid, and 1 mM EDTA (pH 7.0 at 4 °C). Samples were spun at 100,000 × g for 15 min and solubilized proteins were collected from the supernatant. Coomassie blue G-250 was added and the proteins were separated by electrophoresis on a 4–13% gradient Blue Native gel. Gel strips were cut, soaked in 1% SDS for 15 min at 37 °C, and horizontally placed on top of a gel set for 10% SDS-PAGE (10% T, 3% C). After the second electrophoresis, proteins were transferred to a PVDF membrane for Western Blot analysis.
2.6. Western Blot analysis
Primary rabbit polyclonal antibodies to either Drosophila tafazzin or human tafazzin were solid-phase affinity-purified as described previously (Xu et al., 2006b). Primary antibodies to the α-subunit of complex V, the voltage-dependent anion channel (VDAC), the subunit NDUFS3 of complex I, and the flavoprotein of complex II were obtained from Mitosciences (Eugene, OR). PVDF membranes were incubated with primary monoclonal antibodies (1 µg/ml each in 5% NFM-TBS-T). After wash in TBS-T, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz) at a dilution of 1:3,300 in 5% NFM-TBS-T. SuperSignal (Pierce) was used as peroxidase substrate. For quantitative Western Blot, primary antibodies (1 µg/ml) were used in Odyssey blocking buffer containing 0.01% Tween-20. LiCor GAR-IRDye800cw for tafazzin and GAM-IRDye680 for mitochondrial markers were used as the secondary antibody at a dilution of 1:15,000. Tafazzin and mitochondrial markers on the blots were visualized and quantified by the LiCor scanner.
3. Results
3.1. Tafazzin is associated with multiple protein complexes of Drosophila mitochondria
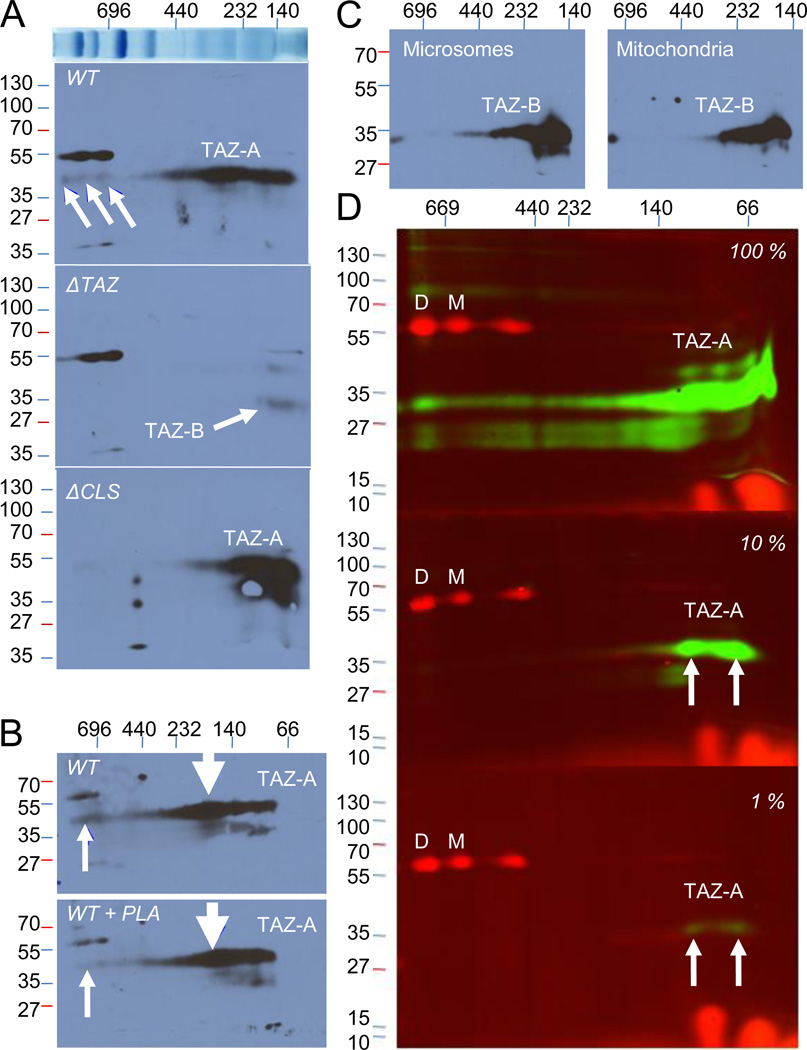
To study the association of tafazzin with protein complexes, we solubilized fly mitochondria with digitonin. Tafazzin was more soluble in the detergent than complex V (Fig. 1A) and solubilized tafazzin could be separated from complex V by density gradient centrifugation (Fig. 1B), which showed that the two proteins were not obligatory components of the same protein complex. However, a small portion of tafazzin was recovered at very high density, which is consistent with previous results in yeast suggesting an association of tafazzin with large supercomplexes containing either complex V or the ADP-ATP-carrier (Claypool et al., 2008). 2D-Blue Native/SDS-PAGE analysis of solubilized tafazzin showed that the major portion of the enzyme was associated with several poorly resolved protein complexes that had an apparent mass of 100–400 kDa while a small portion of the enzyme was bound to three discrete complexes with apparent masses >600 kDa (Fig. 2A). We concluded that Drosophila tafazzin is associated with multiple protein complexes.
Fig. 1. Solubilization and density gradient centrifugation of Drosophila tafazzin.
