Abstract
During the lifespan of cells, their genomic DNA is continuously exposed to the endogenous and exogenous DNA insults. Thus, the appropriate cellular response to DNA damage plays a pivotal role in maintaining genomic integrity and also acts as a molecular barrier towards DNA legion-mediated carcinogenesis. The tumor suppressor p53 participates in an integral part of proper regulation of DNA damage response (DDR). p53 is frequently mutated in a variety of human cancers. Since mutant p53 displays a dominant-negative behavior against wild-type p53, cancers expressing mutant p53 sometimes acquire drug-resistant phenotype, suggesting that mutant p53 prohibits the p53-dependent cell death pathway following DNA damage, and thereby contributing to the acquisition and/or maintenance of drug resistance of malignant cancers. Intriguingly, we have recently found that silencing of pro-oncogenic RUNX2 enhances drug sensitivity of aggressive cancer cells regardless of p53 status. Meanwhile, cancer stem cells (CSCs) have stem cell properties such as drug resistance. Therefore, the precise understanding of the biology of CSCs is quite important to overcome their drug resistance. In this review, we focus on molecular mechanisms behind DDR as well as the serious drug resistance of malignant cancers and discuss some attractive approaches to improving the outcomes of patients bearing drug-resistant cancers.
Keywords: AKT, anti-cancer drug, cancer stem cell, CD133, cell cycle arrest, cell death, DNA damage, drug resistance, p53 family, RUNX family
1. Introduction
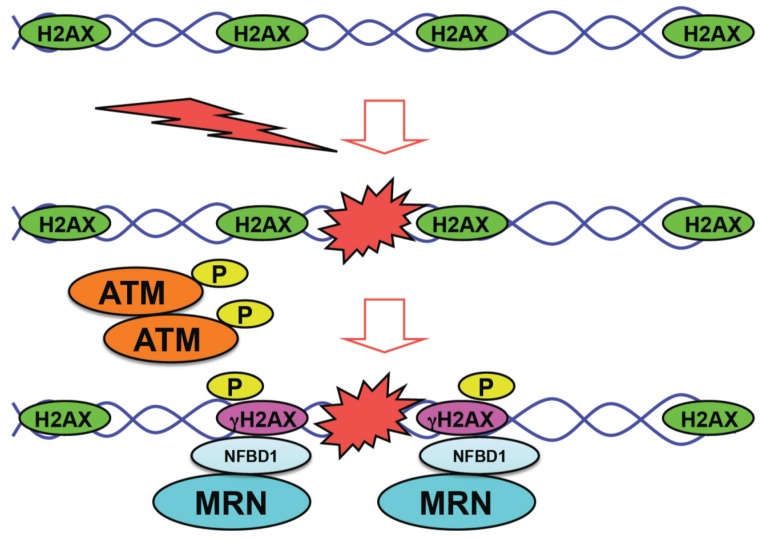
Cells are continuously exposed to endogenous as well as exogenous DNA damage-inducing stimuli including oxidative stress, ultraviolet (UV), ionizing radiation (IR), oncogene activation and/or anti-cancer drugs. The appropriate DNA damage response (DDR), which is a coordinated series of cellular events, contributes to the maintenance of genomic stability and acts as a molecular barrier against carcinogenesis. Thus, the defects in DDR result in genomic instability and then promote the development of cancer. Upon DNA damage, one of the initial cellular events is an auto-phosphorylation (activation) of ATM (ataxia telangiectasia mutated). A phosphorylated form of ATM at Ser-1981 (referred to as p-ATM) then phosphorylates histone variant H2AX at Ser-139 (referred to as γH2AX) to mark the sites of DNA damage (nuclear foci) [1]. The appearance of γH2AX has been widely used as a specific DNA double-strand break (DSB) marker [2]. After p-ATM-mediated phosphorylation of H2AX following DNA damage arising from radiation and/or anti-cancer drugs, MDC1 (mediator of DNA damage checkpoint protein 1)/NFBD1 (nuclear factor with BRCT domains protein 1) (henceforth NFBD1) binds to γH2AX and serves as an anchor protein for the subsequent recruitment of MRN (Mre11-Rad50-Nbs1) DNA damage sensor complex [3,4,5].
Meanwhile, MRN complex is a dynamic macromolecular machinery, which acts at the initial step of DNA double-strand break repair, and has an impact on homologous recombination repair as well as non-homologous end-joining. Among the components of MRN complex, Rad50 is the largest protein with ATPase activity [6]. Mre11 has a single-strand DNA endonuclease and 3'–5' double-strand exonuclease activities [7]. Its C-terminal domain is responsible for protein–protein and protein–DNA interactions. ATP-dependent conformational change in Rad50 ATPase domain regulates Mre11 nuclease activity [8]. Meanwhile, Nbs1 contains a fork head-associated (FHA) domain and two BRCA1. C-terminus (BRCT) domains, which are required for the complex formation with phosphorylated proteins such as ATM, NFBD1 and Mre11 [9]. It is worth noting that MRN complex further stimulates ATM activity, which results in a rapid spreading of γH2AX around the sites of DNA damage, and thereby amplifying DDR signal [10,11,12]. During the early phase of DDR, cell cycle arrest takes place to facilitate DNA repair. When cells are exposed to serious DNA damage, cells undergo cell death. If DNA lesions are not repaired or repaired incorrectly, the genomic instability is induced and causes carcinogenesis.
The representative tumor suppressor p53 is a nuclear sequence-specific transcription factor [13,14,15]. In response to DNA damage, p53 rapidly accumulates and is activated in cell nucleus through the sequential post-translational modifications such as phosphorylation (Ser-15, Ser-20 and Ser-46) and acetylation (Lys-373 and Lys-382). Activated form of p53 transactivates a number of its target genes implicated in the induction of cell cycle arrest (p21WAF1 and 14-3-3σ), DNA repair (GADD45), cellular senescence (p21WAF1) and cell death (BAX, NOXA and PUMA), and thereby exerting its growth-suppressive and pro-apoptotic functions to finally eliminate cells with seriously damaged DNA. Hence, the sequence-specific transactivation ability of p53 is tightly linked to its pro-arrest and pro-apoptotic functions [13,16]. Intriguingly, p53-deficient mice developed spontaneous tumors [17]. Moreover, extensive mutation searches demonstrated that p53 is frequently mutated in over 50% of various human cancers [18]. Among p53 mutations, 90% of its mutations are detectable within the genomic region encoding its sequence-specific DNA-binding domain. As expected, mutant forms of p53 with a longer half-life lack the sequence-specific transactivation ability and participate in the acquisition of the pro-oncogenic potential. Indeed, cancer cells expressing mutant p53, which has a dominant-negative effect on wild-type p53 and/or a pro-oncogenic property (gain-of-function), exhibit drug-resistant phenotype [19,20,21]. Together, p53 stands at the crossroad between cell survival and death in response to DNA damage.
RUNX2 is one of runt-related sequence-specific transcription factors (RUNX family), and has been considered to be a master regulator for osteoblast differentiation as well as bone formation [22,23]. In addition to its role in the regulation of osteogenesis, it has been shown that the expression level of RUNX2 is higher in a variety of human cancer tissues including pancreatic, breast, colon, prostate cancers and osteosarcoma as compared with that in their corresponding normal ones, indicating that RUNX2 has an oncogenic potential [24,25,26]. Consistent with this notion, RUNX2 has an ability to transactivate invasion- and/or metastasis-related genes such as Survivin, MMP-2, MMP-9 and VEGF [27,28,29,30,31,32,33,34]. Recently, we have found for the first time that depletion of RUNX2 in osteosarcoma-derived cells significantly enhances their adriamycin (ADR) sensitivity [35]. Based on our results, RUNX2 attenuated p53-dependent cell death pathway in response to ADR. Together, it is likely that RUNX2 is tightly linked to drug-resistant phenotype of malignant cancers.
The cancer stem cell (CSC) hypothesis has become increasingly accepted to provide a clue to understanding the precise molecular mechanism(s) behind cancer initiation, progression, metastasis and recurrence [36,37,38]. According to this model, a small sub-population of the heterogeneous cancer cells has a greater potential to initiate distant metastasis and acquire drug resistance. Recent extensive studies demonstrated that CSC-like cells are found in brain, breast, colon, lung, liver, pancreas, ovarian, head and neck, melanoma and prostate cancers [39]. Isolation of CSCs is dependent on their specific molecular markers. A growing body of evidence suggests that there exist several molecular markers for CSCs such as CD44, CD24, ESA, CD13 (aminopeptidase N), CD133 (also known as prominin I) and ALDH1 (aldehyde dehydrogenase 1) [40,41,42,43,44]. Among them, the earliest identified marker is CD133 [45]. Although the functional significance of CD133 in CSCs’ biology remains unclear, it has been described that CD133-positive glioblastoma cells are resistant to anti-cancer drugs such as temozolomide, carboplatin, VP16 and taxol [46]. Of note, CSC-enriched fractions prepared from prostate cancer tissues highly expressed RUNX2 as well as its target gene Survivin, implying that RUNX2 might be associated with the biology of CSC in prostate cancer [47,48].
In brief, the initial molecular event during DDR is the auto-phosphorylation of ATM, which phosphorylates histone H2AX at the sites of DNA damage. Subsequently, NFBD1 binds to γH2AX followed by the recruitment of macromolecular DNA repair machinery including MRN complex, and thereby damaged DNA is repaired. Then, cells with accurately repaired DNA re-enter the normal cell cycle (Figure 1).
