Abstract
Spinal cord injury is associated with adaptations to the muscular, skeletal, and spinal systems. Experimental data are lacking regarding the extent to which rehabilitative methods may influence these adaptations. An understanding of the plasticity of the muscular, skeletal, and spinal systems after paralysis may be important as new rehabilitative technologies emerge in the 21st century. Moreover, individuals injured today may become poor candidates for future scientific advancements (cure) if their neuromusculoskeletal systems are irreversibly impaired. The primary purpose of this paper is to explore the physiological properties of skeletal muscle as a result of spinal cord injury; secondarily, to consider associated changes at the skeletal and spinal levels.
Muscular adaptations include a transformation to faster myosin, increased contractile speeds, shift to the right on the torque-frequency curve, increased fatigue, and enhanced doublet potentiation. These muscular adaptations may be prevented in individuals with acute paralysis and partially reversed in individuals with chronic paralysis. Moreover, the muscular changes may be coordinated with motor unit and spinal circuitry adaptations. Concurrently, skeletal adaptations, as measured by bone mineral density, show extensive loss within the first six months after paralysis. The underlying science governing neuromusculoskeletal adaptations after paralysis will help guide professionals as new rehabilitation strategies evolve in the future.
Keywords: catch-like properties, contractile properties, muscle fatigue, neuromuscular plasticity, spinal reflexes
Spinal cord injury occurs in 10 to 20 thousand individuals each year, causes extensive human suffering, and costs society between 4 and 7 billion dollars annually.41 Much of the cost can be attributed to secondary complications associated with degradation of the musculoskeletal system of the paralyzed extremities. For many appropriate reasons, the muscular and skeletal systems below the level of the spinal cord injury receive limited attention during rehabilitation. Indeed, most rehabilitative strategies involve unloading the paralyzed extremities through wheelchairs and providing passive movement of the paralyzed limbs through stretching and range of motion exercises. Rehabilitative programs advance individuals with spinal cord injury to higher levels of functional independence, which improves their overall well being. However, we must be cognizant that we may be embarking on an era where the goal of preserving the muscular, skeletal, and spinal systems below the level of the lesion rises to a higher level of significance. Accordingly, an understanding of the plasticity of these systems is warranted as we advance rehabilitative treatment techniques into the 21st century.
Experimental data are lacking in humans about the extent to which rehabilitative methods may influence the muscular, skeletal, and spinal systems after spinal cord injury. Because individuals with spinal cord injury approach life spans similar to individuals not physically challenged, healthcare practitioners are able to witness, in their lifetimes, the deleterious effects of adaptations to the muscular, skeletal, and spinal circuitry as a result of paralysis. 41 As importantly, it is conceivable that a cure for spinal cord injury will emerge in the 21st century. However, individuals injured today may be very poor candidates for future scientific advancements if their neuromusculoskeletal systems are irreversibly impaired from adaptations attributable to chronic paralysis. The extent to which rehabilitation specialists can make wise decisions regarding future interventions will rely on a sound scientific understanding of the plasticity of these systems in humans following paralysis.
Fractures in individuals with spinal cord injury reflect an accelerated bone demineralization presumably from the loss of osteogenic loads on the skeletal system.20 Paralyzed muscle atrophies, takes on faster properties, and shows increased fatigue consistent with type IIb muscle fibers.10,22,38 Spasticity, a complex motor disorder characterized by a velocity-dependent increase in muscle resistance to passive stretch, suggests that an altered spinal circuitry develops after spinal cord injury.19 Recent observations in animal models support that coordination exists among muscle fibers, motor neurons, and synaptic transmission.23,24 Moreover, this coordination may depend on retrograde signaling from muscle activity levels.13,24 Hence, the extent to which spinal cord reorganization, muscle plasticity, and bone demineralization covary as the result of disuse following spinal cord injury in humans is important to understand and currently is the focus of our neuromuscular research laboratory. Unlike animal models, where the muscular system can be studied independent of the skeletal system, human models of disuse require that we understand the interactions among the various systems to insure safety to the individual. For example, activating paralyzed skeletal muscle without understanding the extent of bone loss could lead to deleterious fractures for individuals with chronic paralysis.3,32
As the science of rehabilitation continues to evolve, it would seem imperative that practitioners develop a keen understanding of the plasticity of the tissues that their treatments potentially influence. Scientific data outlining the temporal factors associated with adaptations after spinal cord injury in humans are essential to understanding if windows for intervention exist. Moreover, the extent to which a treatment, and its appropriate dose, can influence these adaptations are crucial to making well informed decisions regarding future rehabilitation technology. Accordingly, the purposes for this paper are to explore the physiological properties of skeletal muscle as a result of spinal cord injury and to consider associated changes at the skeletal and spinal levels that may be influenced by changes in muscle activity levels.
