Summary
MLL-fusion proteins can induce acute myeloid leukemias (AML) from either hematopoietic stem cells (HSC) or granulocyte macrophage progenitors (GMP), but it remains unclear if the cell of origin influences the biology of the resultant leukemia. MLL-AF9 transduced single HSC or GMP could be continuously replated, but HSC-derived clones were more likely than GMP-derived clones to initiate AML in mice. Leukemia stem cells derived from either HSC or GMP had a similar immunophenotype consistent with a maturing myeloid cell (LGMP). Gene expression analyses demonstrated that LGMP inherited gene expression programs from the cell of origin including high-level Evi-1 expression in HSC derived LGMP. The gene expression signature of LGMP derived from HSC was enriched in poor prognosis human MLL-rearranged AML in three independent data sets. Moreover, global 5’-mC levels were elevated in HSC-derived leukemias as compared to GMP-derived leukemias. This mirrored a difference seen in 5-mC between MLL-rearranged human leukemias that are either EVI1-positive or EVI1-negative. Finally, HSC derived leukemias were more resistant to chemotherapy than GMP-derived leukemias. These data demonstrate that the cell of origin influences the gene expression profile, the epigenetic state, and the drug response in AML, and that these differences can account for clinical heterogeneity within a molecularly defined group of leukemias.
Significance
Human AMLs are heterogeneous even within subtype defined by a specific genetic lesion such as MLL-translocations and this leads to variable clinical outcomes. The developmental stage (or epigenetic state) of the cell in which leukemogenic transformation is initiated may contribute to the ultimate disease phenotype. We used a well established model of MLL-AF9 mediated AML and transformation of single cells to test the relevance of the leukemia cell of origin on AML development, gene expression profiles, DNA methylation and chemotherapy response. We show that HSC derived AML models human high-risk/poor outcome AML pool, and that we can recapitulate important clinical subtypes of human MLL-rearranged AML through the transformation of different cell types. These data demonstrate that the cell of origin can influence the phenotype of a resultant leukemia even if the initiating genetic lesion is the same.
Keywords: MLL, leukemia, cell of origin, gene expression, DNA methylation, chemotherapy, drug resistance
Introduction
Acute Myeloid Leukemia (AML) is a hematopoietic disorder that results in accumulation of abnormal immature myeloid cells in the bone marrow, spleen and peripheral blood. Over 100 genetic alterations found in AML manifest in a wide range of clinical outcomes (Schlenk and Dohner, 2009). Some of the genetic alterations can be used as prognostic factors. However, cytogenetic markers indicate a trend rather than providing an absolute prediction of clinical outcome (Lowenberg, 2008; Ross et al., 2004). Recently, two groups independently identified gene expression signatures associated with poor prognosis that are reminiscent of programs found in hematopoietic stem cells (Eppert et al., 2011; Gentles et al., 2010; Mullighan et al., 2009) which suggests that gene expression programs associated with normal hematopoietic development can influence patient outcome. However, the mechanisms that influence the expression of these programs are poorly defined.
Leukemias bearing translocations involving the Mixed Lineage Leukemia (MLL) gene are found in 5–10% of AML. Over 50 different MLL fusion partners have been identified, but particular translocations show lineage specificity with the t(9;11) most common in AML (Krivtsov and Armstrong, 2007). MLL-rearranged leukemias are most often associated with clinically intermediate to unfavorable prognosis in clinical trials with 5-year disease free survival rates of 30–60% (Balgobind et al., 2009; Krauter et al., 2009). Studies nevertheless demonstrate heterogeneity of clinical outcomes within groups of patients with MLL-rearrangements, which might be due to underlying biology of the disease. Such underlying biological differences in a cytogenetically homogenous group of AML may merit re-adjustment of therapeutics or treatment for individual patients based on additional risk assignment using biomarkers. Experiments with mouse models demonstrated MLL-rearranged AML (MLL-AML) may arise from hematopoietic stem as well as myeloid progenitor cells (Cozzio et al., 2003), making the leukemia cell of origin a possible source for some of the heterogeneity of clinical outcome.