Figure 1.
Initial molecular events in response to DNA damage. Upon DNA damage, activated form of ATM (p-ATM) phosphorylates histone H2AX (γH2AX) to mark the sites of DNA damage (nuclear foci). After nuclear foci formation, NFBD1 binds to γH2AX and then MRN complex is efficiently recruited onto the sites of DNA damage.
Here, we give an overview of the mechanistic basis underlying the development of serious drug-resistant properties of malignant cancers, and also discuss novel, promising strategies for their treatment.
2. The Representative Tumor Suppressor p53
The question is how cell fate determination (cell survival or death) could be regulated in response to DNA damage. One of the key proteins, which stands at the crossroad between cell survival and death, is the tumor suppressor p53.
p53 is a nuclear sequence-specific transcription factor containing an N-terminal transactivation domain (TA), central sequence-specific DNA-binding domain (DB) and C-terminal oligomerization domain (OD) [13,14,15]. As expected, p53 transactivates various target genes implicated in the induction of cell cycle arrest, DNA repair, cellular senescence and/or cell death. Since p53 is a dangerous protein with a pro-apoptotic potential, p53 is inactivated and also kept at an extremely low level under normal physiological conditions (quite short half-life, 20 min). Upon DNA damage, p53 is quickly stabilized and activated in cell nucleus through the sequential post-translational modifications such as phosphorylation and acetylation. Accumulating evidence strongly indicates that the expression of p53 is largely regulated at protein level but not at mRNA level [49,50,51].
E3 ubiquitin ligase MDM2 binds to N-terminal TA domain of p53 and efficiently ubiquitinates its C-terminal Lys residues. Poly-ubiquitinated p53 is then subjected to proteasome-dependent proteolytic degradation. In addition to MDM2, Pirh2, COP1 and ARF-BP1 have been shown to target p53 by ubiquitination for degradation through proteasome [52,53,54]. MDM2 simultaneously masks TA domain of p53 and inhibits its sequence-specific transactivation ability [55,56]. MDM2 is in turn transcriptionally induced by p53, and forms an auto-regulatory feedback loop, which regulates p53 expression level [57,58]. DNA damage-induced phosphorylation of p53 at Ser-15 mediated by p-ATM promotes the dissociation of MDM2 from p53, and thereby dramatically increases its half-life [59,60]. Besides p-ATM-mediated p53 phosphorylation at Ser-15, histone acetyltransferase p300/CBP, which also acts as a transcriptional co-activator, is associated with TA domain of p53 and then acetylates its C-terminal Lys residues to enhance its transactivation ability following DNA damage [61].
As mentioned above, p53 induces cell cycle arrest and/or cell death in response to DNA damage. It is well known that p53-dependent cell cycle arrest requires transactivation of p21WAF1 (G1/S) and/or 14-3-3σ (G2/M). Whereas, p53-dependent cell death is mediated by mitochondrial dysfunction through the up-regulation of BAX, PUMA, NOXA and/or p53AIP1. Unfortunately, the molecular basis, which could determine the initiation of either p53-mediated cell cycle arrest or cell death program, remains unclear. The earlier studies indicate that cell cycle-related gene promoters are activated by p53 under low levels of DNA damage, whereas p53-target cell death-related gene promoters require a higher level of DNA damage [62]. Oda et al. found that, upon repairable DNA damage, p53 is phosphorylated at Ser-15 as well as Ser-20, and then recruited onto the promoter regions of cell cycle arrest genes such as p21WAF1 [63]. When DNA damage is severe and repair is impossible, p53 is phosphorylated at Ser-46 and the resultant conformational change makes p53 to have a higher affinity to promoters of cell death-related genes such as p53AIP1. Therefore, a clear understanding of how p53 could select the pathways of cell cycle arrest or cell death following DNA damage provides a clue to developing a strategy to enhance drug sensitivity of malignant cancers.
One possibility is that p53-target promoters involved in cell cycle arrest have a higher affinity to p53, whereas the lower affinity target promoters are associated with cell death [63,64]. Another hypothesis is that the core promoter composition of p53-target genes plays a vital role in target gene selectivity [65]. Espinosa et al. have found that the amounts of RNA polymerase II complex bound to cell cycle-related gene promoters are larger than those of cell death-related ones [66]. Scala et al. reported that a highly stabilized p53 induces cell death, whereas a lower level of p53 triggers cell cycle arrest but not cell death in response to DNA damage, indicating that the amounts of activated p53 might determine cell fate [67]. As described above [63], phosphorylation of p53 at Ser-46 has an important role in the regulation of cell death. Consistent with these observations, it has been shown that HIPK2-mediated p53 phosphorylation at Ser-46 in response to UV exposure triggers transcriptional induction of pro-apoptotic p53-target genes [68]. In addition to phosphorylation, acetylation of p53 affects its target gene selection. For example, p53 acetylation at Lys-120 augments p53-dependent cell death [69,70,71], whereas its acetylation at Lys-320 promotes pro-arrest gene transcription such as p21WAF1 but not pro-apoptotic genes [70,71].
Collectively, cell fate determination in response to DNA damage (cell survival or death) might be at least in part dependent on p53-target gene selectivity.
3. Mutant Forms of p53 and Anti-Cancer Drug Resistance
As reported previously [13], the sequence-specific transactivation ability of p53 is tightly linked to its DNA damage-mediated biological processes such as the induction of cell cycle arrest and/or cell death. Extensive mutation searches demonstrated that p53 is frequently mutated in a variety of human cancers [72]. According to their observations, over 90% of p53 mutations are detectable within the genomic region encoding its sequence-specific DNA-binding domain. Among them, there are six hot-spot mutations including Arg-175, Gly-245, Arg-248, Arg-249, Arg-273 and Arg-282. Mutant forms of p53 lack the sequence-specific transactivation ability [73]. Subsequent studies revealed that p53 mutants display a dominant-negative behavior towards wild-type p53 through the formation of hetero-oligomers with wild-type p53 and contribute to the acquisition of pro-oncogenic potential [15]. Transgenic mice expressing mutant p53 exhibited accelerated tumor development [74], and certain cancerous cells bearing p53 mutations sometimes showed drug-resistant phenotype [19,20,21]. In addition, the expression level of mutant p53 in cancers has been shown to link to poor prognosis of the patients [75]. In a sharp contrast to a short-lived wild-type p53, mutant p53 has a prolonged half-life (2–12 h) due to the avoidance of MDM2-mediated proteasomal degradation [76], however, its precise molecular basis has not been fully understood. Hence, it is conceivable that the intracellular balance between the expression levels of wild-type p53 and mutant p53 might determine the cell fate in response to DNA damage.
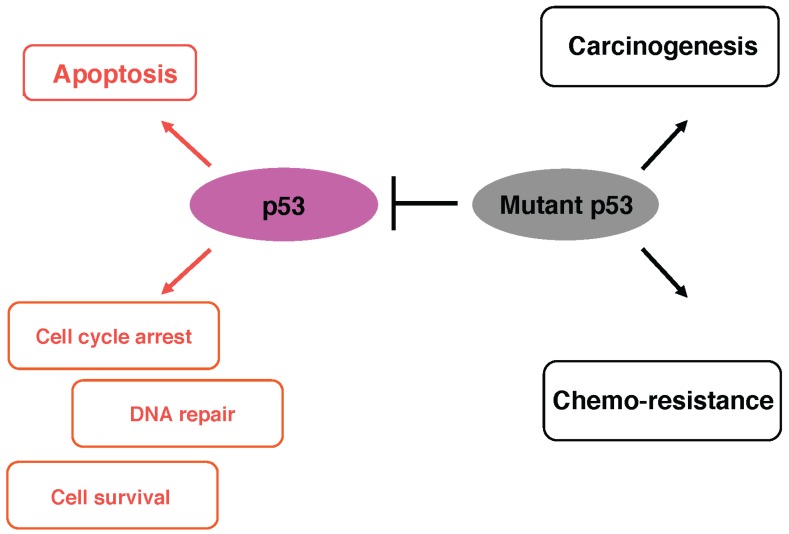
Although mutant p53 lacks the sequence-specific transactivation ability, it has been described that p53 mutant has its own target genes distinct from those of wild-type p53 [77]. For example, mutant p53 regulates a number of genes implicated in carcinogenesis including Myc, Fos, PCNA, IGF1R, EGR1, NFKB2, BCL-xL, IGF2 and VEGFA [78]. Recently, Kolukula et al. found that mutant p53 transactivates pro-oncogenic SLC25A1 [79]. Unfortunately, no defined mutant p53-responsive element has been characterized so far. Further studies are required to adequately address this issue (Figure 2).
Figure 2.
Functional interplay between wild-type p53 and mutant p53. Mutant p53 acts as a dominant-negative inhibitor against wild-type p53 and then contributes to carcinogenesis and drug-resistance.
