Abstract
Eph receptor tyrosine kinases and the corresponding ephrin ligands play a pivotal role in glioma development and progression. Aberrant protein expression levels of the Eph receptors and ephrins are often associated with higher tumor grade and poor prognosis. Their function in tumorigenesis is complex due to the intricate network of possible co-occurring interactions between neighboring tumor cells and tumor microenvironment. Both Ephs and ephrins localize on the surface of tumor cells, tumor vasculature, glioma stem cells tumor cells infiltrating brain and immune cells infiltrating tumors. They can both promote and inhibit tumorigenicity depending on the downstream forward and reverse signalling generated. All the above-mentioned features make the Ephs/ephrins system an intriguing candidate for the development of new therapeutic strategies in glioma treatment. This review will give a general overview on structure and function of Ephs and ephrins, with particular emphasis on the state-of-the-knowledge of their role in malignant gliomas.
Keywords: Eph, Ephrin, Glioma, GBM, Brain Tumor
Introduction
Gliomas account for one third of all primary brain tumors and despite massive effort in developing new therapeutic strategies, they still represent one of the major medical challenges (1, 2). Gliomas are categorized according to their grade: low grade gliomas (WHO grade I-II), like astrocytomas and ependymomas, display benign features and have better prognosis, while more aggressive gliomas (WHO grade III-IV), like oligodendrogliomas or glioblastomas, are usually characterized by anaplastic features and dismal prognosis (3, 4). Standard therapies that include surgery, γ-radiation and temozolomide-based cytotoxic chemotherapy, can only temporarily delay unfavorable prognosis, especially for the higher grades. New therapeutic strategies focus on immunotherapies (5–7), anti-angiogenic agents (8–11), targeted cytotoxins (12–15) or targeting of dysregulated signaling pathways (16–18).
Ephs form the largest known subfamily of receptor tyrosine kinases (RTKs). Extensive studies focusing on these receptors, first isolated from an Erythropoietin-producing human hepatocellular carcinoma line (19), and the corresponding ligands, ephrins (eph receptor-interacting ligands), have generated a complex body of knowledge helping to decipher their role in physiology and pathology (20–24). Ephs and ephrins have a primary role in embryogenesis and development (25–27); they are present virtually in all the developing tissues regulating a complex pattern of developmental processes like cell adhesion, axon guidance, cell migration, cell sorting, platelet aggregation, and others (25, 28). For example, they are involved in establishing neuronal patterning of the auditory system (29) and in guiding the extension and maturation of cortical dendrites (30). But, their expression is often altered in pathological conditions (26, 31), injuries (32–34) and malignancies (35–39) in adulthood.
Mechanism of Ligand-Receptor Activation
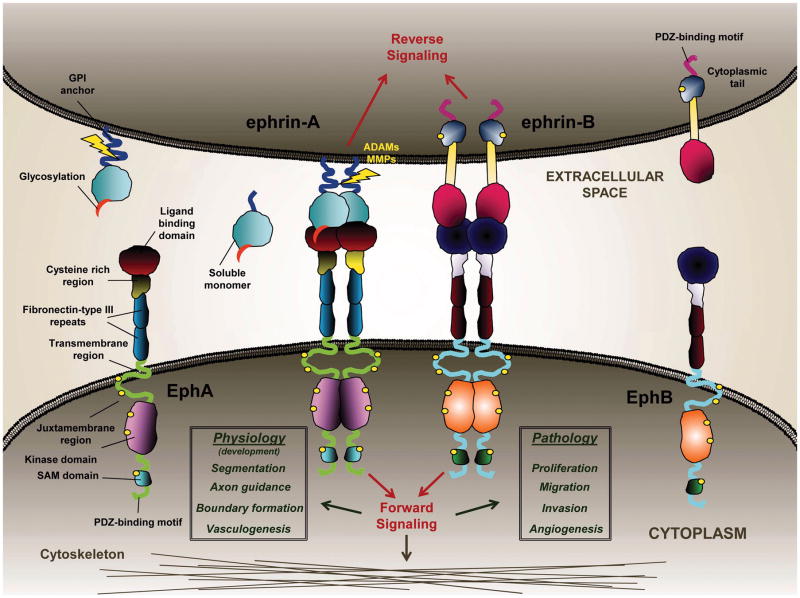
The 16 known members of the Eph family of RTKs are divided into two classes, EphA and EphB (Fig. 1). This classification is based on primary sequence similarity of the ligand binding regions and an affinity for the corresponding ephrin classes A and B, respectively. With few exceptions (40), ligands and receptors of the same subfamily interact with the highest binding affinity and specificity. Both Ephs and ephrins are plasma membrane proteins: ephrin-As are linked to the membrane through a glycophosphatidylinositol (GPI) anchor, while ephrin-Bs contains transmembrane and extended intracellular domains (Fig. 1). The Eph receptors A and B are structurally similar, with an N-terminal ligand binding domain, a cysteine-rich region and two fibronectin type III domains on the extracellular side. The intracellular juxtamembrane region contains two conserved tyrosine residues that undergo autophosphorylation (e.g., Tyr-594 and Tyr-772 for EphA2, (41)), a tyrosine kinase domain with two phosphorylation sites, a sterile alpha motif (SAM), and a PDZ-binding motif (Fig. 1).
Figure 1.
Schematic representation of Ephs and ephrins structure and the mode of interaction between neighboring cells. Ligand-dependent activation induces receptor phosphorylation and a downstream signaling cascade, which acts on the cytoskeleton and regulates cellular proliferation, migration and invasion in glioma cells. Activated clusters of receptors internalize into the receptor-expressing cell and are eventually degraded.
Signal transduction through Eph receptors is generated bi-directionally upon ligand-receptor binding, initiating a “forward signalling” via receptor phosphorylation and a “reverse signalling” via ligand activation (24, 42) (Fig. 1). The intensity of the signal generated in response to receptor activation greatly depends on the nature of ligand stimulation. In a juxtacrine manner, membrane-attached ligands bind the receptors with high affinity evoking receptor clustering and subsequent phosphorylation. During this process, the glycosylation on the ephrin ligand plays a pivotal role in stabilizing Eph and ephrin heterotetramers on the cellular membrane (43). This has been shown for the interaction between ephrin-A1 and -A5 with EphA2, but it is plausible that this is a common mechanism of ligand-receptor interaction as the glycosylation sites are highly conserved, especially among ephrin-As (43). Eph/ephrin complexes are internalized within a few minutes after receptor activation (44) and the receptors are subsequently degraded (43, 45, 46). It is not well understood whether or not the internalized ligands undergo proteolytic degradation as well. Matrix metalloproteinases (MMPs) are actively involved in the internalization process by cleaving specific residues in the extracellular domain of ephrins of the neighbouring cell and thus allowing internalization of the whole ligand-receptor complex (Fig. 1) (47). MMP-1, -2, -9 and -13 (48) have been shown to be the main players responsible for the cleavage together with ADAM10 (47, 49, 50) and ADAM12 (51), two members of the ADAM (A Disintegrin And Metalloproteinase) family of sheddases. To add to the complexity of the system, MMPs can indiscriminately cleave ephrins releasing a soluble monomeric form into the extracellular environment, which maintains the ability to bind and activate Eph receptors. Soluble monomeric ephrin-A1, found in the conditioned media of U-251 MG GBM cells, was able to induce EphA2 receptor internalization and down-regulation (45); the same was observed for recombinant monomeric forms of ephrin-A1 and ephrin-A5 (43). Even a GPI-linked ephrin-A1 initiates the contact with the EphA2 receptor in a monomeric form (52). MMPs-dependent cleavage of Eph receptors has also been reported (53). In addition, due to the plasticity of the cellular membrane, Eph receptors can be cis-or trans-activated depending on the relative composition and density of the Eph/ephrin family members on the lipids rafts. Eph receptors cluster after activation in trans by ligand binding or in cis by ligand-independent receptor-receptor interactions (54).
The activation of Eph receptors is usually coupled with dramatic morphological changes influencing the interactions with the extracellular matrix (ECM) (55) and cellular migration (56). A typical cell-rounded shape that is quickly assumed upon stimulation of the Eph receptors is due to the activation of Src and focal adhesion kinase (FAK), and the subsequent Rho-mediated phosphorylation of myosin light chain II that induces then the contraction of the cell cytoskeleton (50, 57) (Fig. 1). Both Src and FAK are involved in cellular motility, angiogenesis and cancer invasion (58).
