Abstract
Post-transcriptional mechanisms play critical roles in the control of gene expression during neuronal development and maturation as they allow for faster responses to environmental cues and provide spatially-restricted compartments for local control of protein expression. These mechanisms depend on the interaction of cis-acting elements present in the mRNA sequence and trans-acting factors, such as RNA-binding proteins (RBPs) and microRNAs (miRNAs) that bind to those cis-elements and regulate mRNA stability, subcellular localization, and translation. Recent studies have uncovered an unexpected complexity in these interactions, where coding and non-coding RNAs, termed competing endogenous RNAs (ceRNAs), compete for binding to miRNAs. This competition can, thereby, control a larger number of miRNA target transcripts. However, competing RNA networks also extend to competition between target mRNAs for binding to limited amounts of RBPs. In this review, we present evidence that competitions between target mRNAs for binding to RBPs also occur in neurons, where they affect transcript stability and transport into axons and dendrites as well as translation. In addition, we illustrate the complexity of these mechanisms by demonstrating that RBPs and miRNAs also compete for target binding and regulation.
Keywords: RNA-binding proteins, HuD, KSRP, ZBP1 (IMP1), target transcripts, neurons
1. Introduction
1.1. Post-Transcriptional Regulation
Gene expression is a complex and tightly-regulated process. In addition to transcription, post-transcriptional mechanisms, such as mRNA processing, stability, transport, and local translation, are critical for controlling gene expression. Of interest to this review, mechanisms that influence mRNA stability are particularly important for neural genes, as a great number are regulated in this manner. Specific RNA-binding proteins (RBPs), both stabilizing and destabilizing factors, and to some degree microRNAs (miRNAs), which generally function as destabilizers, mediate the stability and, therefore, determine the half-life of a large number of mRNAs. The half-life of a particular mRNA depends on sequences in the transcript itself, generally located in the 3' UTR of the mRNA, as well as the RBPs that bind to these sequences [1,2,3]. Some mRNAs are unstable, with a very short half-life while others are quite stable with very long half-lives [4]. The most studied instability-conferring sequence is the Adenylate-Uridylate Rich Element (ARE) [5,6]. AREs were initially identified in oncogenes, immediate early genes, and cytokines, but they are now recognized across multiple gene families. In fact, a recent database of ARE-containing mRNAs (ARED) [7] lists over 1500 mRNAs. Since the ARED is limited to mRNAs containing canonical ARE sequences, this database represents only a fraction of the total number of unstable mRNAs. Nevertheless, this information has proven very useful as shown by a recent study demonstrating the post-transcriptional regulation of many of the mRNAs listed in ARED [8].
1.2. mRNA Stability in Neurons
About 20% of all neuronal mRNAs in the mouse forebrain contain ARE sequences in their 3' UTR or interact with ARE-binding proteins (ARE-BPs), such as HuD [9], suggesting that these transcripts may be subjected to similar post-transcriptional regulation. In fact, studies from our group and others have demonstrated that HuD stabilizes not only the mRNA for the neuronal GAP-43 protein but also many other neuronal transcripts (for review see [10]). Furthermore, besides GAP-43, many ARE-containing mRNAs listed in the ARED database are expressed in the nervous system, such as the dopamine D1 receptor, SNAP-25, and FMR1 [7,8]. Using RNA immunoprecipitation followed by DNA microarray (RIP-Chip) analyses, Keene’s group identified potential targets of HuB in neuronally-differentiated P19 cells including integrin β, cyclins A2, B1, C, D1 and D2, IGF-2, neurofilament-M, zif268, TGF β, N-cadherin, c-myc, and N-myc mRNAs [11]. In subsequent studies by the same group, other Hu proteins, HuR and HuD, were shown to interact with CaMKIIα, Neuritin, Homer 1, Actin, and Neuroligin 1 and 3 in an activity-dependent manner [12]. Likewise, using affinity chromatography, Malter and co-workers were able to isolate multiple HuR-bound mRNAs from hippocampal tissue post-seizure including APP, MBP, PLP, Bcl2, neurogranin, calmodulin, SV2a, doublecortin, PKC gamma, calcineurin, glutamate NR1 receptor, SCG-10, syntaxin, VAMP-1, and β-catenin [13]. Finally, using a combination of RIP and recombinant protein pull-downs, we identified a large number of HuD target transcripts from mouse forebrain and characterized the recognition motifs and biological pathways enriched with these transcripts [9]. Consistent with the known functions of HuD, gene ontology analyses revealed that its targets are enriched in signaling pathways involved in neuronal differentiation and that many of these mRNAs encode other RBPs, translation factors, and actin-binding proteins. Altogether, these studies suggest that post-transcriptional regulation by changes in mRNA stability may be more widespread in the nervous system than previously thought.
1.3. Mechanisms Controlling mRNA Stability
In mammalian cells, deadenylation is the first step in the degradation of mRNAs containing ARE sequences followed by either 3'–5' exonucleolytic cleavage or decapping and 5' to 3' decay [14]. Like protein degradation, mRNA degradation is a complex process involving several steps and multiprotein complexes, such as the exosome, which is responsible for the 3' to 5' degradation pathway [15,16,17,18]. Alternatively, ARE-containing mRNAs can be degraded via decapping and 5'–3' exonuclease digestion [19,20]. Some RBPs, such as Hu proteins, increase the stability of mRNAs they interact with, whereas others such as TTP, AUF1, and KSRP, act as destabilizing factors [21,22,23]. Furthermore, it is now clear that mRNA degradation is not a default pathway but is regulated by specific extracellular signals [8,24,25,26,27].
Genes regulated by mRNA stability belong to different functional families, from transcription factors and structural proteins to membrane receptors and signaling molecules. After transcription, there are many possible fates for each mRNA and stabilization of an mRNA can synergistically increase the amount of the transcript available for translation. Efficiency of translation can also be regulated to fine tune the amount of protein derived from an mRNA. The combination of transcriptional and posttranscriptional regulation enables not only faster responses but also more flexibility in the control of gene expression in different neuronal populations.
In this review, we will focus on a few RNA-binding proteins that are key players during neuronal development and describe their competitive interactions with each other, their mRNA targets, and miRNAs.
2. RNA-Binding Proteins
2.1. HuD and Other Members of ELAV-Like/Hu Protein Family
Hu proteins comprise a family of RBPs that were first identified as the targets of autoantibodies found in patients with paraneoplastic encephalomyelitis [28]. These proteins are homologs of embryonic lethal abnormal vision (ELAV), a Drosophila RNA-binding protein whose deletion was found to be lethal for flies [29]. There is one ELAV protein in Drosophila, however, four mammalian ELAV-like Hu proteins have been identified, including HuR (also known as HuA), HuB (also known as Hel-N1), HuC, and HuD. Three of these proteins (HuB, HuC, and HuD) are developmentally regulated and expressed in neurons [30]. The fourth, HuR, is expressed in multiple tissues. At the molecular level, all four ELAV-like Hu proteins contain three RNA recognition motifs (RRMs), a highly-conserved 80 amino acid region that was first recognized in splicing factors and poly(A)-binding proteins [31]. Hu proteins bind preferentially to AREs found in the 3'UTRs of many mRNAs involved in cell growth and differentiation [21,32].