4. p53 Family Members p73 and p63
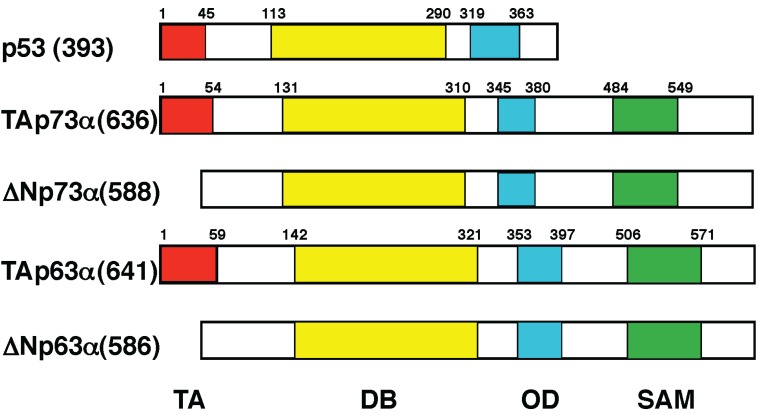
For a long time, p53 has been believed to be a solitary gene. This classical point of view has been challenged by a discovery of novel human p53 homologues termed p73 and p63 [80,81,82]. At present, p53 family is composed of p53, p73 and p63. As deduced from their structural similarity to p53, p73 and p63 are involved in tumor suppression, which regulate cell proliferation, differentiation and death [83]. Like p53, p73 and p63 are induced in response to DNA damage, and transactivate an overlapping set of p53-target genes involved in the induction of cell cycle arrest and/or cell death [84,85]. In contrast to p53, p73 and p63 are rarely mutated in human cancers, suggesting that p73 and p63 are not the classical tumor suppressors [86]. Consistent with these observations, initial studies demonstrated that p73- or p63-deficient mice do not develop spontaneous cancers [87,88,89]. Notably, p73 and p63 encode multiple variants (TAp73, ΔNp73, TAp63 and ΔNp63) arising from alternative splicing and alternative promoter usage [80,81,90]. TAp73 and TAp63 are transcriptionally active valiants containing the intact N-terminal TA domains, whereas ΔNp73 and ΔNp63 are N-terminally truncated ones lacking the sequence-specific transactivation ability (Figure 3).
Figure 3.
Structural comparison among p53 family members. Transcriptionally active p53, TAp73 and TAp63 contain the intact N-terminal transactivation domain (TA), central sequence-specific DNA-binding domain (DB) and C-terminal oligomerization domain (OD). In addition to these functional domains, TAp73 and TAp63 have the sterile α motif domain (SAM) implicated in protein–protein interaction. ΔNp73 and ΔNp63 lack N-terminal TA domain.
Like mutant p53, ΔNp73 and ΔNp63 display dominant-negative behavior towards TAp73 and TAp63 through hetero-oligomer formation, respectively, and are implicated in the acquisition of their oncogenic potential [82]. Consistently, forced expression of ΔNp73 promotes malignant transformation [91,92]. In particular, ΔN isoforms of p73 and p63 are aberrantly expressed in a variety of human cancers and their overexpression is associated with poor prognosis of the patients [93]. We have found for the first time that ΔNp73 transcription is positively regulated by TAp73 through p53/TAp73-responsive element within ΔNp73 promoter region [94]. Similar results were also reported by the other laboratories [95,96]. Therefore, it is likely that there exists a negative feedback regulation of TAp73 by its transcriptional target gene product ΔNp73 to avoid an inappropriate cell death by over-active TAp73 in response to DNA damage.
As described [87,88,89], the initial knockout studies demonstrated that p73- or p63-deficient mice do not develop spontaneous cancers, which might be attributed to the simultaneous disruption of both pro-apoptotic TA and pro-oncogenic ΔN isoforms. With this in mind, subsequent studies were performed using a mouse model in which the selective knockout of TAp73 isoform but not ΔNp73 isoform was designed [97]. According to their results, TAp73-deficient mice exhibited an increased cancer susceptibility and infertility, implying that TAp73 has a tumor suppressive role. Similarly, TAp63 has a critical role in preventing invasiveness and metastasis of epithelial cancers [98]. It is worth noting that p53-dependent cell death in response to DNA damage requires TAp73 and TAp63, whereas TAp73 and TAp63 are capable of triggering DNA damage-mediated cell death in the absence of functional p53 [99]. In fact, forced expression of TAp73 in p53-deficient human pancreatic cancer AsPC-1 cells resulted in massive cell death [100].
Upon DNA damage, TAp73 as well as TAp63 accumulates and is activated in cell nucleus. As described previously [101,102,103], TAp73 is phosphorylated by non-receptor tyrosine kinase c-Abl at Tyr-99 and its stability is significantly enhanced after DNA damage. Unlike p53, MDM2 prohibits TAp73-mediated transcriptional activation but does not affect its protein stability [104,105]. TAp73 and TAp63 are degraded through ubiquitin/proteasome mediated by E3 ubiquitin ligase Itch [106,107]. It remains elusive whether MDM2 could inhibit the transcriptional and pro-apoptotic activities of TAp63 [108,109]. In addition to the regulation of TAp73 at protein level, TAp73 is transcriptionally regulated by E2F-1 [110,111,112,113]. Blattner et al. put forward that E2F-1 is up-regulated and promotes cell death after DNA damage [114]. On the other hand, the transcription of TAp73 is repressed by the zinc finger/homeodomain-containing transcriptional repressor ZEB through ZEB-binding sites within the intron 1 of TAp73 [115]. Together, E2F-1-induced cell death might be mediated at least in part by TAp73.
5. RUNX Family
As mentioned below, mammalian runt-related sequence-specific transcription factor (RUNX) family members including RUNX1, RUNX2 and RUNX3 are closely involved in carcinogenesis, and it is possible that there could exist a functional interaction between RUNX family and p53 family. Each of RUNX family members has a highly conserved sequence-specific DNA-binding domain (known as a Runt domain) and a distinct C-terminus, which contains both inhibitory and activation domains [116]. The Runt domain is associated with core-binding factor subunit-β (CBF-β), which is required for the tight interaction of RUNX proteins with their target sequences (5'-PuACCPuCA-3') (Pu indicates purines) [117]. Accumulating evidence strongly suggests that each of RUNX family members is involved in a distinct biological process. For example, RUNX1 has been identified as part of the t(8; 21) chromosome translocation in acute myeloid leukemia (AML) and is responsible for the establishment of the hematopoietic stem cells [118,119,120]. RUNX1 is the most frequent target for chromosomal translocation in AML, and RUNX1 point mutations are found in hematological diseases such as AML as well as acute lymphocytic leukemia (ALL) [121]. Among them, up to 80% of mutations accumulate within the genomic region encoding its Runt domain, and thereby disrupting its structure [122,123]. Consistent with these observations, RUNX1-deficient mice exhibited a significant defect in hematopoiesis [118,119]. Collectively, it is conceivable that RUNX1 acts as a tumor suppressor for myeloid leukemia.
Unlike RUNX1, RUNX2 plays a pivotal role in the regulation of osteoblast differentiation and bone formation [124,125]. Accordingly, RUNX2 transactivates a number of its target genes implicated in osteogenesis such as type 1 collagen (COL1A1, COL1A2), Osteopontin (OCN, SPP1) and Osteocalcin (OPN, BGLAP) [126]. Notably, RUNX2 is also associated with osteosarcoma [127,128]. In support of this notion, amplification of chromosome 6p21, where RUNX2 exists, was detectable in a subset of osteosarcomas [129]. Several lines of evidence indicate that the dysregulation of RUNX2 expression is frequently detectable in a variety of human cancers and its higher expression level is tightly correlated with poor clinical outcome of the patients [24,25,26]. Indeed, RUNX2 also transactivates numerous genes involved in carcinogenesis [27,28,29,30,31,32]. Recent studies revealed that RUNX2-mdiated carcinogenesis is dependent on the direct activation of survivin expression in prostate cancer cells [33]. Survivin is highly expressed in a wide range of human cancers, which correlates with both accelerated relapse and drug resistance [34]. Thus, it is likely that RUNX2 participates in osteogenic differentiation as well as carcinogenesis in a cell context-dependent manner.
It has been well documented that RUNX3 is required for T cell development during thymopoiesis and plays an essential role in the regulation of the dorsal-root ganglion proprioceptive neuron function [130]. In addition to its capability to modulate the lineage-specific gene expression, RUNX3 has been shown to be obviously involved in the formation of a number of cancers including gastric, colorectal, liver, lung and breast cancers [131,132]. For example, Li et al. have described that RUNX3-deficient mouse gastric mucosa displays hyperplasia due to the enhanced proliferation and suppressed cell death [133]. Based on their results, tumorigenicity of human gastric cancer-derived cells was inversely correlated with RUNX3 expression level and mutation within the genomic region encoding Runt domain of RUNX3 (R122C) abrogated its tumor suppressive activity. It is worth noting that RUNX3 expression is kept at an extremely low level both in gastric cancer-derived cells and gastric cancer tissues, which might be due to the hypermethylation of the CpG island of RUNX3 exon 1 region. Unexpectedly, RUNX3 is rarely mutated in primary gastric cancer tissues. Therefore, DNA methylation-mediated silencing of RUNX3 might contribute to the loss of its tumor-suppressive function.