Eph and ephrins in Gliomas
Prognosis and Survival
Because of their altered expression, Eph and ephrins were suggested as possible molecular markers in gliomas (59–61). Zelinski et al. first showed that EphA2 overexpression was sufficient to transform mammary epithelial cells (62). Since then, EphA2 overexpression was associated with several malignancies like ovarian carcinoma (63), pancreatic cancer (64), and several others (24). This receptor is also expressed in astrocytomas and its expression markedly increases with an increasing pathologic grade (65). About 60% of GBMs overexpress EphA2, while it is not found in normal brain and its overexpression correlated directly with poor prognosis and inversely with patient survival (59, 66, 67). Ephrin-B2 has also been suggested as a strong predictor of short-term survival in malignant astrocytomas because patients with high Ephrin-B2 tumor levels had significantly shorter survival than patients with low levels of this ligand (68). Another clinical study showed that Ephrin-B2 and EphB4 expressions increased according to a histopathological grade of gliomas, and the expression levels were related to progression-free survival in glioblastoma patients (69).
Other Eph receptors were detected in gliomas and were linked to patients’ outcome. Immunohistochemical studies on 32 GBM specimens suggested EphA7 as a new prognostic marker in GBM. The receptor was found to be overexpressed in about 45% of the samples analyzed and was predictive of the adverse outcome in GBM patients. EphA7 stained both tumor and endothelial cells, but not the surrounding connective tissue (60). Moreover, EphA5 expression was detected by semiquantitative PCR in normal brain tissues (61). However, EphA5 expression decreased in low-grade glioma specimens and was further reduced in high-grade gliomas (61). This observation indicates that a decrease in EphA5 expression could be used as a prognostic biomarker of glioma progression and highlights a possible role of EphA5 as tumor suppressor (61). Furthermore, high expression levels of EphB1 appear to be a good prognostic indicator. From the expression profile of 171 glioma specimens, Teng et al. showed that EphB2, B3, and B4 expression levels were significantly higher in GBM than in normal brain whilst EphB1 expression did not vary across tumor grades (70). However, based on Kaplan–Meier survival curves, patients with high EphB1 tumor levels had significantly longer survival than patients with low EphB1 tumor levels, suggesting that high EphB1 expression levels correlate with better patient outcome (70).
Proliferation, Invasion and Migration
Cell division is a tightly regulated mechanism preserving physiological number of cellular divisions and thus preventing uncontrolled cellular proliferation, invasion of the surrounding tissue and migration to distant sites (71). Ephs and ephrins, as membrane proteins, are cellular sensors of the environment and they can modify the cellular behavior. In the developing human brain, Ephs and ephrins are mainly known for their role in axon guidance (72, 73). However, in the adult brain Eph receptors are involved in the regulation of structure and function of excitatory synapses (74). In addition, the subventricular germinal zone of the lateral ventricles expresses Eph receptors B1, B3 and A4, and ephrin ligands B2 and B3. Evidence suggests that EphB2 and ephrin-B2 are involved in the migration of neuroblasts and in the cellular proliferation in the subventricular zone (75).
Ephs and ephrins patterning is often compromised in brain tumors, therefore cellular proliferation and migration are commonly affected in gliomas biology. In vitro studies showed different effects after Eph receptors stimulation by the corresponding ligands. EphA2-overexpressing U-251 MG GBM cells treated with recombinant dimeric ephrin-A1 showed a decrease in migration and proliferation potential (43, 45). Similarly, ligand-dependent EphB1 phosphorylation suppressed migration and invasion in Snb19 and U-251 MG GBM cells (70). This kind of influence of Eph receptors on cellular behavior is not ubiquitous, because phosphorylation of EphA5 did not induce any significant decrease in cell proliferation in U-118 MG GBM cells (44). EphB2 has also been related to invasion and proliferation in glioma cells. EphB2 overexpression in U-251 MG cells stimulated cellular migration and invasion, while reducing cell adhesion (76). GBM neurospheres overexpressing EphB2, when injected intracranially into mice brains, displayed an invasive phenotype at lower proliferative potential. However, EphB2-overexpressed in non-stem-like GBM cells U-87 failed to promote tumor invasion (77).
The importance of ephrin-Bs reverse signaling in glioma cells invasion and migration has been also demonstrated. Invading cells from 19 GBM specimens were collected using laser capture microdissection and EphB/ephrin-B system was identified as the most tightly linked to the invading cell phenotype (68). The protein levels and tyrosine phosphorylation of ephrin-B2 were increased in GBM tissue relative to normal brain (68). Also, in U-87 and U-251 MG GBM cells, ephrin-B2 phosphorylation, induced by addition of a recombinant EphB2 receptor, enhanced cell migration and invasion suggesting ephrin-B2 signaling as a positive regulator of glioma cell migration (68). Overexpression of EphB2 and its corresponding ephrin-B1 ligand were also shown in medulloblastoma, the most frequent childhood malignant brain tumor (78). EphB2 receptor activation by ephrin-B1 resulted in a decrease in cellular adhesion and an increase in invasion in DAOY and Uw-402 medulloblastoma cell lines. Knockdown of EphB2 abolished ephrin-B1 effects on adhesion and invasion in these cells (78).
Tumor Microenvironment, Angiogenesis and Cancer Stem Cells
The development of a tumor depends on the acquisition of several features, collectively known as “the hallmarks of cancer” (79, 80). Tumor/cancer-associated fibroblasts (TAFs/CAFs), tumor-associated macrophages (TAMs), tumor vessels, tumor infiltrating lymphocytes, and extracellular matrix (ECM) all collectively form the tumor “organ” (81–85) (Fig. 2). The mode of interaction between Eph/ephrins and the tumor microenvironment has been relatively less studied (86). In a recent report Jellinghaus et al. showed EphA4 and ephrin-A1 co-localization with CD68, a cellular marker of the macrophage lineage, in advanced human atherosclerotic plaques (87). They also showed that stimulation of human umbilical vein endothelial cells (HUVECs) with soluble ephrin-A1 increased EphA4 receptor tyrosine phosphorylation, which enhanced subsequent adhesion of both the THP-1 monocytic cells and an enriched fraction of CD14+ primary human monocytes. Being that the same was observed for Human Aortic Endothelial Cells (HAEC) and Human Coronary Artery Endothelial Cells (HCAECs), it was concluded that this is possibly a general effect of ephrin-A1 on different types of endothelial cells (87). The increased adhesion induced by EphA4 ligand-dependent forward signaling was dependent on RhoA signaling pathway, which induced significant cytoskeletal changes without affecting transcriptional activity (87). High levels of ephrin-B2 expression were also detected in a murine thymus and spleen suggesting possible role in T cells stimulation (88).
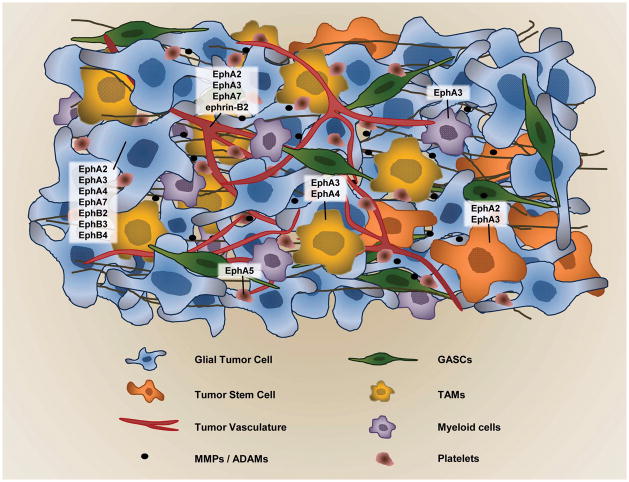
Figure 2.
Ephs and ephrins in glioma tumor environment. Different Eph receptors are overexpressed not only in glioma tumor cells, but also in the surrounding tumor-infiltrating cells like tumor-associated macrophages (TAMs) (84), in tumor vasculature, glioblastoma-associated stromal cells (GASCs)(83), and in the stem-like cell population (103).
Eph receptor A3 is emerging as a potential candidate for regulating the tumor microenvironment. The receptor is overexpressed in ~ 40% of GBM specimens, mainly in the mesenchymal genomic subtype (89). Vey recent findings localized EphA3 predominantly to the stromal tumor microenvironment of lung, prostate and colon cancers and mouse tumor xenografts. The chIIIA4 α-EPHA3 mAb (89) was specific in targeting tumor stroma and vasculature and inhibited tumor growth by disrupting the tumor stromal architecture (90). In GBM specimens, EphA3 co-localized with cells of myeloid origin in the tumor stoma, and in the perivascular regions in particular (Ferluga et al., unpublished data).