SKELETAL ADAPTATIONS FOLLOWING SPINAL CORD INJURY
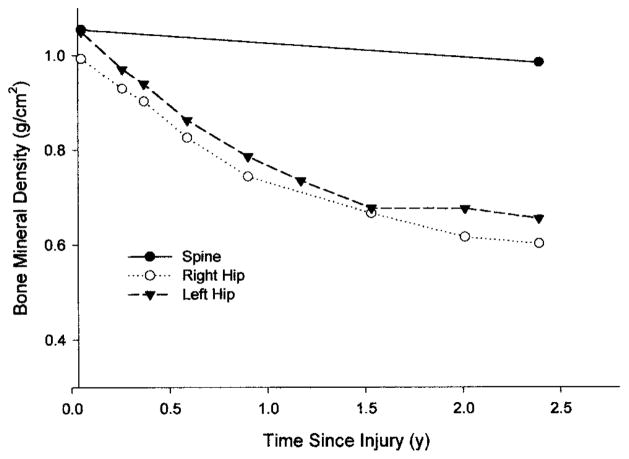
Osteoporosis is a skeletal disease that has a multifactorial pathophysiology; however, the loss of mechanical stimuli to the bone is considered a powerful influence in sustaining bone integrity.20,32 Type I osteoporosis involves the rapid deterioration of the trabecular lattice at a rate of 3–4% each month and is commonly associated with disuse from spinal cord injury.3,4,9,14,43 Complete unloading of the lower extremities, as is the case for most individuals with spinal cord injury in a wheelchair, results in bone loss that is 5 to 20 times greater than losses from purely metabolic etiologies over the same time.23 Bone loss following spinal cord injury is greatest in areas rich in trabecular bone, like the proximal tibia and distal femur.3 An individual who was followed longitudinally (Figure 1) best exemplifies the rate that bone is lost from the femur after spinal cord injury. Notice that the proximal femur shows greater than 15 and 30% loss of bone mineral density by six months and one year, respectively, following paralysis. However, despite the extensive bone demineralization of the lower extremities, there was virtually no change in the lumbar spine (Figure 1). Our interpretation of this finding is that the lumbar spine, while still below the level of the injury, maintains its normal bone density because of the repetitive loading that occurs during sitting in a wheelchair. The dissociation between the lumbar spine and femur also suggests that the loss of mechanical loading, rather than neurogenic factors, is the primary stimulus contributing to the loss of bone density after spinal cord injury.
FIGURE 1.
The bone mineral density (BMD) of the right hip (○), left hip (▼), and the lumbar spine (●) from the onset of injury to 2.5 years following complete quadriplegia. Notice the significant loss of bone density of the hips while the lumbar spine remains normal.
Several methods exist to load the skeletal system of individuals with spinal cord injury. Standing showed a minimal effect; however, the dose of standing is difficult to quantify and was not precisely controlled in the study.18 Hence, the magnitude of the load, duration of the load, and load frequency are all important parameters that may influence the ultimate utility of methods that promote stance after spinal cord injury. Stand-up wheelchairs, standing frames, or suspended treadmill training may influence bone density in individuals with paralysis, but well controlled studies using physiological loads have not yet examined the efficacy of these methods.
Some have attempted to load the skeletal system through muscle contractions.3 However, this requires that the limbs are constrained and the muscular system is capable of exerting loads high enough to be considered osteogenic. The force produced by skeletal muscle works through a very small moment arm. Indeed, to balance the sum of the moments produced by an external load, muscle must generate extremely high forces that can be several times greater than body weight.32 Hence, the preservation of paralyzed muscle may be an important first strategy in striving to maintain the integrity of the skeletal system.
MUSCULAR ADAPTATIONS FOLLOWING SPINAL CORD INJURY
The following presentation is restricted to muscle that is paralyzed but has normal peripheral nerve innervation. The spinal lesion leads to a loss of descending drive but the peripheral nervous system connections remain intact. Denervated muscle, or muscle that has lost its peripheral nerve, will not be the subject of this review.
Histochemistry and Myosin Heavy Chain Analysis of Human Paralyzed Muscle
Myosin, the molecular motor of skeletal muscle, is a protein comprised of two myosin heavy chains (MHC). The MHC is a critical structural and enzymatic component of the contractile apparatus. In human muscle there are three types of MHC—MHC–I, MHC–IIa, MHC–IIx (IIb).42 The type of MHC expressed in human skeletal muscle determines the characteristics of the muscle. The histochemical staining properties of a muscle fiber, and its fiber type classification, are closely associated to the MHC that is expressed.42 Generally, muscle fibers in humans do not express more than one discrete MHC type. However, when muscle is transforming from chronic disuse after spinal cord injury, the number of hybrid fibers coexpressing two MHC types increases dramatically.42
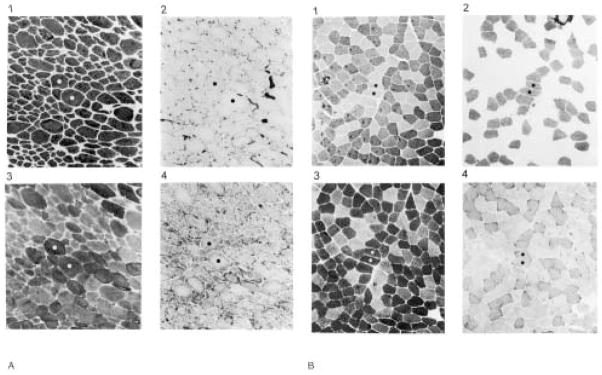
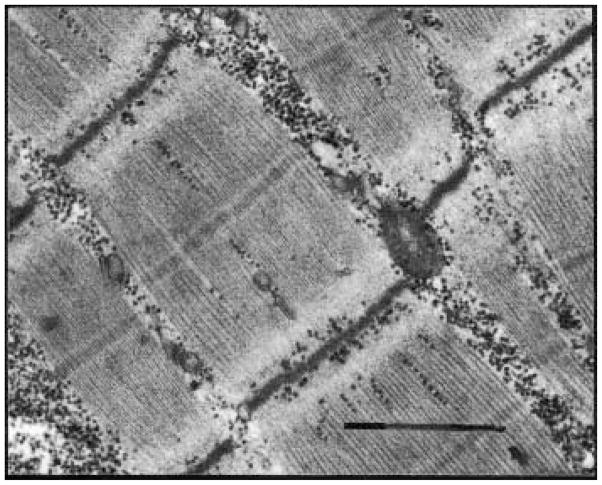
The precise molecular adaptations in human muscle after spinal cord injury have not been fully addressed. In general, the MHC type transforms toward a faster type by one year of paralysis and shows significant increases in MHC–IIx.21 Histochemical fiber-typing studies also support that skeletal muscle shows a dramatic increase in faster (IIa, IIb) fibers after chronic spinal cord injury.22,29,38 The extent of the transformation is evident by the nearly complete appearance of dark type IIb fibers in a biopsy of the rectus femoris muscle taken from an individual with chronic spinal cord injury and from an individual without spinal cord injury. Notice the homogeneity of the fibers under all staining conditions for the paralyzed muscle (Figure 2). Even the ultrastructure of chronically paralyzed muscle shows a narrow z-line (Figure 3), which is a characteristic of sarcomeres making up fast muscle. On average, the z-lines are 20 nanometers narrower than those of slow muscle.