We therefore hypothesized that biomarker and prognostic heterogeneity between MLL-AML patients might be a consequence of leukemogenic transformation occurring in a number of cell types in the hematopoietic hierarchy, including stem or committed progenitors, and that the cell of origin might influence the gene expression and epigenetic programs in the fully developed leukemias. To test this hypothesis we used mouse models of MLL-AF9 AML where leukemias arise from a defined cell type. Our findings show that MLL-AF9 mediated leukemogenic transformation differs when HSC are the cells of origin as compared to GMP. MLL-AF9 transformed HSC are more likely to induce leukemia than are MLL-AF9 transformed GMP. Functionally defined leukemia stem cells (LSC) are immunophenotypically similar when initiated from HSC or GMP, but have differences in gene expression and DNA methylation that are determined by the cell of origin. These differences in gene expression and DNA methylation profiles separate human MLL-rearranged AML cases into two groups with prognostic relevance. Finally, murine AML that originated from HSC were less responsive to chemotherapy treatment in vivo than were leukemias derived from GMP. These data show that the cell of origin can influence gene expression, epigenetic, and drug response profiles in ways that are clinically relevant.
Results
MLL-AF9 transduced HSC induce leukemia more rapidly than MLL-AF9 transduced GMP
We assessed the in vivo kinetics of AML development originating from MLL-AF9 transduced KLS (Lin− CD127− CD117+ Sca1+) and GMP (Lin− CD127− CD117+ Sca1− CD16/32high CD34+) isolated from transgenic mice expressing luciferase under control of the ubiquitin promoter (Stubbs et al., 2008). The expansion of leukemias was monitored using in vivo bioluminescent imaging of recipient mice at multiple time points after transplantation. Transplantation of 5–15×103 MLL-AF9 expressing KLS (KLS:MA9) led to rapid accumulation of leukemic cells, while there was a 4 to 5-week delay in AML development when the same number of GMP expressing MLL-AF9 (GMP:MA9) were transplanted. The median latency for the AML initiated from 5×103 KLS:MA9 was 42 days vs. 76 days for GMP:MA9 (data not shown). When 1.5×104 KLS:MA9 or GMP:MA9 were transplanted the latencies were 37 and 58 days, respectively (Supplemental figure 1A). Next, we transplanted into secondary recipients 5×103 total bone marrow (BM) cells from mice that had succumbed to primary AML which originated from either KLS:MA9 or GMP:MA9. This led to development of histopathologically similar diseases while maintaining a difference in the kinetics of disease development (Supplemental figure 1B). Leukemias derived from KLS possessed more clones than did leukemias initiated from GMP as assessed by Southern blotting (Supplemental figure 1C). Pre-leukemic KLS:MA9 did not have an advantage in homing over GMP:MA9 within the first 48 hours as assayed by bioluminescence tracking (data not shown). Furthermore, mice transplanted with 1.1–3×103 of KLS:MA9 or GMP:MA9 did not have >0.05% of GFP+ cells in the spleen (SP), BM, or peripheral blood (PB) 8 and 10 days after transplantation, similar to previous reports (Somervaille and Cleary, 2006; Wang et al., 2010), while control mice transplanted with 3.3×103 KLS:MIG demonstrated robust engraftment (Supplemental figure 1D). Thus, we could not find evidence that differential homing of pre-leukemic cells plays a role in leukemogenesis in this model. However, these data suggest that the cell of origin does influence development of the AML prompting further assessment.