6. Functional Collaboration between the p53 Family and RUNX Family during DDR
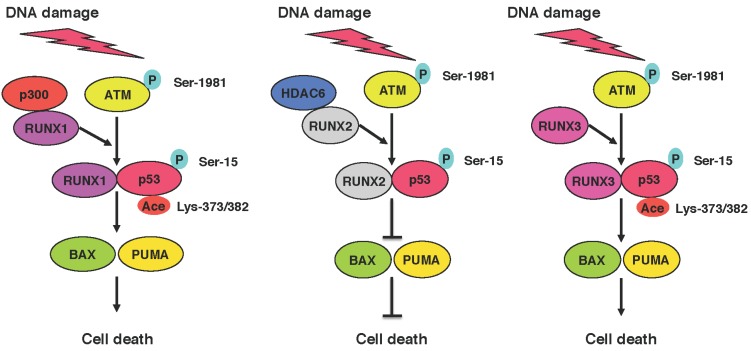
Considering that the p53 family participates in the regulation of DDR pathway, a clear understanding of the mechanistic basis underlying DNA damage-mediated activation and/or inhibition of p53 family-dependent cell death pathway is of particular interest in overcoming drug-resistant phenotype of malignant cancers [134]. In this connection, we have focused on the functional interaction between p53 family and RUNX family in response to DNA damage. As described previously [135], we have found for the first time that RUNX3 is associated with p53, enhancing p-ATM-mediated its phosphorylation at Ser-15, and thereby stimulating DNA damage-induced cell death. Our subsequent studies demonstrated that RUNX1 acts as a molecular bridge or a scaffolding protein for p53-histone acetyltransferase p300, assists p300-mediated acetylation of p53 at Lys-373/382 and further promotes p53-dependent cell death following DNA damage [136]. These observations strongly suggest that RUNX1 as well as RUNX3 serve as a co-activator for pro-apoptotic p53 during DDR.
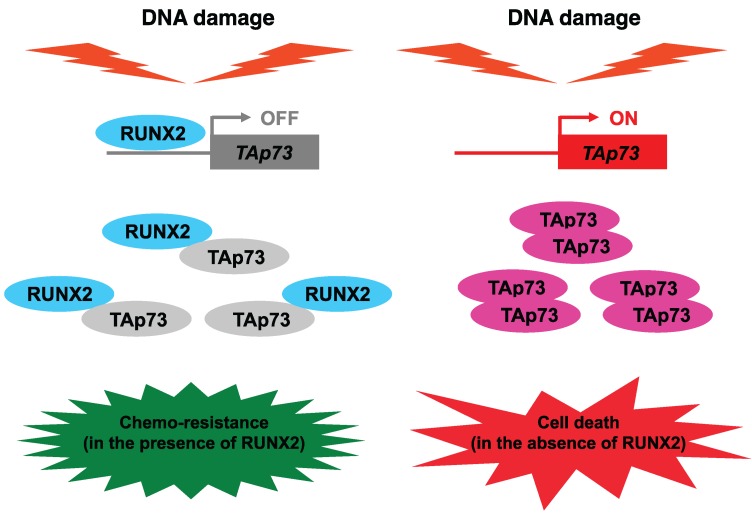
In contrast, we have recently found that pro-oncogenic RUNX2 has an inhibitory role in the regulation of DDR [35]. Based on our results, RUNX2 collaborated with histone deacetylase 6 (HDAC6) and then prohibited pro-apoptotic activity of p53 after DNA damage (Figure 4). In addition to p53, we have also revealed that RUNX2 trans-represses DNA damage-mediated up-regulation of TAp73, and thereby abrogating TAp73-dependent cell death in response to DNA damage [137]. Moreover, RUNX2 formed a complex with TAp73 and attenuated its transcriptional activity. Thus, RUNX2 might be one of the attractive therapeutic targets for malignant cancer treatment.
Figure 4.
Functional interaction between p53 family and RUNX family. In response to DNA damage, RUNX1 and RUNX3 assist p300-mediated acetylation of p53 at Lys-373/382 and p-ATM-mediated phosphorylation of p53 at Ser-15, respectively, and then augment its pro-apoptotic activity. In contrast, RUNX2 is associated with HDAC6, and thereby prohibits pro-apoptotic activity of p53 through its deacetylation.
7. Anti-Cancer Drug Resistance of Cancer Stem Cells
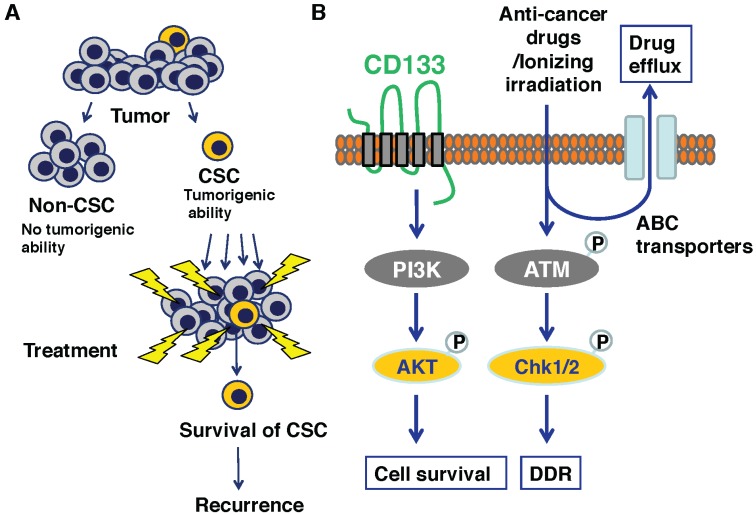
Stem cells (SCs) have the unique property of self-renewal to maintain tissue functions. Their quality must be strictly checked through the specific protective mechanisms that ensure their genomic integrity [138,139]. According to the previous estimation [140], cells including SCs receive 100,000 spontaneous DNA lesions per day. The proper DDR is therefore essential for SCs to prevent dysfunction of specific tissues caused by loss of SC pool and/or carcinogenesis [138]. Recent studies have demonstrated that a small population of cancer cells might possess a highly oncogenic ability when transplanted in immune-deficient mice, and be functionally similar to tissue-specific SCs, so-called cancer stem cells (CSCs). In several malignant cancers including brain, breast and colon cancers, CD133 has been considered to be one of the putative CSCs marker proteins to identify them as well as tissue-specific SCs such as hematopoietic SCs [45,141,142]. In fact, CD133-positive glioma cells are resistant to irradiation [141]. Collectively, increasing evidence indicates that the putative CSC-like cells display resistance to chemotherapy and radiotherapy, implying that CSCs are at least in part responsible for cancer recurrence after treatments [143].
As described [24,25,26], the expression level of RUNX2 was higher in a variety of human cancer tissues including prostate cancer than that in their corresponding normal ones. Intriguingly, CSCs enriched from prostate cancer tissues highly expressed RUNX2 as well as its target gene Survivin [47,48]. In addition, CD133-positive cells isolated from primary colorectal cancers were able to develop cancers in nude mice [139,142]. Consistently, knockdown of CD133 suppressed the xenograft tumor formation and growth of spheres in human colon cancer cells [144]. Together, it is likely that RUNX2 as well as CD133 are closely involved in the acquisition and/or maintenance of drug resistance of CSCs.
We and the other groups demonstrated that CD133 triggers the activation of PI3K/AKT pathway and potentiates oncogenic ability of glioma, hepatocellular carcinoma, neuroblastoma and colon cancer cells [145,146,147]. The oncogenic role of PI3K/AKT axis on cancer growth extends beyond its pro-proliferative and survival effects and includes migration as well as invasion. Notably, it has been shown that there exists a functional relationship between pro-oncogenic PI3K/AKT pathway and RUNX2. For example, Su et al. found that the constitutively active AKT induces RUNX2 expression as well as its target genes involved in carcinogenesis [148]. Consistently, Sase et al. described that RUNX2 immuno-reactivity in colon cancer cells is associated with their aggressive clinical behavior and its higher expression level is mediated by the stimulation of PI3K/AKT pathway [149]. In support of these observations, the activation of PI3K/AKT pathway augmented the sequence-specific DNA-binding ability of RUNX2 [150], suggesting that RUNX2 serves as a substrate of AKT and also an important mediator of pro-oncogenic PI3K/AKT signaling pathway. Thus, it is plausible that PI3K/AKT/RUNX2 regulatory axis participates in the genesis and/or maintenance of drug-resistant CSCs.
Additionally, it has been shown that a variety of tissue-specific SCs and CSCs enrich in side population (SP) of cells originally identified from hematopoietic SCs with a higher efflux rate of Hoechst 33342 [151,152]. The efflux of dye is largely mediated by ATP-binding cassette (ABC) transporters such as ABCB1 (multidrug resistant 1/MDR1), ABCC1 (MDR protein 1/MRP) and ABCG2 (breast cancer-resistant protein1/BCRP1). These ABC transporters have been well known to pump a number of anti-cancer drugs, and might be implicated in the acquisition and/or maintenance of drug-resistant phenotype of CSCs (Figure 5).
Figure 5.
Resistant phenotype of cancer stem cells (CSCs). (A) Schematic drawing of CSC hypothesis; (B) Major signaling pathways for resistant phenotype of CSCs.
8. Attractive Strategies to Overcome Anti-Cancer Drug-Resistant Malignant Cancers
Since MDM2 binds to p53 and abrogates its pro-apoptotic activity, a specific inhibition of the complex formation between MDM2 and p53 might be an attractive strategy to augment pro-apoptotic activity of p53 in response to DNA damage. For example, a small chemical compound termed Nutlin, which binds directly to the p53-binding pocket of MDM2, interrupts p53/MDM2 interaction, and subsequently prohibits MDM2-dependent proteasomal degradation of p53 [153,154]. An alternative approach is mutant p53 reactivation by a small chemical compound, which promotes correct folding of mutant p53. PRIMA-1 and MIRA-1 can restore wild-type conformation to mutant p53, and then induce cell death of cancerous cells [155,156].