In higher vertebrates, Hu proteins are expressed in early development and represent one of the earliest markers expressed in neurons [33]. HuR is the first protein to be expressed in chicken embryos where it is thought to be involved in cell proliferation [34]. HuR is mainly nuclear and shuttles to the cytosol [35,36]. HuC is present in both nuclear and cytosolic fraction while HuB and HuD are primarily cytosolic [37,38,39,40,41]. The expression of HuD coincides with the earliest stages of neuronal differentiation and is maintained through the maturation of neurons [34]. Similar types of expression patterns have been observed in the developing mouse [30] and rat [42]. The involvement of Hu proteins in different stages of neuronal differentiation was confirmed by overexpression and knock-out studies. Overexpression of HuB, HuC or HuD in PC12 cells and in vivo resulted in an increased rate of neuronal differentiation [38,41,43,44,45]. Down-regulation of these proteins in neural cell lines resulted in the opposite phenotype, with cells failing to grow neurites [44,46]. Likewise, HuD KO mice showed increased proliferation of neural precursors but decreased neuronal differentiation and decreased dendritic complexity [47,48]. HuC is also important for proper neuronal maturation as the KO mice were found to have increased brain glutamate levels and exhibit spontaneous seizures [49].
Although Hu proteins were initially described as early markers of development in certain mature neurons, significant levels of Hu proteins persist throughout life, particularly in the cortex and hippocampus. As shown in Bolognani et al. (2004), HuD protein is present in the soma and dendrites of pyramidal cells in the hippocampus and neocortex in close association with polysomes [39]. In contrast, HuD protein is not detected in the mature granule cells [40]. The spatial pattern of HuB expression is similar to that of HuD but distinct from HuC, which is normally expressed at high levels in dentate granule cells [30]. The function of these proteins in mature neurons is not well understood. The findings that HuD protein expression is increased during learning and memory [39,50,51] and after epileptic activity [12,52] suggest that these proteins may also play an important role in mechanisms of synaptic plasticity. Furthermore, genetic manipulations of HuD protein levels in adult mice result in similar learning and memory deficits as demonstrated by cognitive impairment in HuD overexpressing (HuD OE) or KO mice [48,53]. Altogether, these results support the notion that Hu proteins play a critical role in nervous system development and emphasize that HuD levels need to be tightly regulated for proper brain function.
Recently, two reports have suggested that HuD binding to 3' UTR sequences promotes the translation of mRNAs by interacting with cap-binding eIF4A proteins and the poly (A) tail [54,55]. Consistent with the role of poly (A) tail length in mRNA stability, we have previously shown that HuD preferentially binds and stabilizes capped GAP-43 mRNAs with long poly (A) tails [56]. In addition, HuD may relieve miRNA-mediated repression, which promotes dissociation of eIF4A proteins, by restoring the interaction between the mRNA cap and the poly (A) tail [55]. HuD may also exert an additional function in the control of localized mRNAs, as transported RNA-protein complexes are translationally suppressed and HuD has also been linked to transport and localization of neuronal mRNAs. For example, the cis-element required for GAP-43 mRNA localization into PNS axons was mapped to the ARE/HuD binding site in its 3' UTR [57]. HuD has also been implicated in axonal localization of other neuronal mRNAs including Tau, Neuritin/CPG15, Kv.1.1 and CaMKIIα mRNAs [58,59,60,61]. Since the majority of these HuD targets are locally translated in response to specific signaling mechanisms such as those involving mTOR and PKC [60,61,62], it will be critical to determine how HuD’s function can switch from stabilizing and targeting bound mRNAs to facilitating their translation into proteins.
2.2. KSRP
KSRP (K homology Splicing Regulatory Protein) was originally identified as an RNA-binding protein that enhances retention of a neuronal-specific exon in the c-src mRNA [63]. This protein is also known as FBP-2, a member of the Far upstream Binding Protein (FBP) family identified for their binding to the c-myc enhancer. In addition to being localized in the nucleus, KSRP is present in the cytoplasm where it binds to several unstable ARE-containing mRNAs promoting their degradation via an exosome-mediated pathway [17,25,64]. KSRP phosphorylation by p38 decreases the binding of this RNA-binding protein to its targets, suggesting that mRNA degradation is not a default pathway but it is regulated by specific extracellular signals [24,25,26,27]. KSRP is expressed in the nervous system in both neurons and glia [65,66,67] and is a homolog of chicken ZBP-2, a protein that is required for localizing β-actin mRNA to growth cones [68] and rat MARTA-1, a protein that transports MAP-2 mRNA to dendrites [69]. Additionally, KSRP has been shown to bind to the survival motor neuron (SMN) protein and localize to motoneuron axons [70].
KSRP is best characterized as a destabilizing factor for mRNAs, as it recruits the exosome to induce their degradation [17,64]. This has been most extensively studied in the immune system, and more recently in astrocytes, where KSRP promotes the decay of interleukins, cytokines and inducible nitric oxide (iNOS) mRNAs [22,67]. Despite the fact that KSRP is expressed at high levels in neurons [71], the function of KSRP in these cells remains poorly understood. Our recently published work shows a direct role for KSRP in the control of neuronal mRNA stability and rate of axonal outgrowth [72].
In addition to promoting mRNA decay, KSRP has also been shown to promote the biogenesis of a subset of miRNAs, such as let-7a and miR-1 [73]. DNA damage induces the phosphorylation of KSRP by ATM, resulting in increased processing of particular miRNAs [74]. Furthermore, KSRP and hnRNPA1 act antagonistically in regulating the biogenesis of let-7a [75].
2.3. ZBP1/IMP1
In addition to ARE sequences, many mRNAs contain cis-elements in their 3' UTRs that confer subcellular localization through interaction with RBPs. The 54 nucleotide cis-element in β-actin’s 3' UTR that confers localization to dendrites and axons has been termed a “zipcode”. Zipcode binding protein 1 (ZBP1) was first identified as an mRNA transport protein binding to the chicken β-actin 3' UTR zipcode [76]. This protein is the orthologue of the human insulin-like growth factor 2 mRNA-binding protein 1 (IMP-1), which was identified as a RNA-binding protein associated with tau mRNA in axons [58]. Similar to the ELAV-like/Hu proteins, ZBP1 has been shown to bind to many other cellular mRNAs [77,78]. ZBP1 and its orthologues have been implicated in many aspects of RNA regulation, including intracellular RNA localization, stability, and translational control [79,80].