(A) Isolated mitochondria were treated with digitonin and the degree of solubilization was determined by quantitative Western Blot in pellets and supernatants after 100,000 g centrifugation. Tafazzin (TAZ) was more soluble in digitonin than complex V (Co V). Data are mean values with SEM of duplicate determinations. (B) Isolated mitochondria were treated with digitonin (2 g/g protein) and solubilized proteins were separated by continuous sucrose density gradient centrifugation (0.3 – 1.5 M). Gradients were prepared in 2.2 ml tubes by layering sucrose solutions on top of each other, incrementally increasing the sucrose concentration by 0.1 M per layer. Tubes were spun for 12 hours at 100,000 g. Gradient fractions were analyzed by SDS-PAGE followed by Coomassie Blue staining or Western Blot for TAZ and Co V. Solubilized TAZ had a lower density than solubilized Co V, except for a small portion of TAZ that was recovered in the high-density zone of the gradient. Molecular weight markers (kDa) are shown on the left side of the gel.
Fig. 2. Drosophila tafazzin is associated with multiple protein complexes.
Mitochondria were treated with digitonin (6 g/g protein) and solubilized proteins were separated by 2D-Blue Native/SDS-PAGE followed by Western Blot with the Drosophila tafazzin polyclonal antibody. The masses of marker proteins are given in kDa. (A) In wild-type (WT), the full-length isoform of tafazzin (TAZ-A) was associated with protein complexes with an apparent mass of 100–400 kDa. Small amounts of TAZ-A were recovered in larger protein complexes (arrows). Upon deletion of TAZ-A (ΔTAZ), the B isoform of tafazzin, TAZ-B, became more abundant. In a cardiolipin synthase deletion strain (ΔCLS), the size distribution of tafazzin changed towards smaller protein complexes. The top lane shows the distribution of mitochondrial protein complexes in 1-D Blue Native-PAGE. (B) Solubilized Drosophila mitochondria were treated for 30 min with 5 units phospholipase A2 (Naja naja) per 0.8 mg protein in the presence of 4 mM CaCl2 on ice (PLA). PLA treatment decreased the abundance of tafazzin in very large protein complexes (upward arrows) but not in intermediate size complexes (downward arrows). (C) TAZ-B was over-expressed TAZ-B in the ΔTAZ background. TAZ-B was associated with protein complexes in both mitochondria and microsomes. (D) Mitochondria were isolated from TAZ-A expressing and naïve Sf9 cells; the preparations were mixed, adjusting the proportion of TAZ-A expressing mitochondria to 1, 10, or 100 %, in order to vary the intensity of the tafazzin signal. Blots were analyzed with fluorescent secondary antibodies, yielding red fluorescence for complex V and green fluorescence for TAZ-A. Complex V formed monomers (M) and dimers (D), the abundance of which was similar in the three blots. TAZ-A was associated with protein complexes of various sizes but was most abundant in two distinct bands with apparent masses below 140 kDa (marked by arrows).