FIGURE 2.
Chronically paralyzed rectus femoris muscle (A) and a normal rectus femoris muscle (B) stained for the myosin ATPase enzyme with a preincubation at pH 9.4 (1), pH 4.2 (2), and pH 4.6 (3). The oxidative enzyme NADH–TR was stained for in 4. Notice that the paralyzed muscle (A) appears as a homogenous group of type II fibers while the nonparalyzed muscle (B) shows the normal mixed distribution of type I and type II fibers.
FIGURE 3.
A transmission electron micrograph of the chronically paralyzed soleus muscle. From one dark band (z–line) to the next represents one sarcomere. A series of micrographs revealed that the z–lines were, on average, 20 nanometers narrower in chronically paralyzed muscle. Narrow z-lines have previously been described for fast muscle. Magnification = 36,000x. Bar = 1 μm.
The appearance of faster myosin suggests that the muscle would have faster contractile speed properties and ultimately become highly fatigable consistent with physiological properties of fast fatigable motor units. These physiological changes would have a significant impact on the optimal methods to activate paralyzed muscle electrically and the eventual utility of this musculature for functional use in the future.
Physiological Properties of Human Paralyzed Muscle
Most human skeletal muscles have an equal proportion of fast and slow fibers. However, the normal soleus muscle, with its predominance of slow fibers, appears ideal when examining the extent to which slow fibers transform toward fast after spinal cord injury. The physiological evidence that the normally slow soleus muscle transforms to faster fatigable muscle as the result of chronic spinal cord injury is compelling.8,30,31,33–40 Whereas the precise molecular transformations have not been clearly established,42 the changes in physiological properties as a result of chronic paralysis are well documented.34,36,38 We have had the opportunity to monitor the physiological changes in the soleus muscle from one week of spinal cord injury to more than two years.37 We have also had the opportunity to prevent the physiological changes from occurring by training the soleus muscle of one leg while using the opposite side as a control (catch-like property).37 Physiological measurements such as muscle contractile speed,39 fatigue index,38,39 force potentiation,36 delayed onset fatigue,35,39,40 force-frequency relationship,34,35 doublet potentiation,8 sarcolemmal membrane properties,40 and motoneuronal pool suppression31 all support that the neuromuscular system, as determined by examining the soleus muscle, transforms in the direction of fast and fatigable muscle after long-term paralysis.
Contractile Speed Properties and Force (Torque)-Frequency Relationships
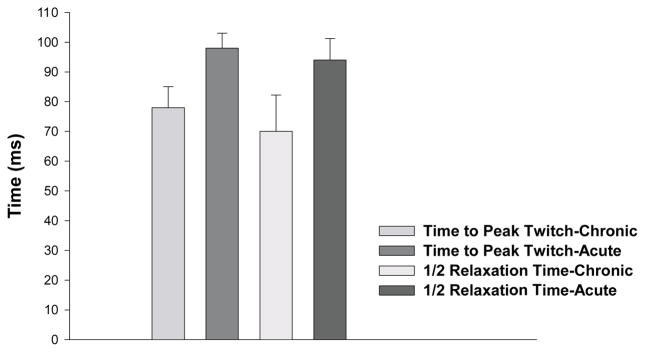
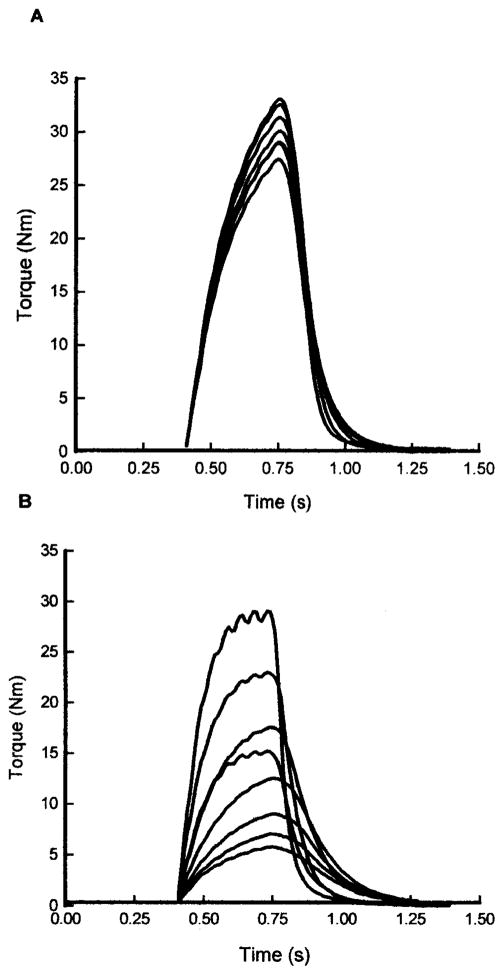
The human chronically paralyzed soleus muscle shows, on average, a 20-ms shorter time to peak twitch torque and a 25% shorter twitch half-relaxation time when compared to the soleus muscle from individuals with acute paralysis (Figure 4). Moreover, one year following paralysis, the soleus muscle becomes highly fatigable when activated each second with a 330-ms 20-Hz train for two minutes (Figure 5). Both of these findings are consistent with the properties expected in a muscle functioning as primarily fast.34,38,39
FIGURE 4.
The mean time to peak twitch and mean half-relaxation time for the chronic and acute paralyzed soleus muscle. Error bars are standard deviations.