3. Competition between mRNAs for Binding to RBPs
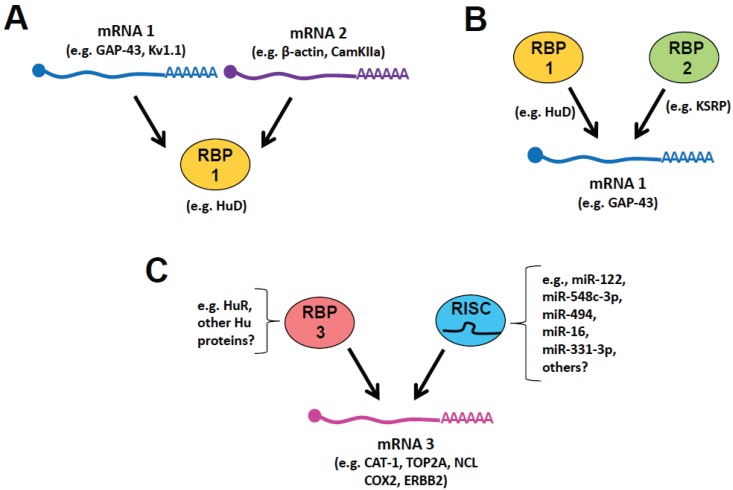
As mentioned above, a significant number of neural mRNAs contain AREs in their 3' UTRs, and they can be bound by various ARE-binding proteins. It is likely that many of these transcripts have similar temporal expression and localization patterns and may, therefore, compete for binding to the same protein. As shown in Figure 1A, two or more mRNAs can compete for the binding of the same RBP. For instance, GAP-43 and β-actin mRNAs compete for binding to ZBP1 that is expressed in limiting quantities in adult neurons [81]. Both transcripts localize into axons; overexpression of the localization element of either β-actin or GAP-43 mRNAs can prevent axonal localization of the other transcript, and increasing ZBP1 protein, rescues this deficit [57,81]. Interestingly, the axonally-synthesized β-actin and GAP-43 proteins generate distinct growth morphologies, with β-actin promoting axonal branching and GAP-43 inducing axonal elongation [82]. In DRG neurons, the GAP-43 gene is transcriptionally induced by axonal injury, but β-actin transcription is not significantly changed; this raises the interesting possibility that the increased levels of endogenous GAP-43 mRNA are able to displace β-actin transcripts from ZBP1, promoting axonal growth and regeneration [57,81]. As noted above, HuD has also been implicated in axonal localization of other neuronal mRNAs [58,59], suggesting that other mRNAs may compete for interaction with HuD in axons.
Figure 1.
Examples of competing RBP target interactions: (A) mRNA1 and mRNA 2 compete for binding to RBP1; (B) RBP1 competes with RBP2 for binding to mRNA 1; and (C) RBP1 competes with the miRNA-associated RISC complex for binding to mRNA 3. Examples of the mRNAs, RBPs, and miRNAs associated with these three types of competitive interactions are indicated in each figure (see text for further details).
Another interesting example of target mRNA competition for binding to HuD is the interaction involving the competition of Kv1.1 and CamKIIα mRNAs for this RBP [60]. Expression of Kv1.1, an ion channel that is locally translated in neuronal processes, is controlled by miR-129 and HuD in a mTORC1 kinase-dependent manner. When mTORC1 is active, miR-129 represses Kv1.1 translation, and when mTORC1 is inhibited, HuD binds to and promotes Kv1.1 translation. HuD binding to Kv1.1 occurs as a result of the degradation of other high-affinity targets of HuD, such as CamKIIα, GAP-43, and Homer 1a, when mTOR is inactive. This was further demonstrated by the finding that overexpression of CamKIIα 3' UTR prevented HuD-mediated increases in Kv1.1 translation. Thus, in this instance, several mRNAs compete for binding of HuD, and HuD competes with miR-129 for binding to Kv1.1 mRNA [60].
4. Competition vs. Cooperation of RBPs
Neuronal ARE-BPs, such as HuD, ZBP1, and KSRP regulate many mRNAs and may compete with one or more proteins for the binding and regulation of their common targets (Figure 1B). There is some evidence for this competition, especially between proteins with antagonistic functions, such as those that affect RNA stability/instability. For example, KSRP’s binding to GAP-43 ARE serves to destabilize the mRNA resulting in its degradation [72]. As HuD stabilizes GAP-43 mRNA and promotes axonal outgrowth, we performed a competitive binding assay and showed that KSRP was able to displace HuD from the GAP-43 mRNA [71]. Overexpression of KSRP inhibited axonal outgrowth of hippocampal neurons, and this effect was reversed by overexpression of GAP-43 or a chimeric construct containing the GAP-43 coding region with a heterologous 3' UTR that confers axonal localization. When KSRP was knocked down or the binding sites for KSRP were removed, GAP-43 mRNA levels and axonal outgrowth were both increased [72].
There are also examples of cooperation among RBPs. ZBP1 and HuD bind together to the GAP-43 3' UTR in an RNA-dependent manner [57], suggesting that the binding sites of the proteins are non-overlapping. HuD and ZBP1 also cooperate for binding to tau mRNA [58]. This is different from the competitive binding of HuD and KSRP to GAP-43 mRNA [72] or the competition of HuD and ZBP1 for binding overlapping, yet distinct, domains in the β-actin mRNA zipcode [83]. Not surprisingly, the different modes of RBP interactions, competitive vs. cooperative, depend on whether these proteins bind overlapping vs. non-overlapping elements that allow both proteins to bind simultaneously to the target transcript such as in the case of HuD and ZPB1 for GAP-43 and tau.
5. Competition vs. Cooperation between RBPs and miRNAs
miRNAs are 22nt single-stranded RNAs that hybridize to complementary sequences primarily in the 3' UTR of mRNAs. This interaction most often serves to either prevent ribosome translation or destabilize the mRNA with the end result being a decrease in protein translation. A recent study indicates that the predominant effect of miRNA binding is mRNA destabilization [84], although depending on the context and complementarity, some miRNAs seem to preferentially act via translational repression [85]. miRNAs function in the context of a ribonucleoprotein complex called RNA-induced silencing complex (RISC), which includes the Argonaute (Ago) proteins. It is easy to imagine that of the approximately 2000 miRNAs that have so far been identified in the human genome, some of them could target similar sequences as RBPs and hinder RBP function (Figure 1C). Indeed, genome-wide analyses show that 3' UTR motifs that are recognized by both ARE-BPs and miRNAs are found on the same transcripts and may overlap [86,87].