According to our recent findings, siRNA-mediated knockdown of pro-oncogenic RUNX2 efficiently augmented p53/TAp73-dependent cell death in p53-proficient human osteosarcoma-derived cells following DNA damage [35,136]. Similar results were also observed in p53-deficient or p53-mutated human pancreatic cancer cells [157], suggesting that siRNA-mediated depletion of RUNX2 promotes the proper DDR regardless of p53 status (Figure 6). Unfortunately, it has been well known that siRNA is unstable and its knockdown effect is transient. Recently, Zorde Khvalevsky et al. developed a local prolonged siRNA delivery system (termed LODER) [158]. Based on their results, LODER system protected siRNA from degradation and released intact siRNA stably and slowly within cancer cells over a few months. Therefore, this LODER system might overcome the current siRNA delivery obstacles, and siRNA-mediated silencing of RUNX2 using LODER delivery system is an original and attractive therapeutic strategy to treat drug-resistant malignant cancers.
Figure 6.
siRNA-mediated silencing of RUNX2 enhances TAp73-dependent cell death pathway in the absence of functional p53. RUNX2 trans-represses DNA damage-mediated induction of TAp73 and forms a complex with TAp73 to prohibit its transcriptional activity. Depletion of RUNX2 further enhances TAp73-dependent cell death pathway in response to DNA damage [136].
9. Conclusions
An appropriate DDR signaling pathway plays a vital role in the regulation of cell fate determination after DNA damage (cell survival or death). Tumor suppressor p53 has a crucial role in eliminating seriously damaged cells, and thereby maintaining genomic integrity. Therefore, its dysfunction arising from mutations renders cancerous cells resistant to anti-cancer drugs. Indeed, p53 mutant acts as a dominant-negative inhibitor against wild-type p53. In contrast to p53, the other p53 family members such as TAp73 and TAp63 are rarely mutated in cancer cells. Importantly, TAp73 and TAp63 are capable of inducing cancer cell death following DNA damage in the absence of functional p53. Consistently, forced expression of TAp73 promotes cell death in p53-deficient as well as p53-mutated cancerous cells. Meanwhile, RUNX1 and RUNX3 enhance p53-dependent cell death in response to DNA damage. On the other hand, RUNX2 attenuates p53/TAp73-mediated cell death following DNA damage. Thus, silencing of RUNX2 might be a promising strategy to improve the efficacy of DNA damage-inducing anti-cancer drugs through the activation of the p53 family-dependent cell death pathway.
Acknowledgments
The authors thank all former and present members of our laboratory for their valuable discussions. This work was supported in part by a research grant (Grant No. 23501278) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to Toshinori Ozaki).
Author Contributions
Toshinori Ozaki, Mizuyo Nakamura and Osamu Shimozato wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Thompson L.H. Recognition, signaling, and repair of DNA double-strand breaks produced by ionizing radiation in mammalian cells: The molecular choreography. Mutat. Res. 2012;751:158–246. doi: 10.1016/j.mrrev.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Falck J., Coates J., Jackson S.P. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg M., Stucki M., Falck J., D’Amours D., Rahman D., Pappin D., Bartek J., Jackson S.P. MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature. 2003;421:952–956. doi: 10.1038/nature01445. [DOI] [PubMed] [Google Scholar]
- 4.Lou Z., Minter-Dykhouse K., Wu X., Chen J. MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature. 2003;421:957–961. doi: 10.1038/nature01447. [DOI] [PubMed] [Google Scholar]
- 5.Stewart G.S., Wang B., Bignell C.R., Taylor A.M., Elledge S.J. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature. 2003;421:961–966. doi: 10.1038/nature01446. [DOI] [PubMed] [Google Scholar]
- 6.Hopfner K.P., Tainer J.A. Rad50/SMC proteins and ABC transporters: Unifying concepts from high-resolution structures. Curr. Opin. Struct. Biol. 2003;13:249–255. doi: 10.1016/S0959-440X(03)00037-X. [DOI] [PubMed] [Google Scholar]
- 7.Hopfner K.P., Karcher A., Craig L., Woo T.T., Carney J.P., Tainer J.A. Structural biochemistry and interaction architecture of the DNA double-strand break repair Mre11 nuclease and Rad50-ATPase. Cell. 2001;105:473–485. doi: 10.1016/S0092-8674(01)00335-X. [DOI] [PubMed] [Google Scholar]
- 8.Paull T.T., Deshpande R.A. The Mre11/Rad50/Nbs1 complex: Recent insights into catalytic activities and ATP-driven conformational changes. Exp. Cell Res. 2014;329:139–147. doi: 10.1016/j.yexcr.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman J.R., Jackson S.P. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 2008;9:795–801. doi: 10.1038/embor.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.H., Paull T.T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 11.Uziel T., Lerenthal Y., Moyal L., Andegeko Y., Mittelman L., Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Difilippantonio S., Nussenzweig A. The NBS1-ATM connection revisited. Cell Cycle. 2007;6:2366–2370. doi: 10.4161/cc.6.19.4758. [DOI] [PubMed] [Google Scholar]
- 13.Horn H.F., Vousden K.H. Coping with stress: Multiple ways to activate p53. Oncogene. 2007;26:1306–1316. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- 14.Kruse J.P., Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meek D.W. Regulation of the p53 response and its relationship to cancer. Biochem. J. 2015;469:325–346. doi: 10.1042/BJ20150517. [DOI] [PubMed] [Google Scholar]
- 16.Pietenpol J.A., Tokino T., Thiagalingam S., el-Deiry W.S., Kinzler K.W., Vogelstein B. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc. Natl. Acad. Sci. USA. 1994;91:1998–2002. doi: 10.1073/pnas.91.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donehower L.A., Harvey M., Slagle B.L., McArthur M.J., Montgomery C.A., Butel J.S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 18.Harris C.C. p53: At the crossroads of molecular carcinogenesis and risk assessment. Science. 1992;262:1980–1981. doi: 10.1126/science.8266092. [DOI] [PubMed] [Google Scholar]
- 19.Vogelstein B., Kinzler K.W. p53 function and dysfunction. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 20.Velculescu V.E., el-Deiry W.S. Biological and clinical importance of the p53 tumor suppressor gene. Clin. Chem. 1996;42:858–868. [PubMed] [Google Scholar]
- 21.Vogelstein B., Lane D., Levine A.J. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 22.Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R.T., Gao Y.H., Inada M., et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 23.Otto F., Thornell A.P., Crompton T., Denzel A., Gilmour K.C., Rosewell I.R., Stamp G.W., Beddington R.S., Mundlos S., Olsen B.R., et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/S0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 24.Brubaker K.D., Vessella R.L., Brown L.G., Corey E. Prostate cancer expression of runt-domain transcription factor Runx2, a key regulator of osteoblast differentiation and function. Prostate. 2003;56:13–22. doi: 10.1002/pros.10233. [DOI] [PubMed] [Google Scholar]
- 25.Kayed H., Jiang X., Keleg S., Jesnowski R., Giese T., Berger M.R., Esposito I., Lohr M., Friess H., Kleeff J. Regulation and functional role of the Runt-related transcription factor-2 in pancreatic cancer. Br. J. Cancer. 2007;97:1106–1115. doi: 10.1038/sj.bjc.6603984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endo T., Ohta K., Kobayashi T. Expression and function of Cbfa-1/Runx2 in thyroid papillary carcinoma cells. J. Clin. Endocrinol. Metable. 2008;93:2409–2412. doi: 10.1210/jc.2007-2805. [DOI] [PubMed] [Google Scholar]
- 27.Barnes G.L., Hebert K.E., Kamal M., Javed A., Einhorn T.A., Lian J.B., Stein G.S., Gerstenfeld L.C. Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases-associated osteolytic disease. Cancer Res. 2004;64:4506–4513. doi: 10.1158/0008-5472.CAN-03-3851. [DOI] [PubMed] [Google Scholar]
- 28.Pratap J., Javed A., Languino L.R., van Wijnen A.J., Stein J.L., Stein G.S., Lian J.B. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol. Cell Biol. 2005;25:8581–8891. doi: 10.1128/MCB.25.19.8581-8591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratap J., Lian J.B., Javed A., Barnes G.L., van Wijnen A.J., Stein J.L., Stein G.S. Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev. 2006;25:589–600. doi: 10.1007/s10555-006-9032-0. [DOI] [PubMed] [Google Scholar]