During tumor development, pre-existing vasculature can be initially utilized for oxygen and nutrients supply whether the tumor forms. While tumor size is increasing, a pro-angiogenic environment is then generated by tumor cells, tumor-associated cells and the release of growth factors and MMPs, thus creating a chemotactive gradient to recruit endothelial cells and pericytes to form tumor neovasculature (91, 92). Vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF) and Tie receptors are all RTKs involved in the communication between host endothelial cells and pro-angiogenic signals produced by tumor cells (93). Eph receptors and ephrins have been also identified as key regulators of angiogenesis during embryonic development and in postnatal vascular remodelling (94).
Ephrin-B2 and EphB4 are primary markers for arterio-venous differentiation, with ephrin-B2 expressed exclusively by arterial endothelial cells and EphB4 by venous endothelial cells (95, 96). An in vivo study identified ephrin-B2 reverse signalling as a positive regulator inducing VEGF receptor-2 internalization and activation of the downstream signalling pathway, eventually controlling endothelial filopodia-mediated vessel sprouting. Ephrin-B2 reverse signalling has been suggested as a therapeutic target in combinatorial anti-angiogenic treatments (97). In agreement with these findings, EphB4 displayed a proangiogenic role by activating ephrin-B2 reverse signalling in the vasculature of a breast cancer mouse model, promoting tumor progression (98). It is noteworthy that EphB4 and ephrin-B2 expression levels increase in clinical glioma samples according to the grade and thus status of neovascularization (20, 69).
EphA2 is also a major player involved in tumor angiogenesis. It was found highly present in GBM tumor vasculature (59). EphA2-deficient endothelial cells displayed impaired survival and tumor-mediated migration, and failed to incorporate into tumor microvessels in vivo (99). Similarly, ephrin-A1 stimulation of EphA2-expressing endothelial cells induced cellular migration and increased survival. In addition, EphA2 ligand-dependent activation was necessary to induce VEGF-dependent angiogenesis (100). In SCID mice in vivo model, EphA5 was present in plasma and platelets. Interestingly, EphA5 levels significantly decreased in plasma of mice bearing angiogenic, fast-growing glioblastoma tumors and increased in mice bearing microscopic dormant glioblastoma (61). Thus, the evidence suggests an active role that Eph/ephrins play in the tumor microenvironment, an avenue that still needs to be better understood (Fig. 2).
Glioma stem-like cells (GSCs) are a small portion of self-renewing glioma tumor cells with high tumorigenic potential and low proliferation rate. GSCs are particularly resistant to chemo-and radiation therapy, being potentially responsible for tumor recurrence after treatment (101–104). Several studies focused on the role of Ephs and ephrins in cancer stem cells (20); however, less is known about their role and expression in GSCs. Binda et al. showed that EphA2 has a regulatory role in tumor-propagating cells (TPCs) with stem-like characteristics (105). By cell sorting they showed that high EphA2 levels are a hallmark of TPCs. EphA2 was prominently down-regulated when TPCs were differentiated, losing stemness and tumorigenicity. Moreover, ephrin-A1-Fc treatment depleted the TPC pool, inhibiting self-renewal and inducing astroglial differentiation (105). A recent study also confirmed that EphA2 knockdown suppressed stem cell properties of GSCs, causing diminished self-renewal, reducing stem marker expression and decreasing tumorigenicity, while its overexpression had opposite effects (106). Similar studies showed that the loss of EphA3 in GBM cells prevents tumorsphere formation, inducing neuronal and glial cell differentiation (89). So, EphA3 appears to be important in maintaining the de-differentiated and tumorigenic state of GSCs (89) (Fig. 2).
Downstream Signalling
Eph receptors dimerize after ligand stimulation, with subsequent phosphorylation of tyrosine and serine residues in the juxtamembrane region, allowing the intracellular tyrosine kinase to convert the receptor into its active form and subsequently activate or repress the downstream signalling (107). The variety of partners that can be regulated by activated-Eph receptors give reason to the complexity of the signalling network generated.
Genome analyses of 22 GBM samples revealed major alterations in genes encoding components of TP53, RB1, and PI3K pathways (108). The tumor suppressor protein p53 is mutated in more than 50% of all human cancers; p53 is activated in response to cellular stresses such as DNA damage, heat, hypoxia and nutrient depletion and is a critical regulator of cell cycle arrest and/or apoptosis (109). Early in vitro studies identified both EphA2 and its ligand ephrin-A1 as targets of p53 (110). In particular, p53, p73, and p63 (two p53 homologues) responsive elements are located within the ephA2 promoter region. EphA2, but not EphA3 or EphB2, expression and protein levels increased upon p53 activation (110). Of interest, ephrin-A1 and EphB4 were up-regulated by p53 in DLD-1 colorectal carcinoma cells (111).
The RAS-RAF-mitogen-activated protein kinase (MAPK)/ERK pathway leads to uncontrolled cellular growth, a necessary step in the development of cancer, and it is commonly activated by RTKs. Pratt et al., first showed that ligand-activated EphA2 signalling starts from the interaction of tyrosine-phosphorylated EphA2 with the SHC adaptor protein (112). SHC bridges EphA2 to GRB2, which facilitates activation and nuclear translocation of the ERK kinases, eventually destabilizing cellular attachment to the ECM (112). Later studies in breast cancer cells demonstrated that EphA2 is a direct transcriptional target of the RAS-RAF-MAPK pathway (94). The authors proposed that EphA2 signalling contributes to a negative feedback loop that negatively regulates RAS activity in a ligand-dependent manner. Briefly, the activated MAPK pathway contributes to the suppression of ephrin-A1 expression, thus increasing the levels of EphA2 and the proliferative and migratory potential of cells (45, 113). On the other hand, the inhibition of the pathway induces ephrin-A1 expression whilst reducing EphA2 levels (113). Similarly, ephrin-B1 binding to EphB2 caused a decrease in the levels of active GTP-bound Ras as well as a decrease in MEK1 and ERK1/2 phosphorylation. The inhibition of the MAPK cascade requires phosphorylation at the conserved juxtamembrane tyrosine residues of EphB2 (114). In NG108 neuronal cells, activated EphB2 was shown to down-regulate the RAS-RAF-MAPK pathway and neurite retraction by recruiting p120RasGAP (115).
There is evidence suggesting the opposite effect on the Ras pathway. Vindis et al. demonstrated that ligand-activated EphB1 activates ERK 1/2 and thus chemotaxis in primary human renal microvascular endothelial cell (97). The proposed model suggests that ligand-activated EphB1 recruits auto-phosphorylated c-Src, which phosphorylates p52Shc, allowing the binding of the SHC PTB domain to Tyr778 of EphB1. Grb2 is also recruited probably through the intermediary of p52Shc. The resulting activation of the ERK cascade enhances cellular migration (116). Therefore, the downstream effect on the MAPK/ERK pathway following Eph ligand-dependent activation is very tissue and cell type dependent.
Of interest, Eph receptors can activate downstream signalling also by a crosstalk with the receptors derived from a different family. One example of such a phenomenon has been documented by Fukai and co-workers; they demonstrated that EphA4 forms a heteroreceptor complex with the fibroblast growth factor receptor 1 (FGFR1) in U-251 MG GBM cells. EphA4 promoted FGFR1-mediated cellular proliferation and migration by activating the MAPK pathway and inducing Akt phosphorylation (117).
The epidermal growth factor receptor (EGFR) acts as an oncoprotein in gliomas. Li et al. showed that ephrin-A5 acts as a tumor suppressor in gliomas. Ephrin-A5 forced expression reduced tumorigenicity of human glioma U373 cells by promoting ubiquitylation and degradation of the EGFR (118).
Upstream activation of RTKs and/or loss of the negative regulator, Phosphatase/tensin homolog deleted on chromosome 10 (PTEN), are the major causes for PI3K/AKT activation, particularly in primary GBM (119). In early studies, ephrin-A1 was demonstrated to stimulate PI3K activity via direct interaction of EphA2 with the p85 subunit of PI3K (120). Migratory glioma cells are known to have high AKT activity (121). Miao and colleagues showed that AKT was highly phosphorylated at both T308 and S473 sites upon serum stimulation in highly migratory U373 MG cells, however co-treatment with ephrin-A1 completely blocked AKT activation, suggesting a direct crosstalk between AKT and EphA2 (122). EphA2 was suggested to be a substrate for AKT, which in turn is negatively regulated by the ligand-activated EphA2. They also demonstrated that AKT-phosphorylated EphA2 on S897 was necessary for ligand-independent promotion of cell migration and invasion and the site became dephosphorylated upon ligand stimulation (122). This offers a possible explanation for the contradictory behaviour of this receptor, which can act as either oncoprotein or tumor suppressor (24). Indeed, later Yang et al., showed that the ligand-dependent activation of EphA2 decreased the growth of PC3 prostate cancer cells and inhibited the AKT-mTORC1 pathway, which was hyperactivated due to loss of PTEN (123). The same pathways have been highlighted by a recent publication, demonstrating that alterations in EphB2 activity induced several changes among the members of the PI3K–AKT–mTOR pathway and the RAS–RAF–MEK–ERK pathway in DAOY medulloblastoma cells (78).