HSC-derived clones initiate leukemia more rapidly and more efficiently than GMP-derived clones
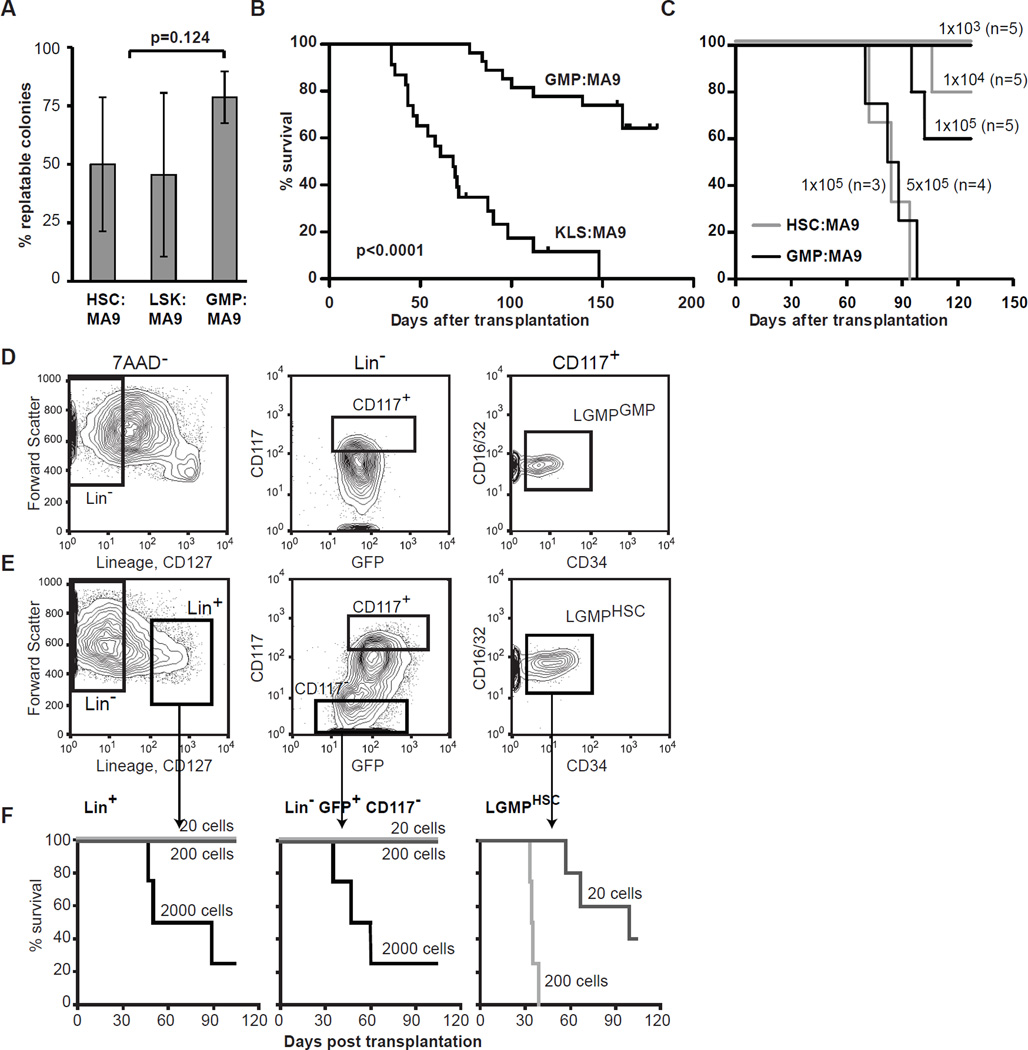
The leukemogenic process starts from a single cell. We transduced KLS, HSC (CD34− KLS) (Osawa et al., 1996) or GMP with the MIG-control or MLL-AF9 retroviruses, and after 40 hours resorted GFP+ cells at 1 cell/well into 96-well plates containing semisolid media. Similar numbers of single-cell sorted KLS:MA9 and GMP:MA9 colonies arose 17.1±8.4% and 21.5±6.7%, respectively. At the end of the 1st week, the clones were solubilized, and 500 cells were replated every 5–7 days for up to 9 weeks (Supplemental figure 2A). MIG-control transduced GMP and KLS exhausted after 1 to 3 replatings (data not shown), while replating efficiency between colonies initiated from HSC:MA9, KLS:MA9 and GMP-MA9 was 50±28.6%; 45.5±35% and 78.6±11.1%, respectively averaged over 5 independent experiments (Figure 1A). These experiments show that at a single cell level MLL-AF9 equally immortalizes HSC, KLS and GMP in culture. Immunophenotypically KLS:MA9 and GMP:MA9 single-cell derived clones (SCC) were indistinguishable by the presence of 6 surface markers including CD11b, Gr1, CD117, Sca1, CD34, and CD16/32 (Supplemental figure 2B). The single cell clones that arose from KLS:MA9 and GMP:MA9 have similar numbers of retroviral integration sites assessed by Southern hybridization (Supplemental figure 2C). Expression levels of MLL-AF9 were slightly but not statistically significantly higher in HSC:MA9 as compared to GMP:MA9 (n=8, each). Also, expression levels of selected MLL-AF9 targets Hoxa9 (n=8, each), Meis1 (n=3, each) and Mef2c (n=3, each) were similar between individual clones of HSC:MA9 and GMP:MA9 as assessed by quantitative(q)-PCR (Supplemental figure 2D). Nevertheless, 86.4% (n=22) of mice transplanted with 1–2×105 KLS:MA9 SCC succumbed to AML with median latency of 68 days, as compared to only 33.3% (n=15) of mice transplanted same number of GMP:MA9 SCC, whose median latency was 100 days (Figure 1B).