- 30.Pratap J., Wixted J.J., Gaur T., Zaidi S.K., Dobson J., Gokul K.D., Hussain S., van Wijnen A.J., Stein J.L., Stein G.S., et al. Runx2 transcriptional activation of Indian Hedgehog and a downstream bone metastatic pathway in breast cancer cells. Cancer Res. 2008;68:7795–7802. doi: 10.1158/0008-5472.CAN-08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendoza-Villanueva D., Deng W., Lopez-Camacho C., Shore P. The Runx transcriptional co-activator, CBFbeta, is essential for invasion of breast cancer cells. Mol. Cancer. 2010 doi: 10.1186/1476-4598-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chimge N.O., Frenkel B. The RUNX family in breast cancer: Relationships with estrogen signaling. Oncogene. 2013;32:2121–2130. doi: 10.1038/onc.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim M., Zhong C., Yang S., Bell A.M., Cohen M.B., Roy-Burman P. Runx2 regulates survivin expression in prostate cancer cells. Lab. Investig. 2010;90:222–233. doi: 10.1038/labinvest.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altieri D.C. Survivin, cancer networks and pathway-directed drug discovery. Nat. Rev. Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 35.Ozaki T., Wu D., Sugimoto H., Nagase H., Nakagawara A. Runt-related transcription factor 2 (RUNX2) inhibits p53-dependent apoptosis through the collaboration with HDAC6 in response to DNA damage. Cell Death Dis. 2013;4:e610. doi: 10.1038/cddis.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 37.Wang J.C., Dick J.E. Cancer stem cells: Lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Dick J.E. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 39.Neuzil J., Stantic M., Zobalova R., Chladova J., Wang X., Prochazka L., Dong L., Andera L., Ralph S.J. Tumour-initiating cells vs. cancer “stem” cells and CD133: What’s in the name? Biochem. Biophys. Res. Commun. 2007;355:855–859. doi: 10.1016/j.bbrc.2007.01.159. [DOI] [PubMed] [Google Scholar]
- 40.Li C., Heidt D.G., Dalerba P., Burant C.F., Zhang L., Adsay V., Wicha M., Clarke M.F., Simeone D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 41.Immervoll H., Hoem D., Sakariassen P.Ø., Steffensen O.J., Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008 doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moriyama T., Ohuchida K., Mizumoto K., Cui L., Ikenaga N., Sato N., Tanaka M. Enhanced cell migration and invasion of CD133+ pancreatic cancer cells cocultured with pancreatic stromal cells. Cancer. 2010;116:3357–3368. doi: 10.1002/cncr.25121. [DOI] [PubMed] [Google Scholar]
- 43.Lee H.J., You D.D., Choi D.W., Choi Y.S., Kim S.J., Won Y.S., Moon H.J. Significance of CD133 as a cancer stem cell markers focusing on the tumorigenicity of pancreatic cancer cell lines. J. Korean Surg. Soc. 2011;81:263–270. doi: 10.4174/jkss.2011.81.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim M.P., Fleming J.B., Wang H., Abbruzzese J.L., Choi W., Kopetz S., McConkey D.J., Evans D.B., Gallick G.E. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS ONE. 2011;6:e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin A.H., Miraglia S., Zanjani E.D., Almeida-Porada G., Ogawa M., Leary A.G., Olweus J., Kearney J., Buck D.W. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 46.Liu G., Yuan X., Zeng Z., Tunici P., Ng H., Abdulkadir I.R., Lu L., Irvin D., Black K.L., Yu J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer. 2006 doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao C.P., Adisetiyo H., Liang M., Roy-Burman P. Cancer-associated fibroblasts enhance the gland-forming capability of prostate cancer stem cells. Cancer Res. 2010;70:7294–303. doi: 10.1158/0008-5472.CAN-09-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao C.P., Adisetiyo H., Liang M., Roy-Burman P. Cancer stem cells and microenvironment in prostate cancer progression. Horm. Cancer. 2010;1:297–305. doi: 10.1007/s12672-010-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haupt Y., Maya R., Kazaz A., Oren M. MDM2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 50.Kubbutat M.H., Jones S.N., Vousden K.H. Regulation of p53 stability by MDM2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 51.Honda R., Tanaka H., Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/S0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 52.Leng R.P., Lin Y., Ma W., Wu H., Lemmers B., Chung S., Parant J.M., Lozano G., Hakem R., Benchimol S. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112:779–791. doi: 10.1016/S0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 53.Dornan D., Wertz I., Shimizu H., Arnott D., Frantz G.D., Dowd P., O’Rourke K., Koeppen H., Dixit V.M. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- 54.Brooks C.L., Gu W. p53 ubiquitination: MDM2 and beyond. Mol. Cell. 2006;21:307–315. doi: 10.1016/j.molcel.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Momand J., Zambetti G.P., Olson D.C., George D., Levine A.J. The MDM-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-R. [DOI] [PubMed] [Google Scholar]
- 56.Oliner J.D., Pietenpol J.A., Thiagalingam S., Gyuris J., Kinzler KW., Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 57.Barak Y., Juven T., Haffner R., Oren M. MDM2 expression is induced by wild type p53 activity. EMBO J. 1993;12:461–468. doi: 10.1002/j.1460-2075.1993.tb05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X., Bayle J.H., Olson D., Levine A.J. The p53-MDM-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 59.Siliciano J.D., Canman C.E., Taya Y., Sakaguchi K., Appella E., Kastan M.B. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shieh S.Y., Ikeda M., Taya Y., Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/S0092-8674(00)80416-X. [DOI] [PubMed] [Google Scholar]
- 61.Gu W., Roeder R.G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/S0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 62.Chen X., Ko L.J., Jayaraman L., Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 63.Oda K., Arakawa H., Tanaka T., Matsuda K., Tanikawa C., Mori T., Nishimori H., Tamai K., Tokino T., Nakamura Y., et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell. 2000;102:849–862. doi: 10.1016/S0092-8674(00)00073-8. [DOI] [PubMed] [Google Scholar]
- 64.Zhao R., Gish K., Murphy M., Yin Y., Notterman D., Hoffman W.H., Tom E., Mack D.H., Levine A.J. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 2000;14:981–993. [PMC free article] [PubMed] [Google Scholar]
- 65.Morachis J.M., Murawsky C.M., Emerson B.M. Regulation of the p53 transcriptional response by structurally diverse core promoters. Genes Dev. 2010;24:135–147. doi: 10.1101/gad.1856710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espinosa J.M., Verdun R.E., Emerson B.M. p53 functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. Mol. Cell. 2003;12:1015–1027. doi: 10.1016/S1097-2765(03)00359-9. [DOI] [PubMed] [Google Scholar]
- 67.Scala F., Brighenti E., Govoni M., Imbrogno E., Fornari F., Treré D., Montanaro L., Derenzini M. Direct relationship between the level of p53 stabilization induced by rRNA synthesis-inhibiting drugs and the cell ribosome biogenesis rate. Oncogene. 2015 doi: 10.1038/onc.2015.147. in press. [DOI] [PubMed] [Google Scholar]
- 68.D’Orazi G., Cecchinelli B., Bruno T., Manni I., Higashimoto Y., Saito S., Gostissa M., Coen S., Marchetti A., del Sal G., et al. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat. Cell Biol. 2002;4:11–19. doi: 10.1038/ncb714. [DOI] [PubMed] [Google Scholar]
- 69.Tang Y., Luo J., Zhang W., Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol. Cell. 2006;24:827–839. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 70.Sykes S.M., Mellert H.S., Holbert M.A., Li K., Marmorstein R., Lane W.S., McMahon S.B. Acetylation of the p53 DNA binding domain regulates apoptosis induction. Mol. Cell. 2006;24:841–851. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Charvet C., Wissler M., Brauns-Schubert P., Wang S.J., Tang Y., Sigloch F.C., Mellert H., Brandenburg M., Lindner S.E., Breit B., et al. Phosphorylation of Tip60 by GSK-3 determines the induction of PUMA and apoptosis by p53. Mol. Cell. 2011;42:584–596. doi: 10.1016/j.molcel.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hollstein M., Sidransky D., Vogelstein B., Harris C.C. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 73.Raycroft L., Wu H.Y., Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990;249:1049–1051. doi: 10.1126/science.2144364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harvey M., Vogel H., Morris D., Bradley A., Bernstein A., Donehower L.A. A mutant p53 transgene accelerates tumour development in heterozygous but not nullizygous p53-deficient mice. Nat. Genet. 1995;9:305–131. doi: 10.1038/ng0395-305. [DOI] [PubMed] [Google Scholar]
- 75.Petitjean A., Achatz M.I., Borresen-Dale A.L., Hainaut P., Olivier M. TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 76.Buschmann T., Minamoto T., Wagle N., Fuchs S.Y., Adler V., Mai M., Ronai Z. Analysis of JNK, MDM2 and p14(ARF) contribution to the regulation of mutant p53 stability. J. Mol. Biol. 2000;295:1009–1021. doi: 10.1006/jmbi.1999.3387. [DOI] [PubMed] [Google Scholar]
- 77.Vaughan C., Pearsall I., Yeudall A., Deb S.P., Deb S. p53: Its mutations and their impact on transcription. Subcell. Biochem. 2014;85:71–90. doi: 10.1007/978-94-017-9211-0_4. [DOI] [PubMed] [Google Scholar]