FIGURE 5.
The torque curve sampled every 30 seconds during a 20-Hz fatigue protocol (every second for 330 ms) for an individual with acute (A) and chronic (B) paralysis. Notice the extensive fatigue and contractile speed slowing at the end of the fatigue protocol in the chronically paralyzed soleus.
Fast fatigable motor units show progressive slowing during fatigue induced by repetitive activation.28 Consistent with properties of faster muscle and motor units, the “transformed soleus muscle” from individuals with chronic paralysis demonstrates a near doubling of the half-relaxation time during fatigue.39 This indicates that as the muscle fatigues, the rate that calcium is taken up becomes compromised or the rate that cross-bridges can detach to release force becomes impaired, or both. The extent to which the chronically paralyzed muscle shows increased contractile speeds in the fresh state and progressive slowing of the contractile speeds during fatigue is illustrated in Figure 5. The unfused nature of the torque curve in the chronically paralyzed soleus verifies the faster contractile speed before fatigue (Figure 5). As fatigue progresses, the more fused condition occurs, which demonstrates that the muscle is having a difficult time relaxing quickly. Hence, as the muscle fatigues, there is clear evidence of contractile speed slowing (Figure 5).
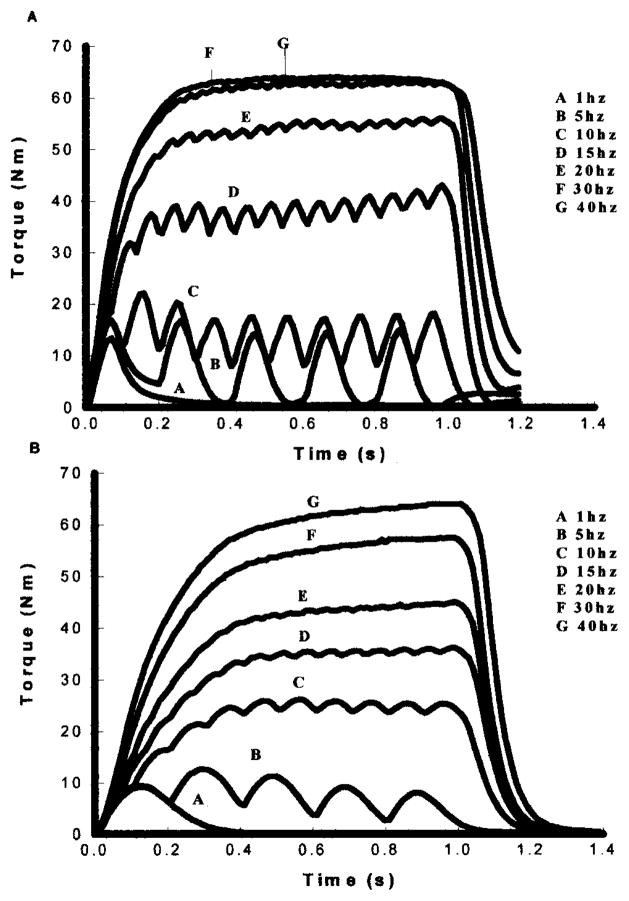
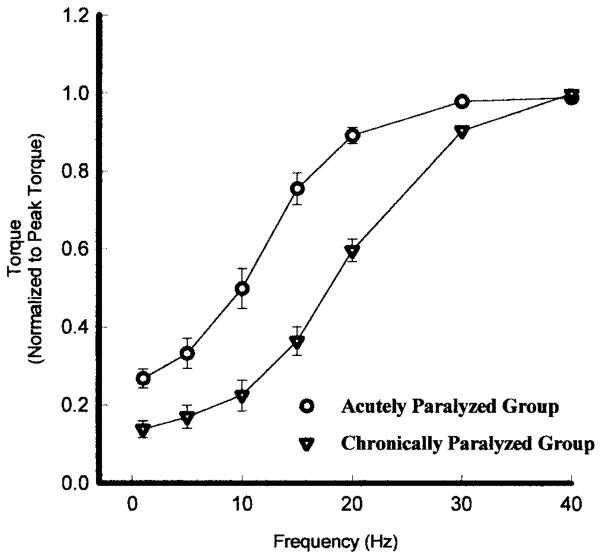
A well established sigmoidal relationship between muscle force (torque) and frequency of electrical stimulation exists in motor units and whole skeletal muscle.28,34 That is, as frequency of the electrical stimulus increases, the muscle contractions progressively become more fused and the muscle generates greater torque (Figure 6). Once the muscle is fully fused an additional increase in frequency causes negligible changes in torque. The torque-frequency relationship should be closely associated with the contractile speeds of the muscle. Thus, a muscle that has slower contractile speeds (Figure 6B) will fuse at a lower frequency when compared to a muscle that has a faster contractile speed (Figure 6A). The torque-frequency curve for a slow muscle will be shifted to the left of the torque-frequency curve for a fast muscle (Figure 7). If the torque produced at each frequency is normalized to each muscle’s peak torque (produced at the highest frequency), then the relative relationship between torque and frequency can be illustrated on the same plot for various muscle types (Figure 7).34
FIGURE 6.
The torque curves obtained with electrical stimulation at 1, 5, 10, 15, 20, 30, and 40 Hz for an individual with chronic paralysis (A) and an individual paralyzed for less than one week (B). Notice the difference in contractile speeds and fusion properties between the acute and chronically paralyzed soleus.
FIGURE 7.
The average torque–frequency curve, normalized for each subjects maximal torque, for an acutely paralyzed group (○) and a chronically paralyzed group (▽). Because of the faster contractile speeds the chronically paralyzed group curve is shifted to the fight. Error bars are standard deviations.