3.2. Cardiolipin is required for the assembly of very large tafazzin complexes in Drosophila
To determine the effect of cardiolipin on the assembly of tafazzin, we analyzed Drosophila mitochondria from a cardiolipin synthase deletion strain by 2D-Blue Native/SDS-PAGE. In the absence of cardiolipin, tafazzin was only present in protein complexes with an apparent mass of 100–300 kDa, suggesting that cardiolipin was required for the assembly of the larger tafazzin complexes (Fig. 2A). This conclusion was supported by another experiment, in which we treated digitonin-solubilized wild-type mitochondria with phospholipase A2. We found that phospholipase treatment dissociated the high-mass complexes (>600 kDa) but did not affect tafazzin complexes of lower mass (Fig. 2B). We concluded that cardiolipin and potentially other phospholipids are required for the stability of large but not of small tafazzin complexes. The latter are therefore formed by protein-protein interactions.
3.3. Isoform B of Drosophila tafazzin assembles similarly in mitochondria and microsomes
To investigate the assembly of tafazzin-B, a minor isoform of the enzyme expressed in Drosophila (Xu et al., 2006b; 2009), we deleted the major isoform, tafazzin-A. Tafazzin-B was only detectable in the low molecular weight range of the Blue Native gel (<200 kDa) but its low abundance made it difficult to exclude any association with larger complexes (Fig. 2A). We have previously shown that tafazzin-B localizes not only to mitochondria but also to the Golgi apparatus and the endoplasmic reticulum (Xu et al., 2009). To compare the assembly pattern of tafazzin-B in different intracellular membranes, we over-expressed the enzyme in the ΔTAZ deletion strain. The assembly pattern of tafazzin-B was similar in mitochondrial and microsomal membranes, suggesting that tafazzin can form complexes not only with mitochondrial but also with microsomal proteins (Fig. 2C). We concluded that tafazzin-B associates non-specifically with different proteins.
3.4. Expression of Drosophila tafazzin in insect cells alters its assembly pattern
To determine the propensity of tafazzin for self-oligomerization, we expressed it at a high level in Sf9 insect cells. Compared to wild-type Drosophila, the proportion of large tafazzin complexes was reduced in insect cells, i.e. most tafazzin was recovered in the <100 kDa region of the Blue Native gel (Fig. 2D). In this region, tafazzin formed two distinct bands (white arrows) that likely represented monomers and dimers. We concluded that tafazzin forms dimers when it is sufficiently concentrated in the membrane but that other proteins are necessary to form the type of complexes seen in normal mitochondria.
3.5. Tafazzin is associated with multiple protein complexes of H9c2 mitochondria
To study tafazzin in mammals, we raised and purified a polyclonal antibody that recognized endogenous tafazzin in Western Blots of human cell cultures (Fig. 3A). The antibody also recognized other proteins, but the identity of the tafazzin band could be established by its absence from lymphoblasts of patients with Barth syndrome (Fig. 3A). Tafazzin knockdown in HEK 293 cells, reduced the abundance of this band to 35.1±3.4 percent (mean±SEM, N=3) as established by densitometry of Western blots. Overexpression of several splice variants of human tafazzin in HEK 293 cells, demonstrated that the endogenous band corresponded to the isoform missing exon 5 (Fig. 3A), which is consistent with previous reports in the literature (Gonzalez 2005; Lu et al., 2004; Vaz et al., 2003). 2D-Blue Native/SDS-PAGE analysis of H9c2 cardiomyoblast mitochondria showed that tafazzin was associated with two distinct complexes that had an apparent mass of 100 and 300–400 kDa respectively (Fig. 3B). We concluded that in H9c2 cells, just like in Drosophila, tafazzin was a resident of multiple protein complexes.