- 78.Brosh R., Rotter V. When mutants gain new powers: News from the mutant p53 field. Nat. Rev. Cancer. 2009;9:701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 79.Kolukula V.K., Sahu G., Wellstein A., Rodriguez O.C., Preet A., Iacobazzi V., D’Orazi G., Albanese C., Palmieri F., Avantaggiati M.L. SLC25A1, or CIC, is a novel transcriptional target of mutant p53 and a negative tumor prognostic marker. Oncotarget. 2014;5:1212–1225. doi: 10.18632/oncotarget.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaghad M., Bonnet H., Yang A., Creancier L., Biscan J.C., Valent A., Minty A., Chalon P., Lelias J.M., Dumont X., et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/S0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 81.Yang A., Kaghad M., Wang Y., Gillett E., Fleming M.D., Dötsch V., Andrews N.C., Caput D., McKeon F. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell. 1998;2:305–316. doi: 10.1016/S1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 82.Melino G., de Laurenzi V., Vousden K.H. p73: Friend or foe in tumorigenesis. Nat. Rev. Cancer. 2002;2:605–615. doi: 10.1038/nrc861. [DOI] [PubMed] [Google Scholar]
- 83.Collavin L., Lunardi A., del Sal G. p53-family proteins and their regulators: Hubs and spokes in tumor suppression. Cell Death Differ. 2010;17:901–911. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- 84.Katoh I., Aisaki K.I., Kurata S.I., Ikawa S., Ikawa Y. p51A (TAp63gamma), a p53 homolog, accumulates in response to DNA damage for cell regulation. Oncogene. 2000;19:3126–3130. doi: 10.1038/sj.onc.1203644. [DOI] [PubMed] [Google Scholar]
- 85.Irwin M.S., Kondo K., Marin M.C., Cheng L.S., Hahn W.C., Kaelin W.G., Jr. Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–410. doi: 10.1016/S1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 86.Ikawa S., Nakagawara A., Ikawa Y. p53 family genes: Structural comparison, expression and mutation. Cell Death Differ. 1999;6:1154–1161. doi: 10.1038/sj.cdd.4400631. [DOI] [PubMed] [Google Scholar]
- 87.Mills A.A., Zheng B., Wang X.J., Vogel H., Roop D.R., Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 88.Yang A., Schweitzer R., Sun D., Kaghad M., Walker N., Bronson R.T., Tabin C., Sharpe A., Caput D., Crum C., et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 89.Yang A., Walker N., Bronson R., Kaghad M., Oosterwegel M., Bonnin J., Vagner C., Bonnet H., Dikkes P., Sharpe A., et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 90.Pozniak C.D., Radinovic S., Yang A., McKeon F., Kaplan D.R., Miller F.D. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science. 2000;289:304–306. doi: 10.1126/science.289.5477.304. [DOI] [PubMed] [Google Scholar]
- 91.Petrenko O., Zaika A., Moll U.M. deltaNp73 facilitates cell immortalization and cooperates with oncogenic Ras in cellular transformation in vivo. Mol. Cell Biol. 2003;23:5540–5555. doi: 10.1128/MCB.23.16.5540-5555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tannapfel A., John K., Mise N., Schmidt A., Buhlmann S., Ibrahim S.M., Pützer B.M. Autonomous growth and hepatocarcinogenesis in transgenic mice expressing the p53 family inhibitor ΔNp73. Carcinogenesis. 2008;29:211–218. doi: 10.1093/carcin/bgm236. [DOI] [PubMed] [Google Scholar]
- 93.Deyoung M.P., Ellisen L.W. p63 and p73 in human cancer: Defining the network. Oncogene. 2007;26:5169–5183. doi: 10.1038/sj.onc.1210337. [DOI] [PubMed] [Google Scholar]
- 94.Nakagawa T., Takahashi M., Ozaki T., Watanabe K., Todo S., Mizuguchi H., Hayakawa T., Nakagawara A. Autoinhibitory regulation of p73 by Delta Np73 to modulate cell survival and death through a p73-specific target element within the Delta Np73 promoter. Mol. Cell Biol. 2002;22:2575–2585. doi: 10.1128/MCB.22.8.2575-2585.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grob T.J., Novak U., Maisse C., Barcaroli D., Lüthi A.U., Pirnia F., Hügli B., Graber H.U., de Laurenzi V., Fey M.F., et al. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ. 2001;8:1213–1223. doi: 10.1038/sj.cdd.4400962. [DOI] [PubMed] [Google Scholar]
- 96.Zaika A.I., Slade N., Erster S.H., Sansome C., Joseph T.W., Pearl M., Chalas E., Moll U.M. DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J. Exp. Med. 2002;196:765–780. doi: 10.1084/jem.20020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tomasini R., Tsuchihara K., Wilhelm M., Fujitani M., Rufini A., Cheung C.C., Khan F., Itie-Youten A., Wakeham A., Tsao M.S., et al. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev. 2008;22:2677–2691. doi: 10.1101/gad.1695308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adorno M., Cordenonsi M., Montagner M., Dupont S., Wong C., Hann B., Solari A., Bobisse S., Rondina M.B., Guzzardo V., et al. A mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 99.Flores E.R., Tsai K.Y., Crowley D., Sengupta S., Yang A., McKeon F., Jacks T. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–564. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- 100.Rödicker F., Pützer B.M. p73 is effective in p53-null pancreatic cancer cells resistant to wild-type TP53 gene replacement. Cancer Res. 2003;63:2737–2741. [PubMed] [Google Scholar]
- 101.Gong J.G., Costanzo A., Yang H.Q., Melino G., Kaelin W.G., Jr., Levrero M., Wang J.Y. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 102.Agami R., Blandino G., Oren M., Shaul Y. Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature. 1999;399:809–813. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- 103.Yuan Z.M., Shioya H., Ishiko T., Sun X., Gu J., Huang Y.Y., Lu H., Kharbanda S., Weichselbaum R., Kufe D. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature. 1999;399:814–817. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- 104.Zeng X., Chen L., Jost C.A., Maya R., Keller D., Wang X., Kaelin W.G., Jr., Oren M., Chen J., Lu H. MDM2 suppresses p73 function without promoting p73 degradation. Mol. Cell Biol. 1999;19:3257–3266. doi: 10.1128/mcb.19.5.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dobbelstein M., Wienzek S., König C., Roth J. Inactivation of the p53-homologue p73 by the MDM2-oncoprotein. Oncogene. 1999;18:2101–2106. doi: 10.1038/sj.onc.1202512. [DOI] [PubMed] [Google Scholar]
- 106.Rossi M., de Laurenzi V., Munarriz E., Green D.R., Liu Y.C., Vousden K.H., Cesareni G., Melino G. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J. 2005;24:836–848. doi: 10.1038/sj.emboj.7600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rossi M., Aqeilan R.I., Neale M., Candi E., Salomoni P., Knight R.A., Croce C.M., Melino G. The E3 ubiquitin ligase Itch controls the protein stability of p63. Proc. Natl. Acad. Sci. USA. 2006;103:12753–12758. doi: 10.1073/pnas.0603449103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Little N.A., Jochemsen A.G. Hdmx and MDM2 can repress transcription activation by p53 but not by p63. Oncogene. 2001;20:4576–4580. doi: 10.1038/sj.onc.1204615. [DOI] [PubMed] [Google Scholar]
- 109.Kojima T., Ikawa Y., Katoh I. Analysis of molecular interactions of the p53-family p51(p63) gene products in a yeast two-hybrid system: Homotypic and heterotypic interactions and association with p53-regulatory factors. Biochem. Biophys. Res. Commun. 2001;281:1170–1175. doi: 10.1006/bbrc.2001.4486. [DOI] [PubMed] [Google Scholar]
- 110.Lissy N.A., Davis P.K., Irwin M., Kaelin W.G., Dowdy S.F. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature. 2000;407:642–645. doi: 10.1038/35036608. [DOI] [PubMed] [Google Scholar]
- 111.Irwin M., Marin M.C., Phillips A.C., Seelan R.S., Smith D.I., Liu W., Flores E.R., Tsai K.Y., Jacks T., Vousden K.H., et al. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature. 2000;407:645–648. doi: 10.1038/35036614. [DOI] [PubMed] [Google Scholar]
- 112.Stiewe T., Pützer B.M. Role of the p53-homologue p73 in E2F1-induced apoptosis. Nat. Genet. 2000;26:464–469. doi: 10.1038/82617. [DOI] [PubMed] [Google Scholar]
- 113.Zaika A., Irwin M., Sansome C., Moll U.M. Oncogenes induce and activate endogenous p73 protein. J. Biol. Chem. 2001;276:11310–11316. doi: 10.1074/jbc.M005737200. [DOI] [PubMed] [Google Scholar]
- 114.Blattner C., Sparks A., Lane D. Transcription factor E2F-1 is upregulated in response to DNA damage in a manner analogous to that of p53. Mol. Cell Biol. 1999;19:3704–1373. doi: 10.1128/mcb.19.5.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fontemaggi G., Gurtner A., Strano S., Higashi Y., Sacchi A., Piaggio G., Blandino G. he transcriptional repressor ZEB regulates p73 expression at the crossroad between proliferation and differentiation. Mol. Cell Biol. 2001;24:8461–8470. doi: 10.1128/MCB.21.24.8461-8470.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ito Y. RUNX genes in development and cancer: Regulation of viral gene expression and the discovery of RUNX family genes. Adv. Cancer Res. 2008;99:33–76. doi: 10.1016/S0065-230X(07)99002-8. [DOI] [PubMed] [Google Scholar]
- 117.Tahirov T.H., Inoue-Bungo T., Morii H., Fujikawa A., Sasaki M., Kimura K., Shiina M., Sato K., Kumasaka T., Yamamoto M., et al. Structural analyses of DNA recognition by the AML1/Runx-1 Runt domain and its allosteric control by CBFbeta. Cell. 2001;104:755–767. doi: 10.1016/S0092-8674(01)00271-9. [DOI] [PubMed] [Google Scholar]