As stated above, Hu proteins most often function as stabilizing factors, and there are several instances in the literature demonstrating competition between HuR and different miRNAs for control of gene expression. As a first example, HuR stabilizes the cationic acid transporter 1 (CAT1) mRNA and competes with miR-122 for control of its expression under stress-related conditions [88]. In Huh-7 hepatoma cells, HuR shuttles from the nucleus and relieves miR-122-mediated repression, resulting in the relocation of CAT1 mRNA from P-bodies to polysomes [88]. HuR also promotes the translation of topoisomerase IIα (TOP2A) mRNA and competes with miR-548c-3p for its expression [89]. In HeLa cells, inhibiting HuR or overexpressing miR-548c-3p alters TOP2A levels and controls the cellular response to the chemotherapeutic agent doxorubicin [89]. Also in HeLa cells, HuR stabilizes nucleolin (NCL) mRNA and relieves repression mediated by miR-494 [90]. In prostate cancer cells, HuR was shown to compete with miR-331-3p for regulation of erb-B2 receptor tyrosine kinase 2 (ERBB2) mRNA [91]. HuR also competes with miR-16, and in colorectal cancer cells, these two factors are antagonists in the regulation of cyclooxygenase-2 (COX2) mRNA [92]. HuR was shown to directly bind miR-16 in vitro and inhibit its activity in these transformed cells [92]. Conversely, miR-16 can prevent the HuR-mediated up-regulation of cyclin E1 in breast cancer cells [93].
In addition to the competition of miRNAs and ARE-BPs, there are a few examples in which these molecules cooperate to repress gene expression. One example is the ARE-targeting miR-16, which contains a sequence complementary to the ARE sequence. miR-16 promotes degradation of multiple mRNAs through its association with the ARE sequence, which requires a physical interaction between RISC and TTP, a destabilizing ARE-BP [94]. Another example of the cooperative activity between an RBP and a miRNA is HuR and let-7, in which HuR uncharacteristically recruits RISC/let-7 to an adjacent site on the c-myc mRNA, inhibiting its expression in HeLa cells [95]. Similarly, HuR and miR-19 cooperate to repress RhoB expression in HaCaT cells exposed to ultraviolet radiation [96]. One possible explanation for this unexpected effect of HuR is that binding of this RBP changes the conformation of the mRNA, helping unmask new miRNA binding sites [95,96]. A more detailed discussion of the above interactions can be found in the excellent review by Jiang and Coller [97]. Additionally, miRNAs could bring two non-contiguous sequences together creating new stem loops and RBP binding sites [98].
Since other Hu proteins have similar binding motifs to HuR, it is reasonable to think that these competitive and cooperative interactions may occur in neural cell types where HuR is less abundant but the other Hu proteins are highly expressed. Indeed, we showed that miR-129 can repress Kv1.1 mRNA in neurons and this repression can be reversed by HuD [60]. We previously identified three major HuD-binding motifs that are comprised of CU-rich (motif 1), GU-rich (motif 2), or AU-rich (motif 3) sequences [9]. Several miRNAs, such as miR-149-3p, miR-495-3p, and miR-590-3p, contain seed sequences that are complementary to these motifs, respectively, and could, therefore, be competitors of HuD under specific conditions. Finally, it is important to consider that miRNAs can directly affect the levels of RBPs such as in the case of miR-16 for HuR [99], miR-375 for HuD [100], and miR-206 for KSRP [101].
6. Conclusions
The competing RNA-protein and mRNA-miRNA networks outlined here have far reaching implications for regulation of gene expression in the nervous system. Several elements contribute to the competition within these networks, including temporal expression of the mRNA, miRNA, and RBPs, localized concentrations of these factors, binding/interaction site availabilities, and binding affinities. Thus, identifying miRNAs that could compete with RBPs may be informed by bioinformatics but cannot completely predict interactions that must take into account cell tropism, temporal expression, subcellular localization and other factors. With miRNAs and RBPs localizing into axons and dendrites [102,103,104], there is a high probability that these molecules compete for binding to targets in these processes, and new competitions could arise locally based on subcellular stoichiometry of different mRNAs, miRNAs, and RBPs. Undoubtedly, more genes are yet to be identified as shared targets of both miRNAs and RBPs, as well as shared targets of different RBPs. Uncovering the biological relevance of these targets will undoubtedly be facilitated by advances in bioinformatics, transcriptomics, and proteomics methodologies. However, heed must also be given to the physiological and cellular contexts of interactions, and unraveling these intricacies will likely require lower throughput cellular and molecular analyses. The highly regulated interactions between RBPs and their target sequences have been shown to contribute to the control of gene expression in a wide variety of physiological and pathological conditions from immune processes and cancer transformation [21] to muscle differentiation [26,27] and, importantly, to neural development and plasticity [105,106,107].
Acknowledgments
Support for the research from the authors’ laboratories reviewed here was provided by NIH NS030255, DA034097 and DA034452 to NPB and NS041596, US Army Medical Research Program (W81XWH-13-1-0308), and the Miriam Adelson and Sheldon G. Adelson Medical Research Foundation to Jeffery L. Twiss.