Therapeutic strategies
Standard treatments of glioma patients include surgery, radiation therapy, and chemotherapy (124, 125). Surgery, or radio-surgery, allows total or partial resection of the tumor depending on the location. However, the treatment does not lead to cures nor to long-survival benefits. Brain tumors are also poorly accessible to circulating drugs because of the blood-brain barrier (BBB) or blood-brain tumor-barrier (BBTB), further increasing the obstacles facing effective treatment. New therapeutic directions focus on the employment of agents specifically targeting tumors while preserving the surrounding healthy brain. In general, the strategies can be divided into active and passive immunotherapies, cytotoxic agents and targeted agents, and small molecule inhibitors. Convection-enhanced delivery (CED) seems to be the most promising approach to deliver drugs locally into the tumor or tumor resection cavity, because it bypasses both BBB and BBTB (15, 126–128).
General RTKs inhibitors, like Dasatinib and Regorafenib, have been tested in brain tumor patients. Dasatinib (Sprycel, BMS-354825, Bristol-Myers Squibb) is an oral inhibitor of multiple targets, including c-KIT, Src, and PDGFRA and B (129). Dasatinib inhibits EphA2 directly with an IC50 of 17 nmol l−1 in sensitive breast cancer cells (130) and EphB2 at similar concentrations (131). It was shown to inhibit ligand-induced EphA2 internalization and subsequent degradation in pancreatic cell lines (132). Of interest, dasatinib inhibited the Src family of kinases, involved in the Eph downstream signalling, significantly suppressing proliferation of primary glioma cells, but it had no measurable inhibitory effect on the growth of glioma stem-like cells (133). The results of a phase I clinical trial of vandetanib, a VEGFR-2 inhibitor, combined with dasatinib, during and after radiotherapy, in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG), were recently published. Unfortunately, the combinatorial therapy did not change the poor prognosis for children with DIPG (134).
Regorafenib (BAY 73–4506, commercial name Stivarga) shows anti-angiogenic activity due to the targeting of VEGFR2 and TIE2 tyrosine kinase inhibition (135). Sorafenib/regorafenib, in combination with lapatinib, killed multiple primary human GBM tumor isolates in a greater than additive manner, in a process that involves induction of endoplasmic reticulum stress, autophagy, and intrinsic and extrinsic apoptotic pathways (136). To our knowledge there are no published studies aiming to decipher possible relationship between Regorafenib and the Eph/ephrin system. Of interest, a recent study identified and characterized doxazosin as a novel small molecule agonist specifically for EphA2 and EphA4. Similarly to ephrin-A1, doxazosin inhibited Akt and ERK kinase activities in an EphA2-dependent manner. Treatment with doxazosin induced receptor internalization suppressing cellular migration of prostate, breast and glioma cancer cells (137).
Different therapeutic strategies have been developed to specifically target Eph receptors that are overexpressed in gliomas. Being that EphA2 overexpression was found in 60% of GBMs and was associated with poor prognosis, Debinski and colleagues produced an ephrin-A1-based cytotoxin to specifically target EphA2 overexpressing cells. The cytotoxin was generated by chemically conjugating dimeric ephrin-A1-Fc with a modified version of the Pseudomonas exotoxin A. The cytotoxin killed GBM cells in vitro with an IC50 of 10−11 mol l−1, and in vivo (12). Because of the very promising in vitro and in vivo preclinical data, the cytotoxin in combination is now in Phase I clinical trial in dogs with spontaneous gliomas.
EphA3 is overexpressed in about 40% of the clinical specimens, on tumor cells, stroma and vasculature. This receptor is specifically targeted by a monoclonal antibody (mAb IIIA4). Recently, Day et al. showed that the in vivo targeting of EphA3 with radiolabelled mAb IIIA4 with the beta-emitting radionucleotide lutetium (177Lu) was very effective in preventing tumor formation possibly by targeting the tumor-initiating cells, with minimal toxicity to normal tissues (89).
A humanized anti-EphB4 monoclonal antibody has also been produced (hAb47), and conjugated to Cy5.5 to produce Cy5.5-hAb47; future clinical applications are envisioned in the near-infrared (NIR) fluorescence imaging of EphB4 expression in tumors (138). Mingyue and colleagues identified an EphB6 variant (EphB6v) analyzing a panel of brain tumor cell lines and GBM specimens. EphB6v has a unique 54 amino acid sequence at the C-terminus that was not found in normal EphB6. EphB6v is preferentially expressed in malignant brain tumors, such as GBM, and anaplastic astrocytomas (139). Two EphB6v-derived peptides have been identified to specifically recognize cytotoxic T lymphocytes (CTLs) in vitro, in the peripheral blood mononuclear cells of HLA-A2(+) glioma patients, suggesting a possible future applications in peptide-based vaccine therapy in glioma patients (139).
High grade gliomas possess immunoediting properties, which refers to the two-faced effect of the immune system: host-protective and tumor-promoting (140). For example, an immunosuppressive environment is generated by secreting immune-inhibitory molecules (e.g., TGF-β, IL-10, VEGF, and others) (141, 142). The failure of an effective immune response is recognized as one of the major reasons for uncontrolled tumor growth (143), generating the rationale for the development of anti-cancer immunotherapies (7, 144).
Tumor-associated antigens (TAAs) are immunogenic, tumor-specific or tumor-associated molecules that are minimally expressed or absent in normal tissue (145). Active and passive immunotherapies are two different strategies used to generate anti-tumor activity. Active immunotherapy employs tumor-specific vaccines, like TAAs, administered in the context of a non-specific immune co-stimulation. These vaccines induce an immune response mainly by activating cytotoxic T lymphocytes (CTLs) that are able to recognize endogenous tumor antigens (146). Passive immunotherapy is also referred to as adoptive immunotherapy. Adoptive immunotherapy, or adoptive cell transfer (ACT), involves infusion of autologous lymphocytes, T lymphocytes and natural killer (NK) cells, into patientsfollowing ex vivo expansion (147). In particular, chimeric antigen receptor (CAR) T cells can be engineered with glioma-specific antigens that provide targets for CAR-based immunotherapy. Among them, the most promising appear to be IL-13 receptor alpha 2 (IL-13RA2), human epidermal growth factor receptor 2 (HER2), and EphA2 (143, 148–151).
Promising results have been obtained using an Eph-dendritic cells (DC)-based vaccine to induce both tumor antigen-specific CTLs and helper-T cells. Human leukocyte antigen (HLA) A2+ peripheral blood mononuclear cells (PBMCs), from healthy donors and glioma patients, have been stimulated with autologous dendritic cells (DCs) loaded with the synthetic EphA2883–891 (TLADFDPRV) peptide. The stimulation induced an antigen-specific, anti-glioma CTL response in HLA-A2+ patient-derived PBMCs (152).
More recently, a phase I/II trial was performed using polarized dendritic cells (αDC1) loaded with synthetic peptides for glioma-associated antigen (GAA) and stabilized by lysine and carboxymethylcellulose (poly-ICLC) (153). GAAs used are EphA2, IL-13Rα2, YKL-40, and gp100. The trial demonstrated not only safety of the vaccination therapy, but also the induction of an anti-glioma immune response, which resulted in GBM progression free for at least 12 months in around 40% of the enrolled patients (153).
Concluding remarks
Ephs and ephrins are certainly one of the most intriguing families of RTKs and corresponding ligands, and one of the most promising candidates in targeted therapies of malignant gliomas (154, 155). It is not surprising to detect Ephs and ephrins abnormal expression in tumor cells, as these proteins are usually expressed during developmental processes. Moreover, progenitor or tumor-initiating cells also rely on the presence and function of these proteins., However, overexpression and downstream signaling of Ephs and ephrins in cancer are highly tissue and cell type specific, generating tremendous biological variability; the same Eph receptor can act as an oncoprotein or as a tumor suppressor. In addition, a crosstalk between Ephs and the receptors of different families potentiate the complexity of the signaling cascade. The major challenge is to contextualize the aberrant expression and signaling of these receptors and ligands within the tumor environment and different subpopulations of cells present within the tumors. EphA2 and EphA3 in particular are the receptors overexpressed not only in glioma tumor cells, but also in tumor vasculature tumor cells infiltrating normal brain and immune cells infiltrating tumors. New therapeutics specifically targeting these receptors showed preliminarily promising results. Moving towards that direction will help to deepen our understanding of the relationship between this ligand-receptor system and the pathobiology of malignant gliomas, and possibly offer urgently needed more effective therapeutic strategies.