Figure 1. Leukemogenic transformation of single KLS, HSC and GMP upon expression of MLL-AF9.
A. Replating efficiency of single cell derived clones originating from KLS, HSC and GMP mean of 5 independent experiments. Bars represent standard deviation, two tailed t-test p=0.124. B. Kaplan-Meyer survival plot of mice transplanted with 1–2×105 cells expanded from single HSC:MA9 or GMP:MA9. The experiments were terminated at day 158 for KLS-derived clones and 180 for GMP-derived clones. Log-rank p-value<0.0001. C. Kaplan-Meyer survival plot assessing survival of recipient mice transplanted with serial dilution of single cell derived HSC:MA9 and GMP:MA9. Calculated leukemia initiating cell frequency in GMP:MA9 and HSC:MA9 cultures are 1/168,427 (±1S.E. 1/104,566–1/271,290)and 1/29,388 (±1S.E. 1/15,275–1/55,275), respectively. D, E. AMLHSC and AMLGMP demonstrate similar immunophenotype and composition. The LGMP population (Lin− CD127− CD117+ CD16/32high CD34low), can be identified in both AMLHSC and AMLGMP. F. LGMP are enriched for LSC in HSC-derived leukemias. Kaplan-Meyer plot of survival of mice transplanted with limiting dilutions 2000 (n=4), 200 (n=5), and 20 (n=5) of Lin− CD127− GFP+ CD117+ CD16/32+ CD34low (LGMPHSC); Lin− GFP+ CD117−; and Lin+ cells.
These data suggested that the while the immunophenotypic appearance of the pre-leukemia cells is very similar the oncogene expressing stem cells might exhibit more potency to further leukemia development. This hypothesis was further tested in direct experiments assessing leukemia initiating cell frequencies (LIC) in GMP:MA9 and HSC:MA9 SCC. LIC frequency assessed in limiting dilutions experiments was 1 in 168,427 (±1S.E. 1/104,566–1/271,290) and 1 in 29,388 (±1S.E. 1/15,275–1/55,275) in GMP:MA9 SCC and HSC:MA9 SCC, respectively (Figure 1C). Quantitative-PCR based assessment demonstrated similar expression levels of MLL-AF9, Meis1 and Mef2c in leukemia cells originated either form HSC (AMLHSC) or GMP (AMLGMP) while Hoxa9 was expressed at higher levels in AMLGMP (Supplemental figure 2E&F). Furthermore, we did not observe correlation between Meis1 expression levels and MLL-AF9 AML (Wong et al., 2007) (Supplemental figure 2F). In our experiments the latency of AML (both in primary and secondary recipient mice) showed strong correlation with the number of transplanted (pre-)leukemic cells. These experiments indicate that MLL-AF9 effectively transforms HSC, KLS and GMP into LSC at the single cell level but that leukemia development is more rapid and efficient from KLS/HSC.