With this background it should be apparent why the torque-frequency curve for chronically paralyzed muscle is shifted to the right of the torque-frequency curve for acutely paralyzed muscle. In essence, to get the same “relative” torque from the chronically paralyzed soleus, it requires a higher frequency because the speed properties are faster. Moreover, if the contractile speeds slow during fatigue of chronically paralyzed muscle, then one should be able to get the same relative force from the muscle with a lower frequency of stimulation.34 After fatigue, other physiological events such as muscle potentiation35 and excitation-contraction coupling impairment28,34 occur, which significantly influences the shape of the torque-frequency curve.28,34
When a muscle is fully fused, there is generally no apparent advantage to activating the muscle with a higher frequency for long periods. The cost of continuously activating a muscle with a higher frequency than necessary for fusion is that failure may eventually occur at the neuromuscular transmission system (neuromuscular junction, sarcolemma propagation).16 This form of fatigue is referred to as high-frequency fatigue. Jones and colleagues16 demonstrated a recovery of force when the frequency was reduced from 80 to 20 Hz during continuous stimulation of a skeletal muscle. Consequently, the ability to get the message to the muscle was the source of the fatigue. When the time between pulses was increased (frequency decreased from 80 to 20 Hz), there was greater recovery time of the neuromuscular transmission system between pulses and, therefore, force increased.16 This principle should be considered when choosing electrical stimulation parameters when activating skeletal muscle for therapeutic purposes. The muscle will not be challenged to adapt if the cause of the fatigue is an inability to get a message to the muscle (neuromuscular transmission impairment). Based on these principles, it is prudent to consider more versatile stimulation protocols to optimize the force-generating ability of skeletal muscle. Before appropriate stimulation parameters are pursued, we must understand the fatigue properties of the musculature that we are interested in repetitively activating. However, studies specific to humans with spinal cord injury are essential. Because studying individuals without pathology is easy, we are often tempted to extend the results from studies of able-bodied individuals to individuals with paralysis, especially with regard to electrical stimulation. Given the plasticity of the paralyzed muscular system and the inability of individuals without paralysis to tolerate supramaximal electrical stimulation, we must be cautious when interpreting studies that are not specific to individuals with paralysis.
Low-Frequency Fatigue
Higher stimulation frequencies than needed to fuse the muscle may induce high-frequency fatigue and is attributable to impairment at the neuromuscular transmission system.16 However, most functional electrical neuromuscular stimulation (FNS) systems employ low-frequency stimulation (15–25 Hz).27 Activating the paralyzed soleus muscle with a 20-Hz stimulation frequency averts high-frequency fatigue while generating approximately 60% of the muscle’s maximum torque capability.34 Repetitive activation of the chronically paralyzed soleus muscle at lower frequencies induces a different type of fatigue called low-frequency fatigue. Because the message to the muscle is not impaired (neuromuscular transmission), the cause of fatigue is internal to the muscle. Low-frequency fatigue is defined as the preferential loss of force at a low frequency that is nearly fully recovered at a high frequency. The cause of failure cannot be attributable to a loss of adenosine triphosphate (ATP) because the force is nearly fully recovered by high stimulation frequencies. The general site of failure is at the muscle’s excitation-contraction coupling system.11,16,27,28
Excitation-contraction coupling is associated with the role calcium plays in triggering the contractile proteins (actin and myosin) to interact. The t-tubular system carries the action potential to voltage-gated receptors, which in turn communicate with receptors on the sarcoplasmic reticulum (SR) (ryanodine).12 When the ryanodine receptor is activated, it induces calcium release from the terminal cisternae. Calcium binds to troponin and allows the globular heads of myosin access to the actin-binding sites. Tension in the muscle develops relative to the number of actin-myosin cross-bridges formed in parallel during this chemical interaction. Interference of the calcium-mediated activation system has been implicated as contributing to muscle fatigue during low-frequency stimulation as well as during prolonged voluntary contractions.12
The excitation-contraction coupling impairment observed during low-frequency fatigue may represent an internal safety mechanism in skeletal muscle to prevent energy supply depletion (ie, ATP) and ultimate muscle rigor. As previously indicated, most of the force is recovered by using a high-frequency stimulus. In bench laboratory experiments, caffeine is also known to restore force loss during low-frequency fatigue.1,45 Caffeine enhances calcium release by improving the communication from the t-tubular activation to the ryanodine receptor of the SR.12 The precise molecular pathway impaired during excitation-contraction coupling failure has not yet been discovered.
Low-frequency fatigue is also characterized by being delayed in onset as well as being long lasting (24–48 hours).11,15 The extent of the low-frequency fatigue induced in the paralyzed soleus muscle immediately, five, and ten minutes after fatigue is compelling.34 Associated with this fatigue is a significant shift to the right of the torque-frequency curve.34 Thus, to get the same relative torque out of the paralyzed soleus muscle that is fatigued during a 20-Hz intermittent stimulation frequency, the muscle must be activated with a higher frequency.34 Low-frequency fatigue was also found to be most prominent in fast intermediate or fast fatigable motor units.15,28 Consequently, it is not surprising that the chronically paralyzed soleus muscle, by virtue of its apparent transformation to fast fatigable muscle, shows extensive delayed onset and long-term low-frequency fatigue.34,36,39
Because most of the muscle force can be recovered with a high frequency, it argues against the prospect of excitation-contraction coupling damage. However, we may be walking a fine line between what constitutes muscle damage and a temporary impairment that can be overcome with caffeine or high stimulation frequencies, or both. As indicated in Warren et al,44 damage may be at the level of excitation-contraction coupling during high-intensity lengthening contractions.