Table 1.
Summary of Ephs and Ephrins expression, functional role and localization in gliomas.
| Ephs/ Ephrins | Expression / Survival | Ligand-Dependent Effect | Downstream Signaling After Ligand-dependent Stimulation | Localization Within the Tumor | Reference |
|---|---|---|---|---|---|
| EphA2 | ↑ / ↓ | ↓ Migration and proliferation. | ↓ The MAPK pathway and blocks Akt activation. | Tumor cells, vasculature and stem cells. | (43, 45, 59, 106, 113, 122) |
| EphA3 | ↑ / ↓ | ↓ Proliferation. | ↓ The MAPK pathway. | Tumor cells, vasculature, stem cells and stroma. | (89, 156) |
| EphA4 | ↑ / - | ↑ Migration and proliferation if FGF stimulated. | ↑ The MAPK pathway and phosphorylates Akt. | Tumor cells. | (117) |
| EphA5 | ↓ / ↑ | No significant decrease in cell proliferation. | - | Plasma and platelets. | (61) |
| EphA7 | ↑ / ↓ | - | - | Tumor microvasculature. | (60) |
| ephrin-A1 | ↓ / - | Down-regulates EphA2. | - | Very low levels on tumor cells. | (45) |
| ephrin-A5 | ↓ / - | Down-regulates EphA2 and EphA3. | ↓ EGFR | Very low levels on tumor cells. | (45, 118) |
| EphB1 | ↓ / ↑ | ↓ Migration and invasion. | ↑ ERK1/2 * | - | (70) |
| EphB2 | ↑ / - | ↑ Invasion. | ↓ The MAPK and the PI3K–Akt– mTOR pathways. | Tumor vasculature. | (76–78) |
| EphB3 | ↑ / - | - | - | Tumor cells. | (70) |
| EphB4 | ↑ / ↓ | - | Pro-angiogenic effect. | Tumor cells. | (69, 98) |
| ephrin-B1 | ↑ / - | ↑ Invasion. | - | - | (68) |
| ephrin-B2 | ↑ / ↓ | ↑ Migration and invasion. | Induce VEGFR2 internalization and the downstream pathways and vessel sprouting. | - | (68, 69, 98) |
| ephrin-B3 | ↑ / - | ↑ Invasion. | - | - | (68) |
Abbreviations: ↑= Increased; ↓= Decreased; - = No data; * = not shown in gliomas; MAPK= mitogen-activated protein kinase; PI3K= phosphatidylinositol-4,5-bisphosphate 3-kinase; EGFR= epidermal growth factor receptor; mTOR= mammalian target of rapamycin; ERK= extracellular signal-regulated kinases; VEGFR2= vascular endothelial growth factor receptor-2.
References
- 1.Wilson TA, Karajannis MA, Harter DH. Glioblastoma multiforme: State of the art and future therapeutics. Surg Neurol Int. 2014;5:64. doi: 10.4103/2152-7806.132138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CBTRUS. http://www.cbtrus.org/2012-NPCR-SEER/CBTRUS_Report_2004-2008_3-23-2012.pdf.
- 3.Altieri R, Agnoletti A, Quattrucci F, Garbossa D, Calamo Specchia FM, Bozzaro M, Fornaro R, Mencarani C, Lanotte M, Spaziante R, Ducati A. Molecular biology of gliomas: present and future challenges. Transl Med UniSa. 2014;10:29–37. [PMC free article] [PubMed] [Google Scholar]
- 4.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reardon DA, Freeman G, Wu C, Chiocca EA, Wucherpfennig KW, Wen PY, Fritsch EF, Curry WT, Jr, Sampson JH, Dranoff G. Immunotherapy advances for glioblastoma. Neuro Oncol. 2014 doi: 10.1093/neuonc/nou212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass R, Synowitz M. CNS macrophages and peripheral myeloid cells in brain tumours. Acta Neuropathol. 2014;128:347–362. doi: 10.1007/s00401-014-1274-2. [DOI] [PubMed] [Google Scholar]
- 7.Wainwright DA, Nigam P, Thaci B, Dey M, Lesniak MS. Recent developments on immunotherapy for brain cancer. Expert Opin Emerg Drugs. 2012;17:181–202. doi: 10.1517/14728214.2012.679929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagane M. Anti-angiogenic therapy for malignant glioma. Gan To Kagaku Ryoho. 2014;41:141–147. [PubMed] [Google Scholar]
- 9.Parmaksiz G, Czabanka M, Vinci M, Vajkoczy P. Antiangiogenic therapy inhibits the recruitment of vascular accessory cells to the perivascular niche in glioma angiogenesis. J Vasc Res. 2014;51:102–109. doi: 10.1159/000357620. [DOI] [PubMed] [Google Scholar]
- 10.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ, Jr, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6:3208–3218. doi: 10.1158/1535-7163.MCT-07-0200. [DOI] [PubMed] [Google Scholar]
- 13.Mintz A, Gibo DM, Madhankumar AB, Debinski W. Molecular targeting with recombinant cytotoxins of interleukin-13 receptor alpha2-expressing glioma. J Neurooncol. 2003;64:117–123. doi: 10.1007/BF02700026. [DOI] [PubMed] [Google Scholar]
- 14.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 15.Debinski W. Local treatment of brain tumors with targeted chimera cytotoxic proteins. Cancer Invest. 2002;20:801–809. doi: 10.1081/cnv-120003545. [DOI] [PubMed] [Google Scholar]
- 16.Daniel P, Filiz G, Brown DV, Hollande F, Gonzales M, D’Abaco G, Papalexis N, Phillips WA, Malaterre J, Ramsay RG, Mantamadiotis T. Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis. 2014;3:e108. doi: 10.1038/oncsis.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gont A, Hanson JE, Lavictoire SJ, Parolin DA, Daneshmand M, Restall IJ, Soucie M, Nicholas G, Woulfe J, Kassam A, Da Silva VF, Lorimer IA. PTEN loss represses glioblastoma tumor initiating cell differentiation via inactivation of Lgl1. Oncotarget. 2013;4:1266–1279. doi: 10.18632/oncotarget.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wick W, Weller M, Weiler M, Batchelor T, Yung AW, Platten M. Pathway inhibition: emerging molecular targets for treating glioblastoma. Neuro Oncol. 2011;13:566–579. doi: 10.1093/neuonc/nor039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Song W, Amato K. Eph receptor tyrosine kinases in cancer stem cells. Cytokine Growth Factor Rev. 2014 doi: 10.1016/j.cytogfr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakada M, Kita D, Teng L, Pyko IV, Watanabe T, Hayashi Y, Hamada J. Receptor tyrosine kinases: principles and functions in glioma invasion. Adv Exp Med Biol. 2013;986:143–170. doi: 10.1007/978-94-007-4719-7_8. [DOI] [PubMed] [Google Scholar]
- 23.Beauchamp A, Debinski W. Ephs and ephrins in cancer: ephrin-A1 signalling. Semin Cell Dev Biol. 2012;23:109–115. doi: 10.1016/j.semcdb.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6:1795–1806. doi: 10.1158/1541-7786.MCR-08-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein R, Kania A. Ephrin signalling in the developing nervous system. Curr Opin Neurobiol. 2014;27:16–24. doi: 10.1016/j.conb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Chatzizacharias NA, Giaginis CT, Agapitos E, Theocharis SE. The role of ephrins’ receptors and ephrins’ ligands in normal placental development and disease. Expert Opin Ther Targets. 2014;18:269–275. doi: 10.1517/14728222.2014.864638. [DOI] [PubMed] [Google Scholar]
- 27.Cavodeassi F, Ivanovitch K, Wilson SW. Eph/Ephrin signalling maintains eye field segregation from adjacent neural plate territories during forebrain morphogenesis. Development. 2013;140:4193–4202. doi: 10.1242/dev.097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov. 2014;13:39–62. doi: 10.1038/nrd4175. [DOI] [PubMed] [Google Scholar]
- 29.Cramer KS, Gabriele ML. Axon guidance in the auditory system: Multiple functions of Eph receptors. Neuroscience. 2014;277C:152–162. doi: 10.1016/j.neuroscience.2014.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clifford MA, Athar W, Leonard CE, Russo A, Sampognaro PJ, Van der Goes MS, Burton DA, Zhao X, Lalchandani RR, Sahin M, Vicini S, Donoghue MJ. EphA7 signaling guides cortical dendritic development and spine maturation. Proc Natl Acad Sci U S A. 2014;111:4994–4999. doi: 10.1073/pnas.1323793111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ende G, Poitz DM, Strasser RH, Jellinghaus S. The role of the Eph/ephrin-system in atherosclerotic plaque development: a complex puzzle. Cardiovasc Pathol. 2014;23:251. doi: 10.1016/j.carpath.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Baumann G, Travieso L, Liebl DJ, Theus MH. Pronounced hypoxia in the subventricular zone following traumatic brain injury and the neural stem/progenitor cell response. Exp Biol Med (Maywood) 2013;238:830–841. doi: 10.1177/1535370213494558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coulthard MG, Morgan M, Woodruff TM, Arumugam TV, Taylor SM, Carpenter TC, Lackmann M, Boyd AW. Eph/Ephrin signaling in injury and inflammation. Am J Pathol. 2012;181:1493–1503. doi: 10.1016/j.ajpath.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 34.Frugier T, Conquest A, McLean C, Currie P, Moses D, Goldshmit Y. Expression and activation of EphA4 in the human brain after traumatic injury. J Neuropathol Exp Neurol. 2012;71:242–250. doi: 10.1097/NEN.0b013e3182496149. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi Y, Itoh M, Nara N, Tohda S. Effect of EPH-ephrin signaling on the growth of human leukemia cells. Anticancer Res. 2014;34:2913–2918. [PubMed] [Google Scholar]
