Figure 1.
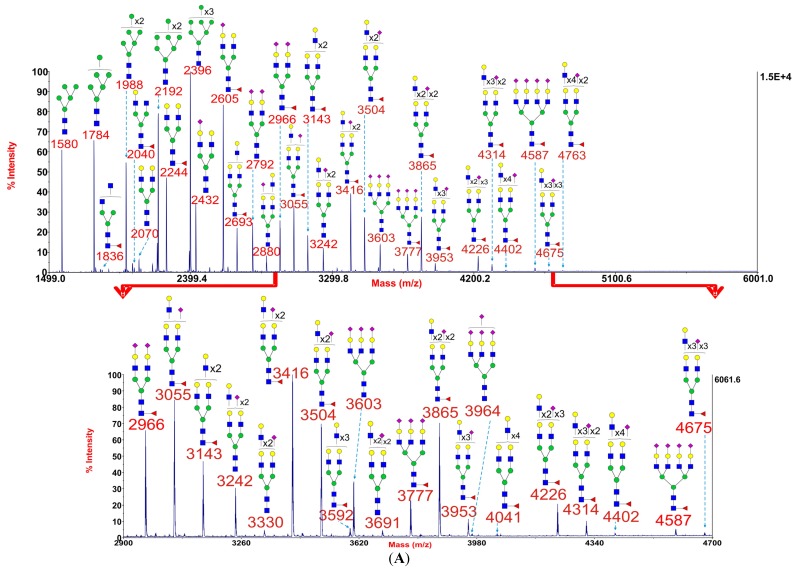
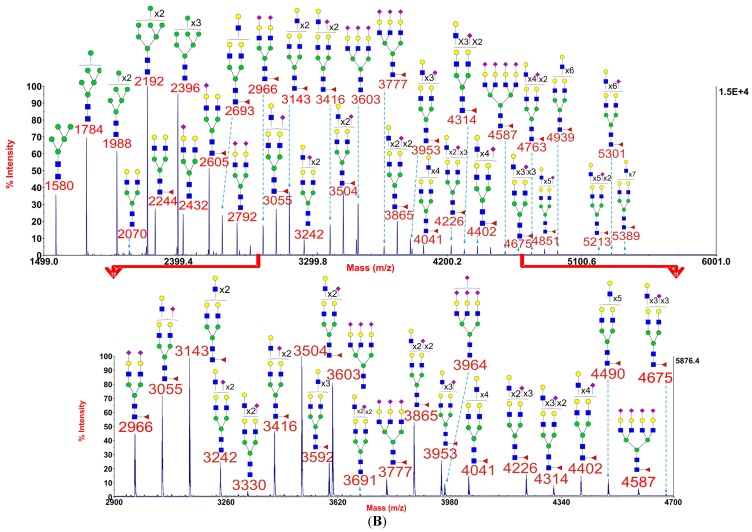
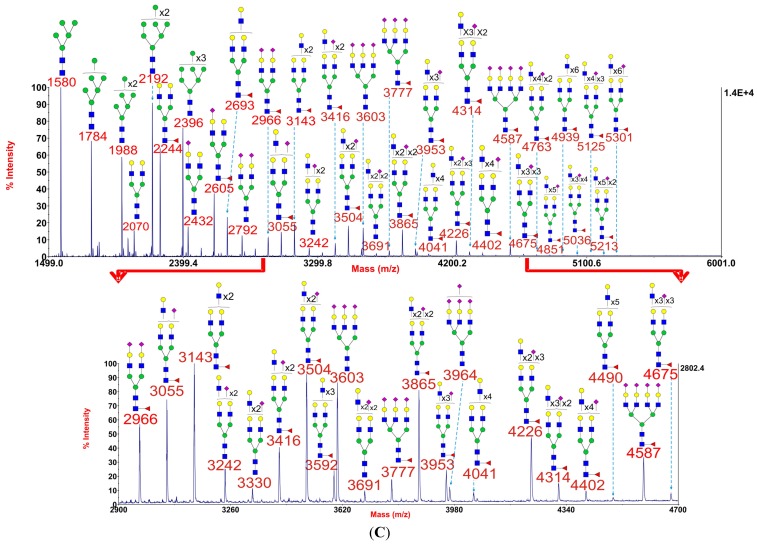
Annotated MALDI-TOF MS spectra of permethylated N-glycans of myoblasts from healthy control 1 (A), GFPT1 patient 1 (B), and the DOK7 patient (C). In each of A, B and C, the top panel shows the full spectrum of glycans and the bottom panel amplifies the mass range where the majority of tri- and tetra-antennary glycans are found, the starting point and ending point of which have been indicated by red arrows. Profiles were obtained from the 50% acetonitrile fraction from a C18 Sep-Pak column. All ions are [M + Na]+. The number indicated in the spectra is the mass to charge ratio (m/z) of the corresponding glycan ion. Since the ion is monocharged, the value of m/z is equal to the molecular weight value of the glycan. Annotations are based on the molecular weight, N-glycan biosynthetic pathway, and MS/MS data. Glycans at m/z 2966, 3777, and 4587 are clearly annotated, which is due to the fact that their structures are unequivocal because each antenna is capped with a sialic acid and thus they are homogeneous bi-, tri-, and tetraantennary glycans. However, the glycan structure is not always as unequivocal as the glycan at m/z 2966 as biosynthetically non-fully sialylated glycan molecular ion species could be made up of mixtures of structural isoforms. Therefore, for those heterogeneous multiantennary structures with extended LacNAc repeats, the annotations are simplified throughout by using biantennary structures with the extensions and NeuAcs listed outside a bracket.  GlcNAc,
GlcNAc,  Man,
Man,  Gal,
Gal,  Fuc,
Fuc,  NeuAc.
NeuAc.