Author Contributions
Amy S. Gardiner, Jeffery L. Twiss and Nora I. Perrone-Bizzozero wrote the manuscript. Amy S. Gardiner and Nora I. Perrone-Bizzozero prepared the figure and NPB designed the graphical abstract.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wilusz C.J., Wormington M., Peltz S.W. The cap-to-tail guide to mRNA turnover. Nat. Rev. Mol. Cell Biol. 2001;2:237–246. doi: 10.1038/35067025. [DOI] [PubMed] [Google Scholar]
- 2.Keene J.D. RNA regulons: Coordination of post-transcriptional events. Nat. Rev. Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 3.Wu X., Brewer G. The regulation of mRNA stability in mammalian cells: 2.0. Gene. 2012;500:10–21. doi: 10.1016/j.gene.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross J. mRNA stability in mammalian cells. Microbiol. Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen C.Y., Shyu A.B. AU-rich elements: Characterization and importance in mRNA degradation. Trends Biochem. Sci. 1995;20:465–470. doi: 10.1016/S0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 7.Bakheet T., Frevel M., Williams B.R., Greer W., Khabar K.S. ARED: Human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 2001;29:246–254. doi: 10.1093/nar/29.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frevel M.A., Bakheet T., Silva A.M., Hissong J.G., Khabar K.S., Williams B.R. p38 Mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol. Cell Biol. 2003;23:425–436. doi: 10.1128/MCB.23.2.425-436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolognani F., Contente-Cuomo T., Perrone-Bizzozero N.I. Novel recognition motifs and biological functions of the RNA-binding protein HuD revealed by genome-wide identification of its targets. Nucleic Acids Res. 2010;38:117–130. doi: 10.1093/nar/gkp863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolognani F., Perrone-Bizzozero N.I. RNA-protein interactions and control of mRNA stability in neurons. J Neurosci. Res. 2008;86:481–489. doi: 10.1002/jnr.21473. [DOI] [PubMed] [Google Scholar]
- 11.Tenenbaum S.A., Carson C.C., Lager P.J., Keene J.D. Identifying mRNA subsets in messenger ribonucleoprotein complexes by using cDNA arrays. Proc. Natl. Acad. Sci. USA. 2000;97:14085–14090. doi: 10.1073/pnas.97.26.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiruchinapalli D.M., Ehlers M.D., Keene J.D. Activity-dependent expression of RNA binding protein HuD and its association with mRNAs in neurons. RNA Biol. 2008;5:157–168. doi: 10.4161/rna.5.3.6782. [DOI] [PubMed] [Google Scholar]
- 13.Westmark C.J., Gourronc F.A., Bartleson V.B., Sayin U., Bhattacharya S., Sutula T., Malter J.S. HuR mRNA ligands expressed after seizure. J. Neuropathol. Exp. Neurol. 2005;64:1037–1045. doi: 10.1097/01.jnen.0000189835.71574.e1. [DOI] [PubMed] [Google Scholar]
- 14.Chen C.Y., Shyu A.B. Mechanisms of deadenylation-dependent decay. Wiley Interdiscip. Rev. RNA. 2011;2:167–183. doi: 10.1002/wrna.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell P., Petfalski E., Shevchenko A., Mann M., Tollervey D. The exosome: A conserved eukaryotic RNA processing complex containing multiple 3'→5' exoribonucleases. Cell. 1997;91:457–466. doi: 10.1016/S0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- 16.Van Hoof A., Parker R. The exosome: A proteasome for RNA? Cell. 1999;99:347–350. doi: 10.1016/S0092-8674(00)81520-2. [DOI] [PubMed] [Google Scholar]
- 17.Chen C.Y., Gherzi R., Ong S.E., Chan E.L., Raijmakers R., Pruijn G.J., Stoecklin G., Moroni C., Mann M., Karin M. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107:451–464. doi: 10.1016/S0092-8674(01)00578-5. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee D., Gao M., O’Connor J.P., Raijmakers R., Pruijn G., Lutz C.S., Wilusz J. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 2002;21:165–174. doi: 10.1093/emboj/21.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoecklin G., Mayo T., Anderson P. ARE-mRNA degradation requires the 5'–3' decay pathway. EMBO Rep. 2006;7:72–77. doi: 10.1038/sj.embor.7400572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenger-Gron M., Fillman C., Norrild B., Lykke-Andersen J. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol. Cell. 2005;20:905–915. doi: 10.1016/j.molcel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Wilusz C.J., Wilusz J. Bringing the role of mRNA decay in the control of gene expression into focus. Trends Genet. 2004;20:491–497. doi: 10.1016/j.tig.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Linker K., Pautz A., Fechir M., Hubrich T., Greeve J., Kleinert H. Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic Acids Res. 2005;33:4813–4827. doi: 10.1093/nar/gki797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gherzi R., Chen C.Y., Trabucchi M., Ramos A., Briata P. The role of KSRP in mRNA decay and microRNA precursor maturation. Wiley Interdiscip. Rev. RNA. 2010;1:230–239. doi: 10.1002/wrna.2. [DOI] [PubMed] [Google Scholar]
- 24.Briata P., Chen C.Y., Ramos A., Gherzi R. Functional and molecular insights into KSRP function in mRNA decay. Biochim. Biophys. Acta. 2013;1829:689–694. doi: 10.1016/j.bbagrm.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Briata P., Ilengo C., Corte G., Moroni C., Rosenfeld M.G., Chen C.Y., Gherzi R. The Wnt/beta-catenin→Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs. Mol. Cell. 2003;12:1201–1211. doi: 10.1016/S1097-2765(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 26.Briata P., Forcales S.V., Ponassi M., Corte G., Chen C.Y., Karin M., Puri P.L., Gherzi R. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol. Cell. 2005;20:891–903. doi: 10.1016/j.molcel.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Amirouche A., Tadesse H., Lunde J.A., Belanger G., Cote J., Jasmin B.J. Activation of p38 signaling increases utrophin A expression in skeletal muscle via the RNA-binding protein KSRP and inhibition of AU-rich element-mediated mRNA decay: Implications for novel DMD therapeutics. Hum. Mol. Genet. 2013;22:3093–3111. doi: 10.1093/hmg/ddt165. [DOI] [PubMed] [Google Scholar]
- 28.Dalmau J., Furneaux H.M., Gralla R.J., Kris M.G., Posner J.B. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer—A quantitative western blot analysis. Ann. Neurol. 1990;27:544–552. doi: 10.1002/ana.410270515. [DOI] [PubMed] [Google Scholar]
- 29.Robinow S., Campos A.R., Yao K.M., White K. The elav gene product of Drosophila, required in neurons, has three RNP consensus motifs. Science. 1988;242:1570–1572. doi: 10.1126/science.3144044. [DOI] [PubMed] [Google Scholar]
- 30.Okano H.J., Darnell R.B. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J. Neurosci. 1997;17:3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenan D.J., Query C.C., Keene J.D. RNA recognition: Towards identifying determinants of specificity. Trends Biochem. Sci. 1991;16:214–220. doi: 10.1016/0968-0004(91)90088-D. [DOI] [PubMed] [Google Scholar]