- 36.Giaginis C, Tsoukalas N, Bournakis E, Alexandrou P, Kavantzas N, Patsouris E, Theocharis S. Ephrin (Eph) receptor A1, A4, A5 and A7 expression in human non-small cell lung carcinoma: associations with clinicopathological parameters, tumor proliferative capacity and patients’ survival. BMC Clin Pathol. 2014;14:8. doi: 10.1186/1472-6890-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin H, Lu C, Tang Y, Wang H, Wang J. Enhanced expression of EphrinB1 is associated with lymph node metastasis and poor prognosis in breast cancer. Cancer Biomark. 2013;13:261–267. doi: 10.3233/CBM-130356. [DOI] [PubMed] [Google Scholar]
- 38.Lisle JE, Mertens-Walker I, Rutkowski R, Herington AC, Stephenson SA. Eph receptors and their ligands: promising molecular biomarkers and therapeutic targets in prostate cancer. Biochim Biophys Acta. 2013;1835:243–257. doi: 10.1016/j.bbcan.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 40.Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 41.Paavilainen S, Grandy D, Karelehto E, Chang E, Susi P, Erdjument-Bromage H, Nikolov D, Himanen J. High-level expression of a full-length Eph receptor. Protein Expr Purif. 2013;92:112–118. doi: 10.1016/j.pep.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daar IO. Non-SH2/PDZ reverse signaling by ephrins. Semin Cell Dev Biol. 2012;23:65–74. doi: 10.1016/j.semcdb.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferluga S, Hantgan R, Goldgur Y, Himanen JP, Nikolov DB, Debinski W. Biological and structural characterization of glycosylation on ephrin-A1, a preferred ligand for EphA2 receptor tyrosine kinase. J Biol Chem. 2013;288:18448–18457. doi: 10.1074/jbc.M113.464008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruce V, Olivieri G, Eickelberg O, Miescher GC. Functional activation of EphA5 receptor does not promote cell proliferation in the aberrant EphA5 expressing human glioblastoma U-118 MG cell line. Brain Res. 1999;821:169–176. doi: 10.1016/s0006-8993(99)01112-9. [DOI] [PubMed] [Google Scholar]
- 45.Wykosky J, Palma E, Gibo DM, Ringler S, Turner CP, Debinski W. Soluble monomeric EphrinA1 is released from tumor cells and is a functional ligand for the EphA2 receptor. Oncogene. 2008;27:7260–7273. doi: 10.1038/onc.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker-Daniels J, Riese DJ, 2nd, Kinch MS. c-Cbl-dependent EphA2 protein degradation is induced by ligand binding. Mol Cancer Res. 2002;1:79–87. [PubMed] [Google Scholar]
- 47.Mancia F, Shapiro L. ADAM and Eph: how Ephrin-signaling cells become detached. Cell. 2005;123:185–187. doi: 10.1016/j.cell.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Beauchamp A, Lively MO, Mintz A, Gibo D, Wykosky J, Debinski W. EphrinA1 is released in three forms from cancer cells by matrix metalloproteases. Mol Cell Biol. 2012;32:3253–3264. doi: 10.1128/MCB.06791-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen JP, Lackmann M, Nikolov DB. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, Groves JT. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science. 2010;327:1380–1385. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ieguchi K, Tomita T, Omori T, Komatsu A, Deguchi A, Masuda J, Duffy SL, Coulthard MG, Boyd A, Maru Y. ADAM12-cleaved ephrin-A1 contributes to lung metastasis. Oncogene. 2014;33:2179–2190. doi: 10.1038/onc.2013.180. [DOI] [PubMed] [Google Scholar]
- 52.Greene AC, Lord SJ, Tian A, Rhodes C, Kai H, Groves JT. Spatial organization of EphA2 at the cell-cell interface modulates trans-endocytosis of ephrinA1. Biophys J. 2014;106:2196–2205. doi: 10.1016/j.bpj.2014.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- 54.Janes PW, Nievergall E, Lackmann M. Concepts and consequences of Eph receptor clustering. Semin Cell Dev Biol. 2012;23:43–50. doi: 10.1016/j.semcdb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Julich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- 56.Meyer S, Hafner C, Guba M, Flegel S, Geissler EK, Becker B, Koehl GE, Orso E, Landthaler M, Vogt T. Ephrin-B2 overexpression enhances integrin-mediated ECM-attachment and migration of B16 melanoma cells. Int J Oncol. 2005;27:1197–1206. [PubMed] [Google Scholar]
- 57.Parri M, Buricchi F, Giannoni E, Grimaldi G, Mello T, Raugei G, Ramponi G, Chiarugi P. EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility. J Biol Chem. 2007;282:19619–19628. doi: 10.1074/jbc.M701319200. [DOI] [PubMed] [Google Scholar]
- 58.Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63:610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3:541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 60.Wang LF, Fokas E, Juricko J, You A, Rose F, Pagenstecher A, Engenhart-Cabillic R, An HX. Increased expression of EphA7 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. BMC Cancer. 2008;8:79. doi: 10.1186/1471-2407-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Almog N, Ma L, Raychowdhury R, Schwager C, Erber R, Short S, Hlatky L, Vajkoczy P, Huber PE, Folkman J, Abdollahi A. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69:836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- 62.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 63.Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN, Kinch MS, Kiener PA, Sood AK. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–5150. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 64.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–1456. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 65.Li X, Wang Y, Zhen H, Yang H, Fei Z, Zhang J, Liu W, Zhang X. Expression of EphA2 in human astrocytic tumors: correlation with pathologic grade, proliferation and apoptosis. Tumour Biol. 2007;28:165–172. doi: 10.1159/000103010. [DOI] [PubMed] [Google Scholar]
- 66.Liu F, Park PJ, Lai W, Maher E, Chakravarti A, Durso L, Jiang X, Yu Y, Brosius A, Thomas M, Chin L, Brennan C, DePinho RA, Kohane I, Carroll RS, Black PM, Johnson MD. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815–10823. doi: 10.1158/0008-5472.CAN-06-1408. [DOI] [PubMed] [Google Scholar]
- 67.Wang LF, Fokas E, Bieker M, Rose F, Rexin P, Zhu Y, Pagenstecher A, Engenhart-Cabillic R, An HX. Increased expression of EphA2 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. Oncol Rep. 2008;19:151–156. [PubMed] [Google Scholar]
- 68.Nakada M, Anderson EM, Demuth T, Nakada S, Reavie LB, Drake KL, Hoelzinger DB, Berens ME. The phosphorylation of ephrin-B2 ligand promotes glioma cell migration and invasion. Int J Cancer. 2010;126:1155–1165. doi: 10.1002/ijc.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tu Y, He S, Fu J, Li G, Xu R, Lu H, Deng J. Expression of EphrinB2 and EphB4 in glioma tissues correlated to the progression of glioma and the prognosis of glioblastoma patients. Clin Transl Oncol. 2012;14:214–220. doi: 10.1007/s12094-012-0786-2. [DOI] [PubMed] [Google Scholar]
- 70.Teng L, Nakada M, Furuyama N, Sabit H, Furuta T, Hayashi Y, Takino T, Dong Y, Sato H, Sai Y, Miyamoto K, Berens ME, Zhao SG, Hamada J. Ligand-dependent EphB1 signaling suppresses glioma invasion and correlates with patient survival. Neuro Oncol. 2013;15:1710–1720. doi: 10.1093/neuonc/not128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;99:1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 72.Coulthard MG, Duffy S, Down M, Evans B, Power M, Smith F, Stylianou C, Kleikamp S, Oates A, Lackmann M, Burns GF, Boyd AW. The role of the Eph-ephrin signalling system in the regulation of developmental patterning. Int J Dev Biol. 2002;46:375–384. [PubMed] [Google Scholar]