Fig. 3. Tafazzin is associated with multiple protein complexes in H9c2 cells.
Western Blots were performed with a polyclonal antibody raised against human tafazzin. Masses of molecular weight markers are given in kDa. (A) Human lymphoblasts (HLB) were obtained from control individuals (C) and from patients with Barth syndrome (BS). The arrow indicates a band near 30 kDa that is absent from BS patients, suggesting it is tafazzin. This band was also visible in native HEK 293 cells (N). HEK cells were transformed to express various isoforms of human tafazzin, including the full-length isoform (FL), the isoform missing exon 5 (Δ5), the isoform missing exon 7 (Δ7), and the isoform missing exons 5 and 7 (Δ5Δ7). Migration of the endogenous band corresponds most closely to Δ5-tafazzin. (B) Mitochondria were isolated from H9c2 rat cardiomyoblasts, solubilized with digitonin (6 g/g protein), and separated by 2D-Blue Native/SDS-PAGE followed by Western Blot. The upper lane shows untransfected cells, the lower lane shows cells transfected with an expression vector for human full-length tafazzin. The arrows indicate the positions of endogenous tafazzin complexes.
3.6. Tafazzin has a short half-life in mammalian cells
To study the turnover of tafazzin in H9c2 cardiomyoblasts, we inhibited protein synthesis by cycloheximide and monitored the abundance of tafazzin and of other mitochondrial proteins. Tafazzin underwent rapid degradation, consistent with a half-life of about 3 hours, whereas complex I, complex V and VDAC, showed little degradation over a period of 24 hours (Fig. 4 A, B). Cycloheximide did not alter the distribution of tafazzin between the two protein complexes, suggesting that both were subject to rapid degradation (Fig. 4C). Since cycloheximide did not shift the tafazzin distribution from the smaller to the larger complex, the smaller complex was probably not an assembly intermediate of the larger one. When we treated human embryonic kidney cells with cycloheximide, we obtained similar results as in H9c2 cells (Fig. 4D). We concluded that the degradation of tafazzin was more rapid than the degradation of mitochondrial marker proteins, such as VDAC and the complexes of oxidative phosphorylation.
Fig. 4. Tafazzin has a short half-life.
(A) H9c2 rat cardiomyoblasts were grown in the presence of 20 µg/mL cycloheximide (CHX) for up to 24 h. Western Blots were performed to determine the abundance of tafazzin (TAZ), complex V (Co V), voltage-dependent anion channel (VDAC), and complex I (Co I). Tafazzin was degraded faster than the other proteins. (B) Signal intensities of the indicated H9c2 proteins were measured by densitometry and plotted versus the incubation time. (C) 2D-Blue Native/SDS-PAGE analysis of tafazzin was performed before and after CHX treatment of H9c2 cells for 6 hours. CHX diminished the abundance of tafazzin without changing its distribution across the blot. (D) HEK 293 cells were grown in the presence of 20 µg/mL CHX. Cells were harvested at the indicated times and Western Blots were performed to determine the abundance of tafazzin (TAZ), complex I (Co I), complex II (Co II), and complex V (Co V). Tafazzin was degraded faster than the other proteins. Data are mean values with SEM of triplicate determinations.
4. Discussion
In the present paper, we examined tafazzin complexes in Drosophila and mammalian cells. We found that tafazzin is associated with multiple protein complexes and that it has a very short half-life. The interactions of tafazzin with membrane proteins seem to lack strong specificity, which may be the reason why tafazzin is assembled in more than one mitochondrial protein complex.
The results confirm and expand previous observations made in yeast, in particular the association of tafazzin with heterogeneous protein complexes, and the requirement for cardiolipin to stabilize the largest of these complexes (Claypool et al., 2008). The latter is consistent with the general role of cardiolipin in promoting supercomplex formation (Mileykovskaya & Dowhan, 2014). Although some variations exist between yeast, flies, and mammals in terms of the assembly pattern of tafazzin, common to all is that tafazzin was recovered mostly in the 100–400 kDa zone of the Blue Native-PAGE gel while only a tiny portion migrated with supercomplexes. We also confirmed that the smaller tafazzin complexes are not assembly intermediates of the larger ones (Claypool et al., 2008).