- 32.Keene J.D. Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc. Natl. Acad. Sci. USA. 1999;96:5–7. doi: 10.1073/pnas.96.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marusich M.F., Furneaux H.M., Henion P.D., Weston J.A. Hu neuronal proteins are expressed in proliferating neurogenic cells. J. Neurobiol. 1994;25:143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- 34.Wakamatsu Y., Weston J.A. Sequential expression and role of Hu RNA-binding proteins during neurogenesis. Development. 1997;124:3449–3460. doi: 10.1242/dev.124.17.3449. [DOI] [PubMed] [Google Scholar]
- 35.Peng S.S.-Y., Chen C.-Y.A., Xu N., Shyu A.-B. RNA stabilization of the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998;17:3461–3470. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan X.C., Steitz J.A. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. PNAS. 1998;95:15293–15298. doi: 10.1073/pnas.95.26.15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinman M.N., Zhou H.L., Sharma A., Lou H. All three RNA recognition motifs and the hinge region of HuC play distinct roles in the regulation of alternative splicing. Nucleic Acids Res. 2013;41:5049–5061. doi: 10.1093/nar/gkt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson K.D., Sengupta J., Morin M., Neve R.L., Valenzuela C.F., Perrone-Bizzozero N.I. Overexpression of HuD accelerates neurite outgrowth and increases GAP-43 mRNA expression in cortical neurons and retinoic acid-induced embryonic stem cells in vitro. Exp. Neurol. 2001;168:250–258. doi: 10.1006/exnr.2000.7599. [DOI] [PubMed] [Google Scholar]
- 39.Bolognani F., Merhege M.A., Twiss J., Perrone-Bizzozero N.I. Dendritic localization of the RNA-binding protein HuD in hippocampal neurons: Association with polysomes and upregulation during contextual learning. Neurosci. Lett. 2004;371:152–157. doi: 10.1016/j.neulet.2004.08.074. [DOI] [PubMed] [Google Scholar]
- 40.Bolognani F., Tanner D.C., Merhege M., Deschenes-Furry J., Jasmin B., Perrone-Bizzozero N.I. In vivo post-transcriptional regulation of GAP-43 mRNA by overexpression of the RNA-binding protein HuD. J. Neurochem. 2006;96:790–801. doi: 10.1111/j.1471-4159.2005.03607.x. [DOI] [PubMed] [Google Scholar]
- 41.Kasashima K., Terashima K., Yamamoto K., Sakashita E., Sakamoto H. Cytoplasmic localization is required for the mammalian ELAV-like protein HuD to induce neuronal differentiation. Genes Cells. 1999;4:667–683. doi: 10.1046/j.1365-2443.1999.00292.x. [DOI] [PubMed] [Google Scholar]
- 42.Clayton G.H., Perez G.M., Smith R.L., Owens G.C. Expression of mRNA for the ELAV-like neural-specific RNA binding protein, HuD, during nervous system development. Dev. Brain Res. 1998;109:271–280. doi: 10.1016/S0165-3806(98)00074-1. [DOI] [PubMed] [Google Scholar]
- 43.Akamatsu W., Okano H.J., Osumi N., Inoue T., Nakamura S., Sakakibara S., Miura M., Matsuo N., Darnell R.B., Okano H. Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems. Proc. Natl. Acad. Sci. USA. 1999;96:9885–9890. doi: 10.1073/pnas.96.17.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mobarak C.D., Anderson K.D., Morin M., Beckel-Mitchener A., Rogers S.L., Furneaux H., King P., Perrone-Bizzozero N.I. The RNA-binding protein HuD is required for GAP-43 mRNA stability, GAP-43 gene expression, and PKC-dependent neurite outgrowth in PC12 cells. Mol. Biol. Cell. 2000;11:3191–3203. doi: 10.1091/mbc.11.9.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson K.D., Morin M.A., Beckel-Mitchener A., Mobarak C.D., Neve R.L., Furneaux H.M., Burry R., Perrone-Bizzozero N.I. Overexpression of HuD, but not of its truncated form HuD I + II, promotes GAP-43 gene expression and neurite outgrowth in PC12 cells in the absence of nerve growth factor. J. Neurochem. 2000;75:1103–1114. doi: 10.1046/j.1471-4159.2000.0751103.x. [DOI] [PubMed] [Google Scholar]
- 46.Dobashi Y., Shoji M., Wakata Y., Kameya T. Expression of HuD protein is essential for initial phase of neuronal differentiation in rat pheochromocytoma cells. Biochem. Biophys. Res. Comm. 1998;244:226–229. doi: 10.1006/bbrc.1998.8247. [DOI] [PubMed] [Google Scholar]
- 47.Akamatsu W., Fujihara H., Mitsuhashi T., Yano M., Shibata S., Hayakawa Y., Okano H.J., Sakakibara S., Takano H., Takano T., et al. The RNA-binding protein HuD regulates neuronal cell identity and maturation. Proc. Natl. Acad. Sci. USA. 2005;102:4625–4630. doi: 10.1073/pnas.0407523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeBoer E.M., Azevedo R., Vega T.A., Brodkin J., Akamatsu W., Okano H., Wagner G.C., Rasin M.R. Prenatal deletion of the RNA-binding protein HuD disrupts postnatal cortical circuit maturation and behavior. J. Neurosci. 2014;34:3674–3686. doi: 10.1523/JNEUROSCI.3703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ince-Dunn G., Okano H.J., Jensen K.B., Park W.Y., Zhong R., Ule J., Mele A., Fak J.J., Yang C., Zhang C., et al. Neuronal Elav-like (Hu) proteins regulate RNA splicing and abundance to control glutamate levels and neuronal excitability. Neuron. 2012;75:1067–1080. doi: 10.1016/j.neuron.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quattrone A., Pascale A., Nogues X., Zhao W., Gusev P., Pacini A., Alkon D.L. Posttranscriptional regulation of gene expression in learning by the neuronal ELAV-like mRNA-stabilizing proteins. Proc. Natl. Acad. Sci. USA. 2001;98:11668–11673. doi: 10.1073/pnas.191388398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pascale A., Gusev P.A., Amadio M., Dottorini T., Govoni S., Alkon D.L., Quattrone A. Increase of the RNA-binding protein HuD and posttranscriptional up-regulation of the GAP-43 gene during spatial memory. Proc. Natl. Acad. Sci. USA. 2004;101:1217–1222. doi: 10.1073/pnas.0307674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolognani F., Tanner D.C., Nixon S., Okano H.J., Okano H., Perrone-Bizzozero N.I. Coordinated expression of HuD and GAP-43 in hippocampal dentate granule cells during developmental and adult plasticity. Neurochem. Res. 2007;32:2142–2151. doi: 10.1007/s11064-007-9388-8. [DOI] [PubMed] [Google Scholar]
- 53.Bolognani F., Qiu S., Tanner D.C., Paik J., Perrone-Bizzozero N.I., Weeber E.J. Associative and spatial learning and memory deficits in transgenic mice overexpressing the RNA-binding protein HuD. Neurobiol. Learn. Mem. 2007;87:635–643. doi: 10.1016/j.nlm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Fukao A., Sasano Y., Imataka H., Inoue K., Sakamoto H., Sonenberg N., Thoma C., Fujiwara T. The ELAV protein HuD stimulates cap-dependent translation in a Poly(A)- and eIF4A-dependent manner. Mol. Cell. 2009;36:1007–1017. doi: 10.1016/j.molcel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Fukao A., Mishima Y., Takizawa N., Oka S., Imataka H., Pelletier J., Sonenberg N., Thoma C., Fujiwara T. MicroRNAs trigger dissociation of eIF4AI and eIF4AII from target mRNAs in humans. Mol. Cell. 2014;56:79–89. doi: 10.1016/j.molcel.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Beckel-Mitchener A.C., Miera A., Keller R., Perrone-Bizzozero N.I. Poly (A) tail length dependent stabilization of GAP-43 mRNA by the RNA binding protein HuD. J. Biol. Chem. 2002;28:27996–28002. doi: 10.1074/jbc.M201982200. [DOI] [PubMed] [Google Scholar]