Catch-Like Property of Paralyzed Muscle
Prolonged muscle tension after a brief excitation was first described for invertebrate muscle46 and is referred to as the catch property. The catch-like property, later described for mammalian muscle, refers to the enhancement of force from a muscle caused by a brief high-frequency train added to a low-frequency background.2,6,26 Burke and colleagues6 found that when two pulses were delivered closely (10 ms), they induced a nonlinear summation that often exceeded what would have developed if two twitches were merely summed individually. Subsequently, stimulation trains with an initial high-frequency burst (variable-frequency stimulation) were found to augment force in human whole muscle5 and paralyzed muscle.8,17,33,40
The mechanisms underlying doublet potentiation remain unclear. Potential causes can be grossly attributed to changes in muscle-tendon stiffness,16 effects on excitation-contraction coupling,2 or cross-bridge dynamics, or all of them. The human chronically paralyzed soleus muscle is fast and fatigable and therefore shows severe excitation-contraction coupling impairment during repetitive activation.8 Following fatigue, variable-frequency stimulation would appear ideal in overcoming low-frequency fatigue without the risk of inducing high-frequency fatigue. In support of this view, a high-frequency doublet (5- to 10-ms interpulse interval) at the start of a train showed a significant torque enhancement (30%) in the chronically paralyzed soleus muscle following a fatiguing protocol.8
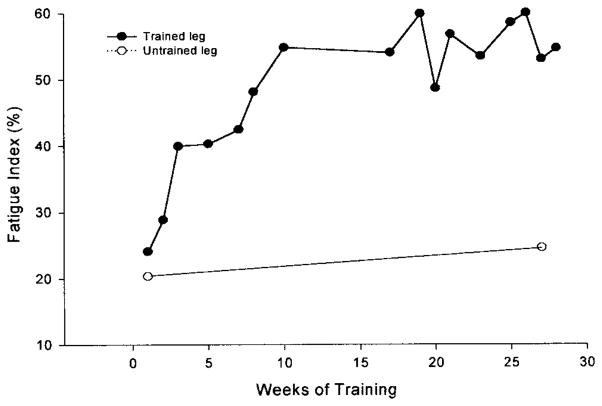
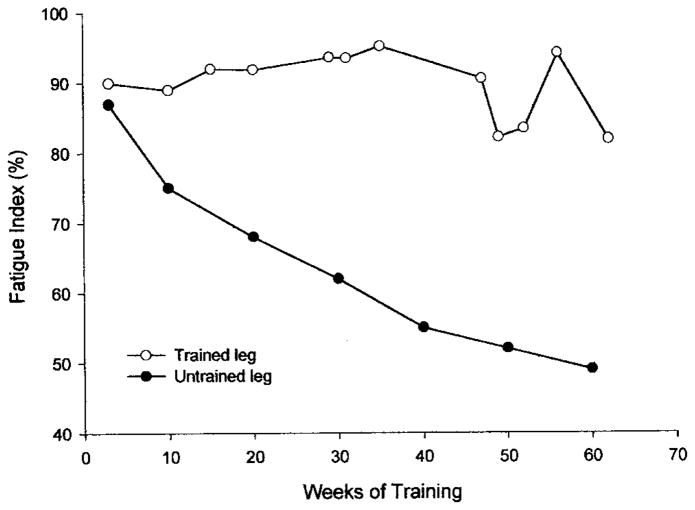
These findings suggest that the optimal method to train paralyzed muscle may depend on using smarter patterns of activation introduced at critical times during the fatigue process. To this end, we developed a feedback control system that introduces various stimulation strategies, contingent on the muscle force, in an attempt to overcome fatigue and accentuate the training of paralyzed musculature.26 We have trained the paralyzed soleus muscle using this feedback control system (initial conditions: 15 Hz, 120 contractions, 1 on, 2 off work-rest cycle) and show more than 100% improvement in the fatigue index after 15 weeks in an individual with chronic paralysis (fatigue index of 23 to 53%) (Figure 8). During the same time, the opposite untrained leg experienced no change. Conversely, training over the same time led to only 30% improvement in the fatigue index (21 to 30%) in an individual who trained with the typical constant-frequency protocol without the online feedback (15 Hz, 120 contractions, 1 on, 2 off work-rest cycle).37
FIGURE 8.
Chronic Paralysis—The fatigue index (final torque/initial torque) from a repetitive stimulation protocol (15 Hz applied each second for 330 ms) for the trained and untrained soleus in an individual with chronic paralysis. Notice that after ten weeks of training the chronic soleus muscle showed more than 100% increase in its endurance while control (untrained) leg changed minimally.
Several individuals, within two weeks of their spinal cord injury, are currently enrolled in a training study using the same stimulation protocol indicated previously for the subjects with chronic paralysis. While one leg serves as a control, the other is exercised to determine the extent to which the soleus muscle can be prevented from becoming more fatigable. While only one subject is presented here, our data support that we can prevent the development of a highly fatigable soleus muscle in individuals with spinal cord injury for more than one year (Figure 9).
FIGURE 9.
Acute Paralysis—The fatigue index (final torque/initial torque) from a repetitive stimulation protocol (15 Hz applied each second for 330 ms) for a trained leg and untrained leg for an individual that began training one leg within two weeks of spinal cord injury. Notice that the trained leg kept the normal soleus fatigue resistance properties after one year of spinal cord injury.
Spinal Reorganization Following Spinal Cord Injury
Immediately following spinal cord injury there is minimal spasticity. However, in the ensuing 6 to 12 months, individuals develop hyperactive deep tendon reflexes and exhibit involuntary motor patterns (flexor withdrawal or mass extension pattern). We recently evaluated the H-reflex suppression in individuals without spinal cord injury and individuals with acute and chronic spinal cord injury.31 In able-bodied subjects and the acutely injured subjects, the H-reflex was suppressed as the frequency with which it was elicited increased. However, the individuals with chronic paralysis lost the ability to suppress the H-reflex at high frequencies. The suppression of H-reflexes, elicited at higher frequencies, is thought to be attributable to presynaptic inhibition.7,31 Thus, our findings suggest that changes occur within the spinal cord that cause H-reflexes to reduce the magnitude of their suppression, perhaps through presynaptic mechanisms.7
Interestingly, if one monitors the muscle fatigability and the H-reflex suppression longitudinally after spinal cord injury, the changes covary (> 0.92). That is, as the muscle is transforming to become faster and fatigable, associated changes in synaptic efficacy appear to be occurring in the spinal cord.13 In our view, this suggests some coordination among the muscle fiber types, the motor unit properties, and synaptic transmission.10,13,24,25 Current studies are underway in our laboratory to document the extent to which spinal reorganization, determined by H-reflex suppression and recruitment curves, can be prevented by early training regimens.