Protein-protein interactions seemed to be involved in the formation of tafazzin complexes in the 100–400 kDa weight range because they remained stable in the absence of cardiolipin and did not disintegrate upon treatment with phospholipase A2. However, the tafazzin-protein interactions were relatively non-specific, prompting complex formation not only in mitochondria but also in microsomes. Therefore, we asked the question whether tafazzin could also interact with itself. Indeed, when we over-expressed tafazzin in insect cells, we found evidence for the presence of tafazzin dimers, but the overall distribution of the enzyme was shifted towards smaller complexes compared to wild-type Drosophila. These data suggest that the formation of tafazzin complexes in wild-type mitochondria was not the result of tafazzin oligomerization but required the involvement of other proteins.
We found that the half-life of tafazzin was only a few hours in H9c2 and HEK 293 cells, which was much shorter than the half-life of other mitochondrial proteins such as VDAC, ATP synthase, and the respiratory complexes. It is known that mitochondrial proteins exhibit different rates of degradation (Kim et al., 2012) and that protein degradation can function as a control mechanism (Price et al., 2010). The short half-life of tafazzin is interesting in light of a recent paper showing that tafazzin nearly vanished from yeast cells in which the MICOS complex was altered by Aim24 deletion combined with Mic22/Mic26 mutations (Harner et al., 2014). Thus, the state of the MICOS complex, a large protein assembly that resides near cristae junctions and bridges the outer and the inner membrane (Harner et al., 2011, Pfanner et al, 2014), has an impact on the steady-state concentration of tafazzin.
Tafazzin degraded rapidly, regardless of the protein complex it was associated with, which indicates it is highly susceptible to proteases. It was shown that the degradation of mutated tafazzins is catalyzed by iAAA (Claypool et al., 2011), but it remains unclear at this point which degradation system is involved in maintaining the steady-state level of wild-type tafazzin. Continuous clearance of tafazzin from the mitochondria ensures that its steady-state concentration can be altered rapidly.
In summary, our data suggest that tafazzin is incorporated into multiple protein complexes but resides there only for a relatively short period of time. During its lifetime, tafazzin exchanges acyl groups between membrane phospholipids, a reaction that is thought to support membrane dynamics or crista assembly by lowering the energy of curvature formation (Schlame et al., 2012). Recent evidence has implicated a protein complex formed by prohibitins and DNAJC19 in cardiolipin remodeling, which suggests that tafazzin may operate in the vicinity of this complex (Richter-Dennerlein et al., 2014). The short-lived nature of tafazzin led us to speculate that tafazzin participates in transient protein assemblies that are formed as intermediates in the biogenesis of mitochondrial membranes.