Leukemias that originate from HSC and GMP have similar LSC frequencies and immunophenotype
Biological differences in the two types of AML might be due to a higher frequency of LSC in AMLHSC than AMLGMP. Thus we performed limiting dilution experiments and found the LSC frequencies were 1/93 cells (±1S.E. 52–165) in AMLHSC and 1/86 (±1S.E. 68–108) in AMLGMP (Supplemental figure 3A&B), similar to previously reported (Krivtsov et al., 2006; Somervaille and Cleary, 2006; Stubbs et al., 2008). Also, the hierarchical organization of AMLHSC resembled that previously reported for AMLGMP (Figure 1D&E) with the GMP-like (LGMP) population at the apex of the hierarchy as assessed by limiting dilution experiments (Figure 1F). The calculated LSC frequencies using limiting dilution analyses were 1/22 (±1 S.E. 1/12–1/39) in LGMPHSC, and 1/1,784 (±1 S.E. 1/992–1/3,205) in GFP+ Lin− Kit− and Lin+ GFP+ populations. The morphology of LGMPHSC and LGMPGMP were also similar with some degree of monocytic differentiation and thus resembled the FAB M4 or M5 AML classification (Supplemental figure 3C&D). These data are consistent with previous reports that LSC activity is not restricted to the LGMP compartment and leukemic cells with Gr1+ Mac1+ immunophenotype are capable of transferring AML to secondary recipients when transplanted in larger numbers (Somervaille and Cleary, 2006). It also demonstrates that LSC derived from HSC are similar in their immunophenotype to downstream committed myeloid cells. Our experiments show that the hierarchies and LSC frequencies are similar between the two types of AML, as well as expression of MLL-AF9 and its selected target genes.
LGMPGMP and LGMPHSC demonstrate different gene expression programs
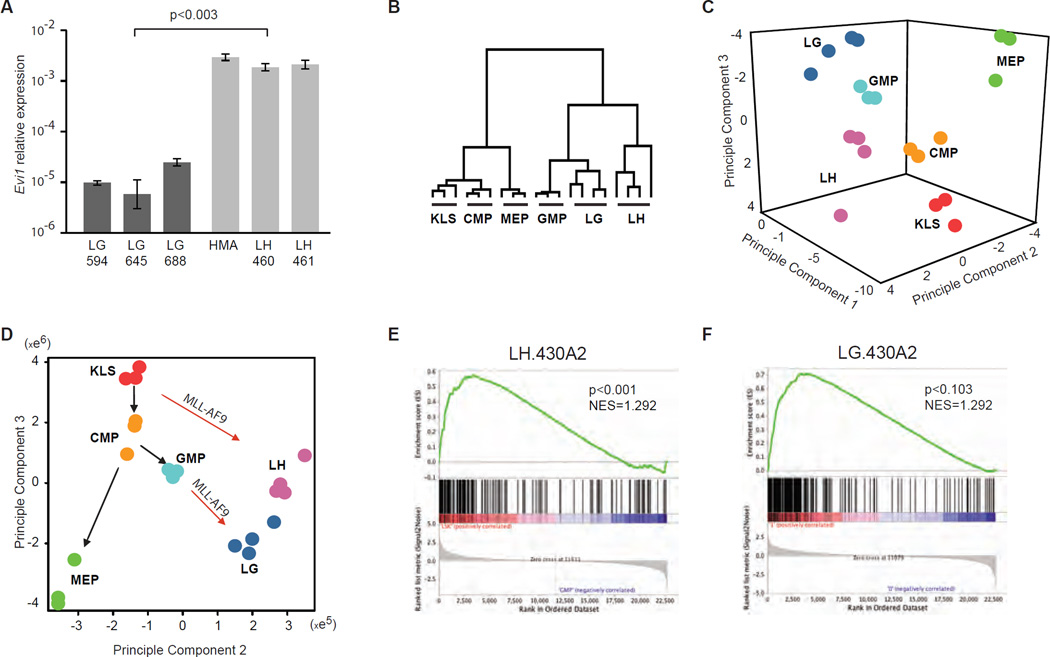
Previous reports (Arai et al., 2010; Bindels et al., 2012) have demonstrated that ectopic MLL-AF9 expression maintains expression of Evi1 when expressed in HSC, however MLL-AF9 does not reactivate Evi1 expression in committed progenitors. Moreover, several reports correlated elevated EVI1 expression with adverse clinical outcome (Haas et al., 2008; Lugthart et al., 2008). Furthermore, MLL-AF9 leukemias can be clearly separated into good and poor prognosis groups based on the presence or absence of EVI-1 expression (Groschel et al., 2012). These previous data point to biological differences between EVI1-positive and EVI1-negative MLL-fusion mediated AML. We therefore assessed expression of Evi1 in LGMP from fully developed AMLHSC and AMLGMP. qPCR analyses (with 3 independent primer pairs) demonstrated that Evi1 expression levels were on average 200 fold higher in LGMPHSC than in LGMPGMP (Figure 2A). To further understand complexity of the two AML types we then assessed gene expression differences between LGMPHSC (LH) and LGMPGMP (LG). RNA from LG, LH, KLS, common myeloid progenitors (CMP), GMP, and megakaryocyte-erythroid progenitors (MEP) was extracted, amplified, labeled, and hybridized to Affymetrix microarrays. Hierarchical clustering analysis of the expression profiles demonstrated that LG and LH are more similar to GMP and to each other than to any other cell type assessed (Figure 2B). Principal component (PC) analysis of the same dataset demonstrated that the LGMPs were most similar to GMP or perhaps a myeloid cell further downstream in myeloid development, but do have distinct gene expression programs dependent on the cell of origin (Figure 2C). Of particular interest, we found that separation of samples along PC2 and PC3, which best separated KLS from more differentiated hematopoietic cells, revealed that LGMPHSC had a gene expression profile that appeared less differentiated than LGMPGMP (Figure 2D). These data demonstrate that while globally most similar to a differentiating myeloid cell, LSC derived from different cells of origin possess reproducible differences in gene expression.