Implications to Rehabilitation Specialists
Muscle activity is, most likely, a powerful stimulus to the overall health of the intact neuromuscular system. Likewise, routine activation of paralyzed musculature may also be an important trigger that prevents abnormal spinal circuitry reorganization while also applying osteogenic forces to the skeletal system. However, we must chart this course with open minds. We must recognize that some adaptations after paralysis are beneficial, and that through our interventions the possibility exists that we induce an adaptation that is detrimental to the overall health of the individual. Accordingly, the ultimate outcome study is paramount to establish if prevention of neural, muscular, and skeletal adaptations translates into an improved quality of life for individuals with paralysis. Ultimately, research must establish the optimal methods to influence neural, muscular, and skeletal adaptations in humans following paralysis. We must keep abreast with scientific breakthroughs so the needs of our clients are met in a timely manner. Indeed, clinical scientists should lead the development of innovative rehabilitation technology so that we are well positioned to meet the needs of our clients in the 21st century.
Acknowledgments
Grant support from the Paralyzed Veterans of American Spinal Cord Research Foundation and National Institutes of Health Grant R01H39445.
Footnotes
This commentary was approved by the Institutional Review Board, University of Iowa, Iowa City, IA.
References
- 1.Allen DG, Lee JA, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. Physiol. 1989;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevan L, Laouris Y, Reinking RM, Stuart DG. The effect of the stimulation pattern on the fatigue of single motor units in adult cats. J Physiol. 1992;449:85–108. doi: 10.1113/jphysiol.1992.sp019076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biering-Sorensen F, Bohr H, Schaadt O. Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia. 1988;26:293–301. doi: 10.1038/sc.1988.44. [DOI] [PubMed] [Google Scholar]
- 4.Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 5.Binder-Macleod ST, Barker CB. Use of a catchlike property of human skeletal muscle to reduce fatigue. Muscle Nerve. 1991;14:850–857. doi: 10.1002/mus.880140909. [DOI] [PubMed] [Google Scholar]
- 6.Burke RE, Rudomin P, Zajac FE. Catch property in single mammalian motor units. Science. 1970;168:122–124. doi: 10.1126/science.168.3927.122. [DOI] [PubMed] [Google Scholar]
- 7.Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr Clin Neurophysiol. 1993;89:177–186. doi: 10.1016/0168-5597(93)90131-8. [DOI] [PubMed] [Google Scholar]
- 8.Chang YJ, Shields RK. Variable versus constant frequency stimulation in human paralyzed muscle. Abstr Soc Neurosci. 1995 [Google Scholar]
- 9.Chantraine A, Nusgens B, Lapiere CM. Bone remodeling during the development of osteoporosis in paraplegia. Calcif Tissue Int. 1986;38:323–327. doi: 10.1007/BF02555744. [DOI] [PubMed] [Google Scholar]
- 10.Cope TC, Bodine SC, Fournier M, Edgerton VR. Soleus motor units in chronic spinal transected cats: physiological and morphological alterations. J Neurophysiol. 1986;55:1202–1220. doi: 10.1152/jn.1986.55.6.1202. [DOI] [PubMed] [Google Scholar]
- 11.Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Foehring R, Munson J. Motoneuron and muscle-unit properties after long-term and direct innervation of soleus muscle by medial gastrocnemius nerve in cat. J Neurophysiol. 1990;64:847–861. doi: 10.1152/jn.1990.64.3.847. [DOI] [PubMed] [Google Scholar]
- 14.Garland DE, Stewart CA, Adkins RH, et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10:371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- 15.Jami L, Murthy KS, Petit J, Zytnicki D. After-effects of repetitive stimulation at low-frequency on fast-contracting motor units of cat muscle. J Physiol. 1983;340:129–143. doi: 10.1113/jphysiol.1983.sp014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones DA. Muscle fatigue due to changes beyond the neuromuscular junction. In: Porter R, Whelan J, editors. Human Muscle Fatigue: Physiological Mechanisms (CIBA Foundation Symposium 82) London, England: Pitman Medical; 1981. pp. 178–196. [DOI] [PubMed] [Google Scholar]
- 17.Karu ZZ, Durfee WK, Barzilai AM. Reducing muscle fatigue in FES applications by stimulating with N-Let pulse trains. IEEE Trans Biomed Eng. 1995;42:809–817. doi: 10.1109/10.398642. [DOI] [PubMed] [Google Scholar]
- 18.Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil. 1993;74:73–78. [PubMed] [Google Scholar]
- 19.Lance J. Pathophysiology of Spasticity and Clinical Experience with Baclofen. Spasticity: Disordered Motor Control. Chicago, IL: Year Book; 1981. [Google Scholar]
- 20.Lanyon LE. Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone. 1996;18:37S–43S. doi: 10.1016/8756-3282(95)00378-9. [DOI] [PubMed] [Google Scholar]
- 21.Lotta S, Scelsi R, Alfonsi E, et al. Morphometric and neurophysiological analysis of skeletal muscle in paraplegic patients with traumatic cord lesion. Paraplegia. 1991;29:247–252. doi: 10.1038/sc.1991.35. [DOI] [PubMed] [Google Scholar]