- 57.Yoo S., Kim H.H., Kim P., Donnelly C.J., Kalinski A.L., Vuppalanchi D., Park M., Lee S.J., Merianda T.T., Perrone-Bizzozero N.I., et al. A HuD-ZBP1 ribonucleoprotein complex localizes GAP-43 mRNA into axons through its 3' untranslated region AU-rich regulatory element. J. Neurochem. 2013;126:792–804. doi: 10.1111/jnc.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atlas R., Behar L., Elliott E., Ginzburg I. The insulin-like growth factor mRNA binding-protein IMP-1 and the Ras-regulatory protein G3BP associate with tau mRNA and HuD protein in differentiated P19 neuronal cells. J. Neurochem. 2004;89:613–626. doi: 10.1111/j.1471-4159.2004.02371.x. [DOI] [PubMed] [Google Scholar]
- 59.Akten B., Kye M.J., Haole T., Wertz M.H., Singh S., Nie D., Huang J., Merianda T.T., Twiss J.L., Beattie C.E., et al. Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc. Natl. Acad. Sci. USA. 2011;108:10337–10342. doi: 10.1073/pnas.1104928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sosanya N.M., Huang P.P., Cacheaux L.P., Chen C.J., Nguyen K., Perrone-Bizzozero N.I., Raab-Graham K.F. Degradation of high affinity HuD targets releases Kv1.1 mRNA from miR-129 repression by mTORC1. J. Cell Biol. 2013;202:53–69. doi: 10.1083/jcb.201212089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sosanya N.M., Cacheaux L.P., Workman E.R., Niere F., Perrone-Bizzozero N.I., Raab-Graham K.F. Mammalian target of rapamycin (mTOR) tagging promotes dendritic branch variability through the Capture of Ca2+/Calmodulin-dependent protein kinase II alpha (CaMKIIalpha) mRNAs by the RNA-binding protein HuD. J. Biol. Chem. 2015;290:16357–16371. doi: 10.1074/jbc.M114.599399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanevski F., Xu B. HuD interacts with BDNF mRNA and is essential for activity-induced BDNF synthesis in dendrites. PLoS ONE. 2015;10:e0117264. doi: 10.1371/journal.pone.0117264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Min H., Turck C.W., Nikolic J.M., Black D.L. A new regulatory protein, KSRP, mediates exon inclusion through an intronic splicing enhancer. Genes Dev. 1997;11:1023–1036. doi: 10.1101/gad.11.8.1023. [DOI] [PubMed] [Google Scholar]
- 64.Gherzi R., Lee K.Y., Briata P., Wegmuller D., Moroni C., Karin M., Chen C.Y. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol. Cell. 2004;14:571–583. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Snee M., Kidd G.J., Munro T.P., Smith R. RNA trafficking and stabilization elements associate with multiple brain proteins. J. Cell Sci. 2002;115:4661–4669. doi: 10.1242/jcs.00137. [DOI] [PubMed] [Google Scholar]
- 66.Li Q., Lee J.-A., Black D.L. Neuronal regulation of alternative pre-mRNA splicing. Nat. Rev. Neurosci. 2007;8:819–831. doi: 10.1038/nrn2237. [DOI] [PubMed] [Google Scholar]
- 67.Li X., Lin W.J., Chen C.Y., Si Y., Zhang X., Lu L., Suswam E., Zheng L., King P.H. KSRP: A checkpoint for inflammatory cytokine production in astrocytes. Glia. 2012;60:1773–1784. doi: 10.1002/glia.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gu W., Pan F., Zhang H., Bassell G.J., Singer R.H. A predominantly nuclear protein affecting cytoplasmic localization of beta-actin mRNA in fibroblasts and neurons. J. Cell Biol. 2002;156:41–51. doi: 10.1083/jcb.200105133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rehbein M., Wege K., Buck F., Schweizer M., Richter D., Kindler S. Molecular characterization of MARTA1, a protein interacting with the dendritic targeting element of MAP2 mRNAs. J. Neurochem. 2002;82:1039–1046. doi: 10.1046/j.1471-4159.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 70.Tadessse H., Deschenes-Furry J., Boisvenue S., Côté J. KH-type splicing regulatory protein interacts with survival motor neuron protein and is misregulated in spinal muscular atrophy. Hum. Mol. Genet. 2008;17:506–524. doi: 10.1093/hmg/ddm327. [DOI] [PubMed] [Google Scholar]
- 71.Lu J.Y., Schneider R.J. Tissue distribution of AU-rich mRNA-binding proteins involved in regulation of mRNA decay. J. Biol. Chem. 2004;279:12974–12979. doi: 10.1074/jbc.M310433200. [DOI] [PubMed] [Google Scholar]
- 72.Bird C.W., Gardiner A.S., Bolognani F., Tanner D.C., Chen C.Y., Lin W.J., Yoo S., Twiss J.L., Perrone-Bizzozero N. KSRP modulation of GAP-43 mRNA stability restricts axonal outgrowth in embryonic hippocampal neurons. PLoS ONE. 2013;8:e79255. doi: 10.1371/journal.pone.0079255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trabucchi M., Briata P., Garcia-Mayoral M., Haase A.D., Filipowicz W., Ramos A., Gherzi R., Rosenfeld M.G. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature. 2009;459:1010–1014. doi: 10.1038/nature08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X., Wan G., Berger F.G., He X., Lu X. The ATM kinase induces microRNA biogenesis in the DNA damage response. Mol. Cell. 2011;41:371–383. doi: 10.1016/j.molcel.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michlewski G., Caceres J.F. Antagonistic role of hnRNP A1 and KSRP in the regulation of let-7a biogenesis. Nat. Struct. Mol. Biol. 2010;17:1011–1018. doi: 10.1038/nsmb.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross A.F., Oleynikov Y., Kislauskis E.H., Taneja K.L., Singer R.H. Characterization of a beta-actin mRNA zipcode-binding protein. Mol. Cell Biol. 1997;17:2158–2165. doi: 10.1128/MCB.17.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jonson L., Vikesaa J., Krogh A., Nielsen L.K., Hansen T., Borup R., Johnsen A.H., Christiansen J., Nielsen F.C. Molecular composition of IMP1 ribonucleoprotein granules. Mol. Cell Proteom. 2007;6:798–811. doi: 10.1074/mcp.M600346-MCP200. [DOI] [PubMed] [Google Scholar]