References
- Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, Khuchua Z. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem. 2011a;286:899–908. doi: 10.1074/jbc.M110.171439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acehan D, Malhotra A, Xu Y, Ren M, Stokes DL, Schlame M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J. 2011b;100:2184–2192. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth PG, Scholte HR, Berden JA, Van der Klei-Van Moorsel JM, Luyt-Houwen IE, Van 't Veer-Korthof ET, Van der Harten JJ, Sobotka-Plojhar MA. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leukocytes. J Neurol Sci. 1983;62:327–355. doi: 10.1016/0022-510x(83)90209-5. [DOI] [PubMed] [Google Scholar]
- Bione S, D'Adamo P, Maestrini E, Gedeon AK, Bolhuis PA, Toniolo D. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat Genet. 1996;12:385–389. doi: 10.1038/ng0496-385. [DOI] [PubMed] [Google Scholar]
- Brandner K, Mick DU, Frazier AE, Taylor RD, Meisinger C, Rehling P. Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: Implications for Barth syndrome. Mol Biol Cell. 2005;16:5202–5214. doi: 10.1091/mbc.E05-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool SM, McCaffery JM, Koehler CM. Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J Cell Biol. 2006;174:379–390. doi: 10.1083/jcb.200605043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool SM, Boontheung P, McCaffery JM, Loo JA, Koehler CM. The cardiolipin transacylase, tafazzin, associates with two distinct respiratory components providing insight into Barth syndrome. Mol Biol Cell. 2008;19:5143–5155. doi: 10.1091/mbc.E08-09-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool SM, Whited K, Srijumnong S, Han X, Koehler CM. Barth syndrome mutations that cause tafazzin complex lability. J Cell Biol. 2011;192:447–462. doi: 10.1083/jcb.201008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, Rehling P, Meisinger C, Ryan MT, Wiedemann N, Greenberg ML, Pfanner N. Mitochondrial cardiolipin involved in outer-membrane biogenesis: Implications for Barth syndrome. Curr Biol. 2009;19:2133–2139. doi: 10.1016/j.cub.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez IL. Barth syndrome: TAZ gene mutations, mRNAs, and evolution. Am J Med Gen. 2005;134A:409–414. doi: 10.1002/ajmg.a.30661. [DOI] [PubMed] [Google Scholar]
- Gu Z, Valianpour F, Chen S, Vaz FM, Hakkaart GA, Wanders RJ, Greenberg ML. Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth syndrome. Mol Microbiol. 2004;51:149–158. doi: 10.1046/j.1365-2958.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- Harner M, Korner C, Walther D, Mokranjac D, Kaesmacher J, Welsch U, Griffith J, Mann M, Reggiori F, Neupert W. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J. 2011;30:4356–4370. doi: 10.1038/emboj.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harner ME, Unger AK, Izawa T, Walther DM, Özbalci C, Geimer S, Reggiori F, Brügger B, Mann M, Westermann B, Neupert W. Aim24 and MICOS modulate respiratory function, tafazzin-related cardiolipin modification and mitochondrial architecture. eLife. 2014;3:e01684. doi: 10.7554/eLife.01684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RI, Cheatham JP, Clark BJ, Nigro MA, Powell BR, Sherwood GW, Sladky JT, Swisher WP. X-linked dilated cardiomyopathy with neutropenia, growth retardation, and 3-methyglutaconic aciduria. J Pediatr. 1991;119:738–747. doi: 10.1016/s0022-3476(05)80289-6. [DOI] [PubMed] [Google Scholar]
- Kim T-Y, Wang D, Kim AK, Lau E, Lin AJ, Liem DA, Zhang J, Zong NC, Lam MPY, Ping P. Metabolic labeling reveals proteome dynamics of mouse mitochondria. Mol Cell Proteomics. 2012;11:1586–1594. doi: 10.1074/mcp.M112.021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lu B, Kelher MR, Lee DP, Lewin TM, Coleman RA, Choy PC, Hatch GM. Complex expression pattern of the Barth syndrome gene product tafazzin in human cell lines and murine tissues. Biochem Cell Biol. 2004;82:569–576. doi: 10.1139/o04-055. [DOI] [PubMed] [Google Scholar]