- 118.Okuda T., van Deursen J., Hiebert S.W., Grosveld G., Downing J.R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/S0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 119.Wang Q., Stacy T., Binder M., Marin-Padilla M., Sharpe A.H., Speck N.A. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl. Acad. Sci. USA. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miyoshi H., Shimizu K., Kozu T., Maseki N., Kaneko Y., Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc. Natl. Acad. Sci. USA. 1991;88:10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mangan J.K., Speck N.A. RUNX1 mutations in clonal myeloid disorders: From conventional cytogenetics to next generation sequencing, a story 40 years in the making. Crit. Rev. Oncog. 2011;16:77–91. doi: 10.1615/CritRevOncog.v16.i1-2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Osato M., Yanagida M., Shigesada K., Ito Y. Point mutations of the RUNX1/AML1 gene in sporadic and familial myeloid leukemias. Int. J. Hematol. 2001;74:245–251. doi: 10.1007/BF02982056. [DOI] [PubMed] [Google Scholar]
- 123.Osato M. Point mutations in the RUNX1/AML1 gene: Another actor in RUNX leukemia. Oncogene. 2004;23:4284–4296. doi: 10.1038/sj.onc.1207779. [DOI] [PubMed] [Google Scholar]
- 124.Welch R.D., Jones A.L., Bucholz R.W., Reinert C.M., Tjia J.S., Pierce W.A., Wozney J.M., Li X.J. Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goat tibial fracture model. J. Bone Miner. Res. 1998;13:1483–1490. doi: 10.1359/jbmr.1998.13.9.1483. [DOI] [PubMed] [Google Scholar]
- 125.Thomas D.M., Johnson S.A., Sims N.A., Trivett M.K., Slavin J.L., Rubin B.P., Waring P., McArthur G.A., Walkley C.R., Holloway A.J., et al. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J. Cell Biol. 2004;167:925–934. doi: 10.1083/jcb.200409187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nathan S.S., Pereira B.P., Zhou Y.F., Gupta A., Dombrowski C., Soong R., Pho R.W., Stein G.S., Salto-Tellez M., Cool S.M., et al. Elevated expression of Runx2 as a key parameter in the etiology of osteosarcoma. Mol. Biol. Rep. 2009;36:153–158. doi: 10.1007/s11033-008-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lau C.C., Harris C.P., Lu X.Y., Perlaky L., Gogineni S., Chintagumpala M., Hicks J., Johnson M.E., Davino N.A., Huvos A.G., et al. Frequent amplification and rearrangement of chromosomal bands 6p12-p21 and 17p11.2 in osteosarcoma. Genes Chromosomes Cancer. 2004;39:11–21. doi: 10.1002/gcc.10291. [DOI] [PubMed] [Google Scholar]
- 128.Inoue K., Ozaki S., Shiga T., Ito K., Masuda T., Okado N., Iseda T., Kawaguchi S., Ogawa M., Bae S.C., et al. Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat. Neurosci. 2002;5:946–954. doi: 10.1038/nn925. [DOI] [PubMed] [Google Scholar]
- 129.Ito Y. Oncogenic potential of the RUNX gene family: “Overview”. Oncogene. 2004;23:4198–4208. doi: 10.1038/sj.onc.1207755. [DOI] [PubMed] [Google Scholar]
- 130.Subramaniam M.M., Chan J.Y., Yeoh K.G., Quek T., Ito K., Salto-Tellez M. Molecular pathology of RUNX3 in human carcinogenesis. Biochim. Biophys. Acta. 2009;1796:315–331. doi: 10.1016/j.bbcan.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 131.Li Q.L., Ito K., Sakakura C., Fukamachi H., Inoue K., Chi X.Z., Lee K.Y., Nomura S., Lee C.W., Han S.B., et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–124. doi: 10.1016/S0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 132.Carvajal L.A., Manfredi J.J. Another fork in the road—Life or death decisions by the tumour suppressor p53. EMBO Rep. 2013;14:414–421. doi: 10.1038/embor.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamada C., Ozaki T., Ando K., Suenaga Y., Inoue K., Ito Y., Okoshi R., Kageyama H., Kimura H., Miyazaki M., et al. RUNX3 modulates DNA damage-mediated phosphorylation of tumor suppressor p53 at Ser-15 and acts as a co-activator for p53. J. Biol. Chem. 2010;285:16693–16703. doi: 10.1074/jbc.M109.055525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu D., Ozaki T., Yoshihara Y., Kubo N., Nakagawara A. Runt-related transcription factor 1 (RUNX1) stimulates tumor suppressor p53 protein in response to DNA damage through complex formation and acetylation. J. Biol. Chem. 2013;288:1353–1364. doi: 10.1074/jbc.M112.402594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ozaki T., Sugimoto H., Nakamura M., Hiraoka K., Yoda H., Sang M., Fujiwara K., Nagase H. Runt-related transcription factor 2 attenuates the transcriptional activity as well as DNA damage-mediated induction of pro-apoptotic TAp73 to regulate chemosensitivity. FEBS J. 2015;282:114–128. doi: 10.1111/febs.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rossi D.J., Jamieson C.H., Weissman I.L. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 137.Orford K.W., Scadden D.T. Deconstructing stem cell self-renewal: Genetic insights into cell-cycle regulation. Nat. Rev. Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 138.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 139.Ricci-Vitiani L., Lombardi D.G., Pilozzi E., Biffoni M., Todaro M., Peschle C., de Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 140.Singh S.K., Hawkins C., Clarke I.D., Squire J.A., Bayani J., Hide T., Henkelman R.M., Cusimano M.D., Dirks P.B. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 141.Bao S., Wu Q., McLendon R.E., Hao Y., Shi Q., Hjelmeland A.B., Dewhirst M.W., Bigner D.D., Rich J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 142.O’Brien C.A., Pollett A., Gallinger S., Dick J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 143.Blanpani C., Mohrin M., Sotiropoulou P.A., Passegue E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8:16–29. doi: 10.1016/j.stem.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 144.Shimozato O., Waraya M., Nakashima K., Souda H., Takiguchi N., Yamamoto H., Takenobu H., Uehara H., Ikeda E., Matsushita S., et al. Receptor-type protein tyrosine phosphatase kappa directly dephosphorylates CD133 and regulates downstream AKT activation. Oncogene. 2015;34:1949–1960. doi: 10.1038/onc.2014.141. [DOI] [PubMed] [Google Scholar]
- 145.Ma S., Lee T.K., Zheng B.J., Chan K.W., Guan X.Y. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 146.Takenobu H., Shimozato O., Nakamura T., Ochiai H., Yamaguchi Y., Ohira M., Nakagawara A., Kamijo T. CD133 suppresses neuroblastoma cell differentiation via signal pathway modification. Oncogene. 2011;30:97–105. doi: 10.1038/onc.2010.383. [DOI] [PubMed] [Google Scholar]
- 147.Wei Y., Jiang Y., Zou F., Liu Y., Wang S., Xu N., Xu W., Cui C., Xing Y., Liu Y., et al. Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proc. Natl. Acad. Sci. USA. 2013;110:6829–6834. doi: 10.1073/pnas.1217002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Su B., Gao L., Baranowski C., Gillard B., Wang J., Ransom R., Ko H.K., Gelman I.H. A genome-wide RNAi screen identifies FOXO4 as a metastasis-suppressor through counteracting PI3K/AKT signal pathway in prostate cancer. PLoS ONE. 2014;9:e101411. doi: 10.1371/journal.pone.0101411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sase T., Suzuki T., Miura K., Shiiba K., Sato I., Nakamura Y., Takagi K., Onodera Y., Miki Y., Watanabe M., et al. Runt-related transcription factor 2 in human colon carcinoma: A potent prognostic factor associated with estrogen receptor. Int. J. Cancer. 2012;131:2284–2293. doi: 10.1002/ijc.27525. [DOI] [PubMed] [Google Scholar]
- 150.Fujita T., Azuma Y., Fukuyama R., Hattori Y., Yoshida C., Koida M., Ogita K., Komori T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J. Cell Biol. 2004;166:85–95. doi: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Goodell M.A., Brose K., Paradis G., Conner A.S., Mulligan R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kondo T., Setoguchi T., Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc. Natl. Acad. Sci. USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vassilev L.T., Vu B.T., Graves B., Carvajal D., Podlaski F., Filipovic Z., Kong N., Kammlott U., Lukacs C., Klein C., et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 154.Tovar C., Rosinski J., Filipovic Z., Higgins B., Kolinsky K., Hilton H., Zhao X., Vu B.T., Qing W., Packman K., et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: Implications for therapy. Proc. Natl. Acad. Sci. USA. 2006;103:1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bykov V.J., Issaeva N., Shilov A., Hultcrantz M., Pugacheva E., Chumakov P., Bergman J., Wiman K.G., Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat. Med. 2002;8:282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 156.Bykov V.J., Issaeva N., Zache N., Shilov A., Hultcrantz M., Bergman J., Selivanova G., Wiman K.G. Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J. Biol. Chem. 2005;280:30384–30391. doi: 10.1074/jbc.M501664200. [DOI] [PubMed] [Google Scholar]
- 157.Sugimoto H., Nakamura M., Yoda H., Hiraoka H., Shinohara K., Sang M., Fujiwara K., Shimozato O., Nagase H., Ozaki T. Silencing of RUNX2 enhances gemcitabine sensitivity of p53-deficient human pancreatic cancer AsPC-1 cells through the stimulation of TAp63-mediated cell death. Cell Death Discov. 2015 doi: 10.1038/cddiscovery.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zorde Khvalevsky E., Gabai R., Rachmut I.H., Horwitz E., Brunschwig Z., Orbach A., Shemi A., Golan T., Domb A.J., Yavin E., et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2013;100:20723–20728. doi: 10.1073/pnas.1314307110. [DOI] [PMC free article] [PubMed] [Google Scholar]