Figure 2. Gene expression profiles of normal KLS and progenitor cells, LGMPHSC, LGMPGMP.
A. Relative expression of Evi1 in LSC derived from GMP (LG) and HSC (LH) assessed by qPCR with Sybrgreen. Bars represent ΔΔCt averaged over 3 independent samples; brackets represent standard deviation between 3 replicates, p-value calculated using t-test. B. Hierarchical clustering using approximately 16000 filtered probesets demonstrates that LSC derived from GMP (LG) and HSC (LH) possess gene expression profiles most similar to GMP. C. Three dimensional projection of principal component analysis (PCA) of approximately 8000 probesets expressed in normal hematopoietic precursors (HSC; CMP; MEP; GMP), LH and LG demonstrate that the leukemia stem cells possess unique gene expression programs. D. Two dimensional projection of the same PCA analysis plotted as principal component 2 vs. principal component 3. E. GSEA demonstrates enrichment of LH-signature in KLS vs. GMP. F. GSEA demonstrates a trend of enrichment of LG-signature in GMP vs. KLS.
LH-signature is enriched in normal HSC and is correlated with poor clinical outcome
While expression of direct MLL-AF9 target genes was similar between LH and LG (Supplemental figure 4A), comparative marker selection analysis demonstrated that 173 probesets (134 genes) have higher expression in LH (LH-signature) while 1217 probesets showed higher expression in LG, the top 200 (197 genes) of which were selected for further analyses (LG-signature) (Supplemental figure 4B). Gene set enrichment analysis (GSEA) demonstrated that the LH-signature is expressed at higher levels in murine KLS when compared to GMP (p<0.001) (Figure 2E). The LH-signature was then converted to human (HG_U133A) probesets or gene IDs and was also found to be enriched in human HSC when compared to GMP (Bruns et al., 2009) (p<0.001) (Supplemental figure 4C). The LG-signature showed a trend toward higher expression levels in mouse GMP as compared to KLS (p<0.103) (Figure 2F), but did not show any significant enrichment in human GMP vs. HSC (Supplemental figure 4D). These data demonstrate that the reason HSC-derived LSC are different from GMP-derived LSC is that they express a more extensive HSC signature.