- Mileykovskaya E, Dowhan W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem Phys Lipids. 2014;179:42–48. doi: 10.1016/j.chemphyslip.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N, van der Laan M, Amati P, Capaldi RA, Caudy AA, Chacinska A, Darshi M, Deckers M, Hoppins S, Icho T, Jakobs S, Ji J, Kozjak-Pavlovic V, Meisinger C, Odgren PR, Park SK, Rehling P, Reichert AS, Sheikh MS, Taylor SS, Tsuchida N, van der Bliek AM, van der Klei IJ, Weissman JS, Westermann B, Zha J, Neupert W, Nunnari J. Uniform nomenclature for the mitochondrial contact site and cristae organizing system. J Cell Biol. 2014;204:1083–1086. doi: 10.1083/jcb.201401006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoon CKL, Acehan D, Schlame M, Stokes DL, Edelman-Novemsky I, Yu D, Xu Y, Viswanathan N, Ren M. Tafazzin knockdown in a transgenic mouse model leads to a developmental cardiomyopathy with ventricular myocardial noncompaction. J Am Heart Assoc. 2012 doi: 10.1161/JAHA.111.000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JC, Guan s, Burlingame A, Prusiner SB, Ghaemmaghami A. Analysis of proteome dynamics in the mouse brain. Proc Natl Acd Sci USA. 2010;107:14508–14513. doi: 10.1073/pnas.1006551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Dennerlein R, Korwitz A, Haag M, Tatsuta T, Dargazanli S, Baker M, Decker T, Lamkemeyer T, Rugarli EI, Langer T. DNAJC19, a mitochondrial cochaperone associated with cardiomyopathy, forms a complex with prohibitins to regulate cardiolipin remodeling. Cell Metabolism. 2014;20:1–14. doi: 10.1016/j.cmet.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Schlame M, Towbin JA, Heerdt PM, Jehle R, DiMauro S, Blanck TJJ. Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann Neurol. 2002;51:634–637. doi: 10.1002/ana.10176. [DOI] [PubMed] [Google Scholar]
- Schlame M, Acehan D, Berno B, Xu Y, Valvo S, Ren M, Stokes DL, Epand RM. The physical state of lipid substrates provides transacylation specificity for tafazzin. Nat Chem Biol. 2012;8:862–869. doi: 10.1038/nchembio.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soustek MS, Falk D, Mah C, Toth M, Schlame M, Lewin A, Byrne B. Characterization of a transgenic short hairpin RNA-induced murine model of tafazzin deficiency. Human Gene Therapy. 2011;22:865–871. doi: 10.1089/hum.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CT, Bryant RM, Day J, Gonzalez I, Colan SD, Thompson WR, Berthy J, Redfearn SP, Byrne BJ. Cardiac and clinical phenotype in Barth syndrome. Pediatrics. 2006;118:e337–e346. doi: 10.1542/peds.2005-2667. [DOI] [PubMed] [Google Scholar]
- Valianpour F, Mitsakos V, Schlemmer D, Towbin JA, Taylor JM, Ekert PG, Thorburn DR, Munnich A, Wanders RJ, Barth PG, Vaz FM. Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J Lipid Res. 2005;46:1182–1195. doi: 10.1194/jlr.M500056-JLR200. [DOI] [PubMed] [Google Scholar]
- Vaz FM, Houtkooper RH, Valianpour F, Barth PG, Wanders RJA. Only one splice variant of the human TAZ gene encodes a functional protein with a role in cardiolipin metabolism. J Biol Chem. 2003;278:43089–43094. doi: 10.1074/jbc.M305956200. [DOI] [PubMed] [Google Scholar]
- Vreken P, Valianpour F, Nijtmans LG, Grivell LA, Plecko B, Wanders RJA, Barth PG. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem Biophys Res Comm. 2000;279:378–382. doi: 10.1006/bbrc.2000.3952. [DOI] [PubMed] [Google Scholar]
- Wittig I, Braun HP, Schägger H. Blue native PAGE. Nature Protocols. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sutachan JJ, Plesken H, Kelley RI, Schlame M. Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab. Invest. 2005;85:823–830. doi: 10.1038/labinvest.3700274. [DOI] [PubMed] [Google Scholar]
- Xu Y, Malhotra A, Ren M, Schlame M. The enzymatic function of tafazzin. J Biol Chem. 2006a;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Condell M, Plesken H, Edelman-Novemsky I, Ma J, Ren M, Schlame M. A Drosophila model of Barth syndrome. Proc Natl Acad Sci USA. 2006b;103:11584–11588. doi: 10.1073/pnas.0603242103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang S, Malhotra A, Edelman-Novemsky I, Ma J, Kruppa A, Cernicica C, Blais S, Neubert TA, Ren M, Schlame M. Characterization of tafazzin splice variants from humans and fruit flies. J Biol Chem. 2009;284:29230–29239. doi: 10.1074/jbc.M109.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]