- 73.Palmer A, Klein R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev. 2003;17:1429–1450. doi: 10.1101/gad.1093703. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi Y, Pasquale EB. Eph receptors in the adult brain. Curr Opin Neurobiol. 2004;14:288–296. doi: 10.1016/j.conb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 76.Nakada M, Niska JA, Miyamori H, McDonough WS, Wu J, Sato H, Berens ME. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004;64:3179–3185. doi: 10.1158/0008-5472.can-03-3667. [DOI] [PubMed] [Google Scholar]
- 77.Wang SD, Rath P, Lal B, Richard JP, Li Y, Goodwin CR, Laterra J, Xia S. EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene. 2012;31:5132–5143. doi: 10.1038/onc.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sikkema AH, den Dunnen WF, Hulleman E, van Vuurden DG, Garcia-Manero G, Yang H, Scherpen FJ, Kampen KR, Hoving EW, Kamps WA, Diks SH, Peppelenbosch MP, de Bont ES. EphB2 activity plays a pivotal role in pediatric medulloblastoma cell adhesion and invasion. Neuro Oncol. 2012;14:1125–1135. doi: 10.1093/neuonc/nos130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 80.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 81.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2012;60:502–514. doi: 10.1002/glia.21264. [DOI] [PubMed] [Google Scholar]
- 83.Clavreul A, Guette C, Faguer R, Tetaud C, Boissard A, Lemaire L, Rousseau A, Avril T, Henry C, Coqueret O, Menei P. Glioblastoma-associated stromal cells (GASCs) from histologically normal surgical margins have a myofibroblast phenotype and angiogenic properties. J Pathol. 2014;233:74–88. doi: 10.1002/path.4332. [DOI] [PubMed] [Google Scholar]
- 84.Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012;14:958–978. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 86.Brantley-Sieders D, Schmidt S, Parker M, Chen J. Eph receptor tyrosine kinases in tumor and tumor microenvironment. Curr Pharm Des. 2004;10:3431–3442. doi: 10.2174/1381612043383160. [DOI] [PubMed] [Google Scholar]
- 87.Jellinghaus S, Poitz DM, Ende G, Augstein A, Weinert S, Stutz B, Braun-Dullaeus RC, Pasquale EB, Strasser RH. Ephrin-A1/EphA4-mediated adhesion of monocytes to endothelial cells. Biochim Biophys Acta. 2013;1833:2201–2211. doi: 10.1016/j.bbamcr.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 88.Yu G, Luo H, Wu Y, Wu J. Ephrin B2 induces T cell costimulation. J Immunol. 2003;171:106–114. doi: 10.4049/jimmunol.171.1.106. [DOI] [PubMed] [Google Scholar]
- 89.Day BW, Stringer BW, Al-Ejeh F, Ting MJ, Wilson J, Ensbey KS, Jamieson PR, Bruce ZC, Lim YC, Offenhauser C, Charmsaz S, Cooper LT, Ellacott JK, Harding A, Leveque L, Inglis P, Allan S, Walker DG, Lackmann M, Osborne G, Khanna KK, Reynolds BA, Lickliter JD, Boyd AW. EphA3 maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme. Cancer Cell. 2013;23:238–248. doi: 10.1016/j.ccr.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 90.Vail ME, Murone C, Tan A, Hii L, Abebe D, Janes PW, Lee FT, Baer M, Palath V, Bebbington C, Yarranton G, Llerena C, Garic S, Abramson D, Cartwright G, Scott AM, Lackmann M. Targeting EphA3 inhibits cancer growth by disrupting the tumor stromal microenvironment. Cancer Res. 2014;74:4470–4481. doi: 10.1158/0008-5472.CAN-14-0218. [DOI] [PubMed] [Google Scholar]
- 91.Baglole CJ, Ray DM, Bernstein SH, Feldon SE, Smith TJ, Sime PJ, Phipps RP. More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunol Invest. 2006;35:297–325. doi: 10.1080/08820130600754960. [DOI] [PubMed] [Google Scholar]
- 92.Ziyad S, Iruela-Arispe ML. Molecular mechanisms of tumor angiogenesis. Genes Cancer. 2011;2:1085–1096. doi: 10.1177/1947601911432334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 94.Cheng N, Brantley DM, Chen J. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 2002;13:75–85. doi: 10.1016/s1359-6101(01)00031-4. [DOI] [PubMed] [Google Scholar]
- 95.Oike Y, Ito Y, Hamada K, Zhang XQ, Miyata K, Arai F, Inada T, Araki K, Nakagata N, Takeya M, Kisanuki YY, Yanagisawa M, Gale NW, Suda T. Regulation of vasculogenesis and angiogenesis by EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells. Blood. 2002;100:1326–1333. [PubMed] [Google Scholar]
- 96.Bai J, Wang YJ, Liu L, Zhao YL. Ephrin B2 and EphB4 selectively mark arterial and venous vessels in cerebral arteriovenous malformation. J Int Med Res. 2014;42:405–415. doi: 10.1177/0300060513478091. [DOI] [PubMed] [Google Scholar]
- 97.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 98.Noren NK, Lu M, Freeman AL, Koolpe M, Pasquale EB. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc Natl Acad Sci U S A. 2004;101:5583–5588. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brantley-Sieders DM, Fang WB, Hicks DJ, Zhuang G, Shyr Y, Chen J. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J. 2005;19:1884–1886. doi: 10.1096/fj.05-4038fje. [DOI] [PubMed] [Google Scholar]
- 100.Cheng N, Brantley DM, Liu H, Lin Q, Enriquez M, Gale N, Yancopoulos G, Cerretti DP, Daniel TO, Chen J. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol Cancer Res. 2002;1:2–11. [PubMed] [Google Scholar]
- 101.Germano IM, Binello E. Stem cells and gliomas: past, present, and future. J Neurooncol. 2014;119:547–555. doi: 10.1007/s11060-014-1498-y. [DOI] [PubMed] [Google Scholar]
- 102.Altaner C. Glioblastoma and stem cells. Neoplasma. 2008;55:369–374. [PubMed] [Google Scholar]
- 103.Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, Greve JM, Soriano RH, Gilmour LL, Rivers CS, Modrusan Z, Nacu S, Guerrero S, Edgar KA, Wallin JJ, Lamszus K, Westphal M, Heim S, James CD, VandenBerg SR, Costello JF, Moorefield S, Cowdrey CJ, Prados M, Phillips HS. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–375. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 104.Carruthers R, Ahmed SU, Strathdee K, Gomez-Roman N, Amoah-Buahin E, Watts C, Chalmers AJ. Abrogation of radioresistance in glioblastoma stem-like cells by inhibition of ATM kinase. Mol Oncol. 2014 doi: 10.1016/j.molonc.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Binda E, Visioli A, Giani F, Lamorte G, Copetti M, Pitter KL, Huse JT, Cajola L, Zanetti N, DiMeco F, De Filippis L, Mangiola A, Maira G, Anile C, De Bonis P, Reynolds BA, Pasquale EB, Vescovi AL. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. Cancer Cell. 2012;22:765–780. doi: 10.1016/j.ccr.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miao H, Gale NW, Guo H, Qian J, Petty A, Kaspar J, Murphy AJ, Valenzuela DM, Yancopoulos G, Hambardzumyan D, Lathia JD, Rich JN, Lee J, Wang B. EphA2 promotes infiltrative invasion of glioma stem cells in vivo through cross-talk with Akt and regulates stem cell properties. Oncogene. 2014 doi: 10.1038/onc.2013.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hong B, van den Heuvel AP, Prabhu VV, Zhang S, El-Deiry WS. Targeting tumor suppressor p53 for cancer therapy: strategies, challenges and opportunities. Curr Drug Targets. 2014;15:80–89. doi: 10.2174/1389450114666140106101412. [DOI] [PubMed] [Google Scholar]
- 110.Dohn M, Jiang J, Chen X. Receptor tyrosine kinase EphA2 is regulated by p53-family proteins and induces apoptosis. Oncogene. 2001;20:6503–6515. doi: 10.1038/sj.onc.1204816. [DOI] [PubMed] [Google Scholar]
- 111.Yu J, Zhang L, Hwang PM, Rago C, Kinzler KW, Vogelstein B. Identification and classification of p53-regulated genes. Proc Natl Acad Sci U S A. 1999;96:14517–14522. doi: 10.1073/pnas.96.25.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pratt RL, Kinch MS. Activation of the EphA2 tyrosine kinase stimulates the MAP/ERK kinase signaling cascade. Oncogene. 2002;21:7690–7699. doi: 10.1038/sj.onc.1205758. [DOI] [PubMed] [Google Scholar]
- 113.Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, Gray JW, McCormick F. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 114.Elowe S, Holland SJ, Kulkarni S, Pawson T. Downregulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol Cell Biol. 2001;21:7429–7441. doi: 10.1128/MCB.21.21.7429-7441.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tong J, Elowe S, Nash P, Pawson T. Manipulation of EphB2 regulatory motifs and SH2 binding sites switches MAPK signaling and biological activity. J Biol Chem. 2003;278:6111–6119. doi: 10.1074/jbc.M208972200. [DOI] [PubMed] [Google Scholar]