- 22.Martin TP, Stein RB, Hoeppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol. 1992;72:1401–1406. doi: 10.1152/jappl.1992.72.4.1401. [DOI] [PubMed] [Google Scholar]
- 23.Mazess RB, Whedon GD. Immobilization and bone. Calcif Tissue Int. 1983;35:265–267. doi: 10.1007/BF02405043. [DOI] [PubMed] [Google Scholar]
- 24.Mendell LM, Taylor JS, Johnson RD, Munson JB. Rescue of motoneuron and muscle afferent function in cats by regeneration into skin. II. Ia-motoneuron synapse. J Neurophysiol. 1995;73:662–673. doi: 10.1152/jn.1995.73.2.662. [DOI] [PubMed] [Google Scholar]
- 25.Munson JB, Foehring RC, Mendell LM, Gordon T. Fast-to-slow conversion following chronic low-frequency activation of medial gastrocnemius muscle in cats. II. Motoneuron properties. J Neurophysiol. 1997;77:2605–2615. doi: 10.1152/jn.1997.77.5.2605. [DOI] [PubMed] [Google Scholar]
- 26.Parmigianni F, Stein RB. Nonlinear summation of contractions in cat muscles. II. Later facilitation and stiffness changes. J Gen Physiol. 1981;78:295–311. doi: 10.1085/jgp.78.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips W, Burkett LN, Munro R, Davis M, Pomeroy K. Relative changes in blood flow with functional electrical stimulation during exercise of the paralyzed lower limbs. Paraplegia. 1995;33:90–93. doi: 10.1038/sc.1995.21. [DOI] [PubMed] [Google Scholar]
- 28.Powers RK, Binder M. Effects of low-frequency stimulation on the tension-frequency relations of fast–twitch motor units in the cat. J Neurophysiol. 1991;66:905–918. doi: 10.1152/jn.1991.66.3.905. [DOI] [PubMed] [Google Scholar]
- 29.Rochester L, Barron MJ, Chandler CS, Sutton RA, Miller S, Johnson MA. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 2. Morphological and histochemical properties. Paraplegia. 1995;33:514–522. doi: 10.1038/sc.1995.112. [DOI] [PubMed] [Google Scholar]
- 30.Schindler-Ivens S, Shields RK. H-reflex torque relationship in humans with and without spinal cord injury. Abstr Soc Neurosci. 2000 [Google Scholar]
- 31.Schindler-Ivens S, Shields RK. Low-frequency depression of H-reflexes in humans with acute and chronic spinal–cord injury. Exp Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultheis L. The mechanical control system of bone in weightless spaceflight and in aging. Exp Gerontol. 1991;26:203–214. doi: 10.1016/0531-5565(91)90012-b. [DOI] [PubMed] [Google Scholar]
- 33.Shields RK, Carr M, Fessler A, Hanson D, Spieler K. Torque frequency relationship during variable and constant frequency stimulation of the soleus muscle in humans with spinal cord injury. Abstr Soc Neurosci. 2000 [Google Scholar]
- 34.Shields RK, Chang YJ. The effects of fatigue on the torque frequency curve of the human paralyzed soleus muscle. J Electromyogr Kinesiol. 1997;7:3–13. doi: 10.1016/s1050-6411(96)00015-6. [DOI] [PubMed] [Google Scholar]
- 35.Shields RK, Chang YJ, Littmann A. Torque potentiation before and after fatigue of the paralyzed soleus in humans. Abstr Soc Neurosci. 1997 [Google Scholar]
- 36.Shields RK, Chang YJ, Ross M. Neuromuscular propagation after fatiguing contractions of the paralyzed soleus muscle in humans. Muscle Nerve. 1998;21:776–787. doi: 10.1002/(sici)1097-4598(199806)21:6<776::aid-mus10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 37.Shields RK. Effects of training on torque, fatigue index, and H-reflex suppression in individuals with acute and chronic SCI. Abstr Soc Neurosci. 1999 [Google Scholar]
- 38.Shields RK. Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol. 1995;73:2195–2206. doi: 10.1152/jn.1995.73.6.2195. [DOI] [PubMed] [Google Scholar]
- 39.Shields RK, Law L, Reiling B, Sass K, Wilwert J. Effects of electrically induced fatigue on the twitch and tetanus of paralyzed soleus muscle in humans. J Appl Physiol. 1997;82:1499–1507. doi: 10.1152/jappl.1997.82.5.1499. [DOI] [PubMed] [Google Scholar]
- 40.Shields RK. Optimizing paralyzed muscle force using adaptive feedback control. Abstr Soc Neurosci. 1996 [Google Scholar]
- 41.Stover SL, DeLisa JS, Whiteneck GG. Spinal Cord Injury: Clinical Outcomes from the Model Systems. Gaithersburg, MD: Aspen Publishers, Inc; 1995. [Google Scholar]
- 42.Talmadge RJ. Myosin heavy chain isoform expression following reduced neuromuscular activity: Potential regulatory mechanisms. Muscle Nerve. 2000;23:661–679. doi: 10.1002/(sici)1097-4598(200005)23:5<661::aid-mus3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 43.Uebelhart D, Demiaux–Domeneche B, Roth M, Chantraine A. Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia. 1995;33:669–673. doi: 10.1038/sc.1995.140. [DOI] [PubMed] [Google Scholar]
- 44.Warren GL, Ingalls CP, Lowe DA, Armstrong RB. What mechanisms contribute to the strength loss that occurs during and in the recovery from skeletal muscle injury? J Orthop Sports Phys Ther. 2002;32:58–64. doi: 10.2519/jospt.2002.32.2.58. [DOI] [PubMed] [Google Scholar]
- 45.Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol. 1993;75:382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]
- 46.Wilson DM, Larimer JL. The catch property of ordinary muscle. Proc Natl Acad Sci. 1968;61:909–916. doi: 10.1073/pnas.61.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]