- 78.Patel V.L., Mitra S., Harris R., Buxbaum A.R., Lionnet T., Brenowitz M., Girvin M., Levy M., Almo S.C., Singer R.H., et al. Spatial arrangement of an RNA zipcode identifies mRNAs under post-transcriptional control. Genes Dev. 2012;26:43–53. doi: 10.1101/gad.177428.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huttelmaier S., Zenklusen D., Lederer M., Dictenberg J., Lorenz M., Meng X., Bassell G.J., Condeelis J., Singer R.H. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438:512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- 80.Stohr N., Lederer M., Reinke C., Meyer S., Hatzfeld M., Singer R.H., Huttelmaier S. ZBP1 regulates mRNA stability during cellular stress. J. Cell Biol. 2006;175:527–534. doi: 10.1083/jcb.200608071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Donnelly C.J., Willis D.E., Xu M., Tep C., Jiang C., Yoo S., Schanen N.C., Kirn-Safran C.B., van Minnen J., English A., et al. Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 2011;30:4665–4677. doi: 10.1038/emboj.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Donnelly C.J., Park M., Spillane M., Yoo S., Pacheco A., Gomes C., Vuppalanchi D., McDonald M., Kim H.H., Merianda T.T., et al. Axonally synthesized beta-actin and GAP-43 proteins support distinct modes of axonal growth. J. Neurosci. 2013;33:3311–3322. doi: 10.1523/JNEUROSCI.1722-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim H.H., Lee S.J., Gardiner A.S., Perrone-Bizzozero N.I., Yoo S. Different motif requirements for the localization zipcode element of beta-actin mRNA binding by HuD and ZBP1. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eichhorn S.W., Guo H., McGeary S.E., Rodriguez-Mias R.A., Shin C., Baek D., Hsu S.H., Ghoshal K., Villen J., Bartel D.P. mRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues. Mol. Cell. 2014;56:104–115. doi: 10.1016/j.molcel.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee T., Wang N., Houel S., Couts K., Old W., Ahn N. Dosage and temporal thresholds in microRNA proteomics. Mol. Cell Proteom. 2015;14:289–302. doi: 10.1074/mcp.M114.043851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacobsen A., Wen J., Marks D.S., Krogh A. Signatures of RNA binding proteins globally coupled to effective microRNA target sites. Genome Res. 2010;20:1010–1019. doi: 10.1101/gr.103259.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mukherjee N., Corcoran D.L., Nusbaum J.D., Reid D.W., Georgiev S., Hafner M., Ascano M., Tuschl T., Ohler U., Keene J.D. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol. Cell. 2011;43:327–339. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhattacharyya S.N., Habermacher R., Martine U., Closs E.I., Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 89.Srikantan S., Abdelmohsen K., Lee E.K., Tominaga K., Subaran S.S., Kuwano Y., Kulshrestha R., Panchakshari R., Kim H.H., Yang X., et al. Translational control of TOP2A influences doxorubicin efficacy. Mol. Cell Biol. 2011;31:3790–3801. doi: 10.1128/MCB.05639-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tominaga K., Srikantan S., Lee E.K., Subaran S.S., Martindale J.L., Abdelmohsen K., Gorospe M. Competitive regulation of nucleolin expression by HuR and miR-494. Mol. Cell Biol. 2011;31:4219–4231. doi: 10.1128/MCB.05955-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Epis M.R., Barker A., Giles K.M., Beveridge D.J., Leedman P.J. The RNA-binding protein HuR opposes the repression of ERBB-2 gene expression by microRNA miR-331-3p in prostate cancer cells. J. Biol. Chem. 2011;286:41442–41454. doi: 10.1074/jbc.M111.301481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Young L.E., Moore A.E., Sokol L., Meisner-Kober N., Dixon D.A. The mRNA stability factor HuR inhibits microRNA-16 targeting of COX-2. Mol. Cancer Res. 2012;10:167–180. doi: 10.1158/1541-7786.MCR-11-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo X., Connick M.C., Vanderhoof J., Ishak M., Hartley R.S. MicroRNA-16 modulates HuR regulation of cyclin E1 in breast cancer cells. Int. J. Mol. Sci. 2015;16:7112–7132. doi: 10.3390/ijms16047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jing Q., Huang S., Guth S., Zarubin T., Motoyama A., Chen J., di Padova F., Lin S.C., Gram H., Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 95.Kim H.H., Kuwano Y., Srikantan S., Lee E.K., Martindale J.L., Gorospe M. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 2009;23:1743–1748. doi: 10.1101/gad.1812509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glorian V., Maillot G., Poles S., Iacovoni J.S., Favre G., Vagner S. HuR-dependent loading of miRNA RISC to the mRNA encoding the Ras-related small GTPase RhoB controls its translation during UV-induced apoptosis. Cell Death Differ. 2011;18:1692–1701. doi: 10.1038/cdd.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang P., Coller H. Functional interactions between microRNAs and RNA binding proteins. MicroRNA. 2012;1:70–79. doi: 10.2174/2211536611201010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doyle F., Tenenbaum S.A. Trans-regulation of RNA-binding protein motifs by microRNA. Front. Genet. 2014 doi: 10.3389/fgene.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu F., Zhang X., Lei Y., Liu Z., Tong T., Wang W. Loss of repression of HuR translation by miR-16 may be responsible for the elevation of HuR in human breast carcinoma. J. Cell Biochem. 2010;111:727–734. doi: 10.1002/jcb.22762. [DOI] [PubMed] [Google Scholar]
- 100.Abdelmohsen K., Hutchison E.R., Lee E.K., Kuwano Y., Kim M.M., Masuda K., Srikantan S., Subaran S.S., Marasa B.S., Mattson M.P., et al. miR-375 inhibits differentiation of neurites by lowering HuD levels. Mol. Cell Biol. 2010;30:4197–4210. doi: 10.1128/MCB.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amirouche A., Tadesse H., Miura P., Belanger G., Lunde J.A., Cote J., Jasmin B.J. Converging pathways involving microRNA-206 and the RNA-binding protein KSRP control post-transcriptionally utrophin A expression in skeletal muscle. Nucleic Acids Res. 2014;42:3982–3997. doi: 10.1093/nar/gkt1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sasaki Y., Gross C., Xing L., Goshima Y., Bassell G.J. Identification of axon-enriched microRNAs localized to growth cones of cortical neurons. Dev. Neurobiol. 2014;74:397–402. doi: 10.1002/dneu.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Natera-Naranjo O., Aschrafi A., Gioio A.E., Kaplan B.B. Identification and quantitative analyses of microRNAs located in the distal axons of sympathetic neurons. RNA. 2010;18:1516–1529. doi: 10.1261/rna.1833310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schratt G. MicroRNAs at the synapse. Nat. Rev. Neurosci. 2009;10:842–849. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- 105.Perrone-Bizzozero N.I., Bolognani F. Role of HuD and other RNA-binding proteins in neural development and plasticity. J. Neurosci. Res. 2002;68:121–126. doi: 10.1002/jnr.10175. [DOI] [PubMed] [Google Scholar]
- 106.Perrone-Bizzozero N.I., Tanner D.C., Mounce J., Bolognani F. Increased expression of axogenesis-related genes and mossy fiber length in dentate granule cells from adult HuD overexpressor mice. ASN Neuro. 2011;3:259–270. doi: 10.1042/AN20110015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gomes C., Merianda T.T., Lee S.J., Yoo S., Twiss J.L. Molecular determinants of the axonal mRNA transcriptome. Dev. Neurobiol. 2014;74:218–232. doi: 10.1002/dneu.22123. [DOI] [PMC free article] [PubMed] [Google Scholar]