- 116.Vindis C, Cerretti DP, Daniel TO, Huynh-Do U. EphB1 recruits c-Src and p52Shc to activate MAPK/ERK and promote chemotaxis. J Cell Biol. 2003;162:661–671. doi: 10.1083/jcb.200302073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fukai J, Yokote H, Yamanaka R, Arao T, Nishio K, Itakura T. EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol Cancer Ther. 2008;7:2768–2778. doi: 10.1158/1535-7163.MCT-07-2263. [DOI] [PubMed] [Google Scholar]
- 118.Li JJ, Liu DP, Liu GT, Xie D. EphrinA5 acts as a tumor suppressor in glioma by negative regulation of epidermal growth factor receptor. Oncogene. 2009;28:1759–1768. doi: 10.1038/onc.2009.15. [DOI] [PubMed] [Google Scholar]
- 119.McNamara MG, Sahebjam S, Mason WP. Emerging biomarkers in glioblastoma. Cancers (Basel) 2013;5:1103–1119. doi: 10.3390/cancers5031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pandey A, Lazar DF, Saltiel AR, Dixit VM. Activation of the Eck receptor protein tyrosine kinase stimulates phosphatidylinositol 3-kinase activity. J Biol Chem. 1994;269:30154–30157. [PubMed] [Google Scholar]
- 121.Joy AM, Beaudry CE, Tran NL, Ponce FA, Holz DR, Demuth T, Berens ME. Migrating glioma cells activate the PI3-K pathway and display decreased susceptibility to apoptosis. J Cell Sci. 2003;116:4409–4417. doi: 10.1242/jcs.00712. [DOI] [PubMed] [Google Scholar]
- 122.Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, Sloan AE, Cohen ML, Wang B. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang NY, Fernandez C, Richter M, Xiao Z, Valencia F, Tice DA, Pasquale EB. Crosstalk of the EphA2 receptor with a serine/threonine phosphatase suppresses the Akt-mTORC1 pathway in cancer cells. Cell Signal. 2011;23:201–212. doi: 10.1016/j.cellsig.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 125.Stupp R, HM, Gorlia T, et al. Standard chemoradiotherapy ± cilengitide in newly diagnosed glioblastoma (GBM): Updated results and subgroup analyses of the international randomized phase III CENTRIC trial (EORTC trial #26071–22072/Canadian Brain Tumor Consortium). Program and abstracts of the European Cancer Congress; 2013; Amsterdam, The Netherlands. 2013. p. Abstract 3302. [Google Scholar]
- 126.Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009;9:1519–1527. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yun J, Rothrock RJ, Canoll P, Bruce JN. Convection-enhanced delivery for targeted delivery of antiglioma agents: the translational experience. J Drug Deliv. 2013;2013:107573. doi: 10.1155/2013/107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vogelbaum MA, Iannotti CA. Convection-enhanced delivery of therapeutic agents into the brain. Handb Clin Neurol. 2012;104:355–362. doi: 10.1016/B978-0-444-52138-5.00023-2. [DOI] [PubMed] [Google Scholar]
- 129.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 130.Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, Lee F, Shaw P, Clark E. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 131.Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Kocher T, Superti-Furga G. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 132.Chang Q, Jorgensen C, Pawson T, Hedley DW. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer. 2008;99:1074–1082. doi: 10.1038/sj.bjc.6604676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han X, Zhang W, Yang X, Wheeler CG, Langford CP, Wu L, Filippova N, Friedman GK, Ding Q, Fathallah-Shaykh HM, Gillespie GY, Nabors LB. The role of Src family kinases in growth and migration of glioma stem cells. Int J Oncol. 2014;45:302–310. doi: 10.3892/ijo.2014.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Broniscer A, Baker SD, Wetmore C, Pai Panandiker AS, Huang J, Davidoff AM, Onar-Thomas A, Panetta JC, Chin TK, Merchant TE, Baker JN, Kaste SC, Gajjar A, Stewart CF. Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin Cancer Res. 2013;19:3050–3058. doi: 10.1158/1078-0432.CCR-13-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, Thierauch KH, Zopf D. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 136.Hamed HA, Tavallai S, Grant S, Poklepovic A, Dent P. Sorafenib/Regorafenib and Lapatinib interact to kill CNS tumor cells. J Cell Physiol. 2014 doi: 10.1002/jcp.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Petty A, Myshkin E, Qin H, Guo H, Miao H, Tochtrop GP, Hsieh JT, Page P, Liu L, Lindner DJ, Acharya C, MacKerell AD, Jr, Ficker E, Song J, Wang B. A small molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor cell migration in vitro and prostate cancer metastasis in vivo. PLoS One. 2012;7:e42120. doi: 10.1371/journal.pone.0042120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leung K. Cy5.5-Anti-ephrin receptor B4 (EphB4) humanized monoclonal antibody hAb47. 2004 [PubMed] [Google Scholar]
- 139.Jin M, Komohara Y, Shichijo S, Yamanaka R, Nikawa J, Itoh K, Yamada A. Erythropoietin-producing hepatocyte B6 variant-derived peptides with the ability to induce glioma-reactive cytotoxic T lymphocytes in human leukocyte antigen-A2+ glioma patients. Cancer Sci. 2008;99:1656–1662. doi: 10.1111/j.1349-7006.2008.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 141.Golik AN. Characteristics of premorbid (pre-manifest) personality traits in schizophrenia in children and adolescents with psychopathic disorders. Zh Nevropatol Psikhiatr Im S S Korsakova. 1991;91:90–93. [PubMed] [Google Scholar]
- 142.Pellegatta S, Cuppini L, Finocchiaro G. Brain cancer immunoediting: novel examples provided by immunotherapy of malignant gliomas. Expert Rev Anticancer Ther. 2011;11:1759–1774. doi: 10.1586/era.11.102. [DOI] [PubMed] [Google Scholar]
- 143.Bielamowicz K, Khawja S, Ahmed N. Adoptive cell therapies for glioblastoma. Front Oncol. 2013;3:275. doi: 10.3389/fonc.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wainwright DA, Dey M, Chang A, Lesniak MS. Targeting Tregs in Malignant Brain Cancer: Overcoming IDO. Front Immunol. 2013;4:116. doi: 10.3389/fimmu.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ruella M, Kalos M. Adoptive immunotherapy for cancer. Immunol Rev. 2014;257:14–38. doi: 10.1111/imr.12136. [DOI] [PubMed] [Google Scholar]
- 148.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5:985–990. [PubMed] [Google Scholar]
- 149.Wykosky J, Gibo DM, Stanton C, Debinski W. Interleukin-13 receptor alpha 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14:199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]
- 150.Hatano M, Kuwashima N, Tatsumi T, Dusak JE, Nishimura F, Reilly KM, Storkus WJ, Okada H. Vaccination with EphA2-derived T cell-epitopes promotes immunity against both EphA2-expressing and EphA2-negative tumors. J Transl Med. 2004;2:40. doi: 10.1186/1479-5876-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y, Grossman RG, Powell S, Lee D, Ahmed N, Gottschalk S. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21:629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hatano M, Eguchi J, Tatsumi T, Kuwashima N, Dusak JE, Kinch MS, Pollack IF, Hamilton RL, Storkus WJ, Okada H. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, Lieberman FS. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Day BW, Stringer BW, Boyd AW. Eph receptors as therapeutic targets in glioblastoma. Br J Cancer. 2014;111:1255–1261. doi: 10.1038/bjc.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nakada M, Hayashi Y, Hamada J. Role of Eph/ephrin tyrosine kinase in malignant glioma. Neuro Oncol. 2011;13:1163–1170. doi: 10.1093/neuonc/nor102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jeon HM, Kim SH, Jin X, Park JB, Joshi K, Nakano I, Kim H. Crosstalk between Glioma-Initiating Cells and Endothelial Cells Drives Tumor Progression. Cancer Res. 2014;74:4482–4492. doi: 10.1158/0008-5472.CAN-13-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]