To assess the potential clinical relevance of the cell of origin associated gene expression programs on the resulting leukemia we tested the correlation of expression of LG- and LH-signatures with clinical outcome in a group of 35 adult patients diagnosed with MLL-rearranged AML (MLL-AML) for which two sets of microarray data were available (Bullinger et al., 2008; Noordermeer et al., 2011). 72 out of 121 LH-signature and 113 out of 162 LG-signature genes were present in the combined human dataset, which were then used to cluster samples. Hierarchical clustering was used to separate gene expression profiles from 35 patients into those with high- and low- level expression of the LH- or LG-signatures (Supplemental figure 5A&B). Kaplan-Meier survival analysis demonstrated that patients with low-level expression of LH-signature have longer relapse free survival than those expressing high levels (Figure 4A). The expression of the LG-signature was less associated with the relapse free survival in the same patients, even though there was a trend toward better survival for patients with leukemias that expressed higher levels of the LG signature (Figure 4B). We further assessed the correlation of LH- and LG-signatures with clinical outcome in two previously published MLL-AML datasets (Ross et al., 2004; Valk et al., 2004). Similarly, hierarchical clustering was used to separate gene expression profiles from St. Jude Children’s Research Hospital (CRH) and Erasmus University Medical Center (UMC) patients into those with high- and low- level expression of the LH-signature. Kaplan-Meier survival demonstrated a trend toward longer relapse free survival for patients whose leukemias had low LH gene expression than those expressing higher levels. But due to small numbers in both sets the correlation was not significant (Supplemental figure6A and 6B). Next, we used GSEA to assess whether expression levels of LH- or LG- signatures would demonstrate correlation with clinical outcome. For patients (n=21) treated at St. Jude CRH poor outcome was considered relapse of the disease and good outcome – no relapse. For patients (n=15) treated at Erasmus UMC, good outcome group contains patients who were in remission after the first cycle of treatment and poor outcome contains patients who were not. Expression of the LG-signature again did not correlate with clinical outcome in either dataset. However, expression of the LH-signature correlated with poor clinical outcome in both St.Jude CRH (Supplemental figure 6C) and Erasmus UMC MLL-AML (Supplemental figure 6D) datasets. Of interest, the LH-signature only correlated with poor clinical outcome in MLL-AMLs, and not in other select groups of AML samples (Table 1).
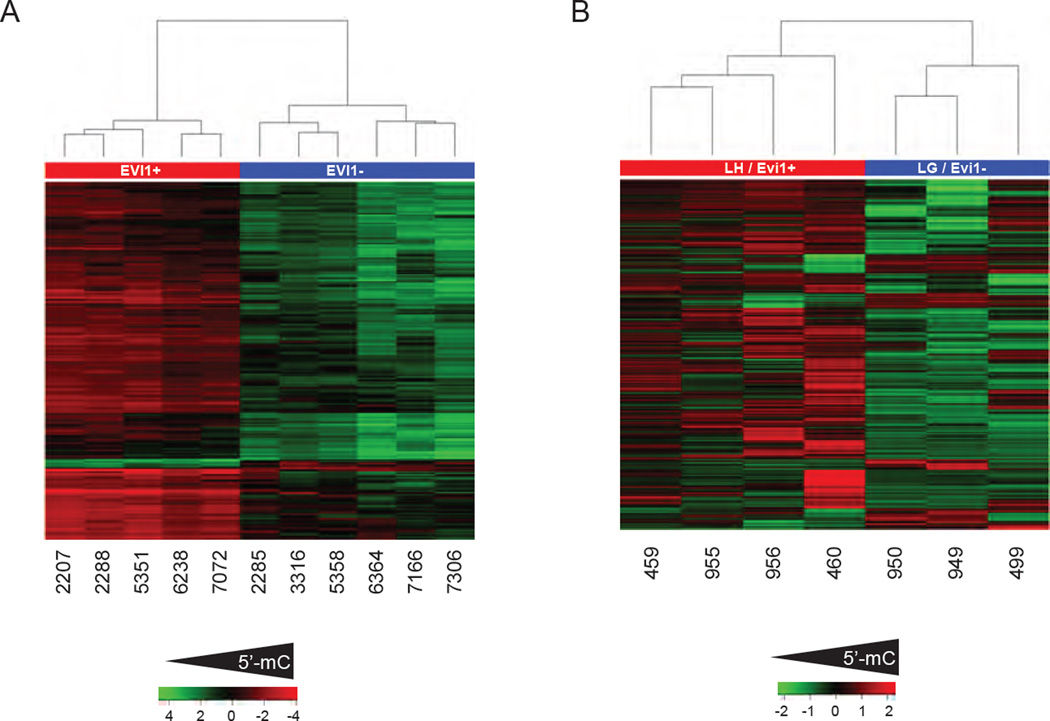
Figure 4. HSC-derived AML are hypermethylated compared to progenitor derived AML.
A. Heat-map representation of differential DNA methylation (5’-mC) between human primary MLL-AMLs with over expression of Evi1 (red) vs. MLL-AMLs without Evi1 over expression (Blue) as measured by the HELP assay. Each rows represents a probeset on the HELP microarray while columns represent individual cases. B. Heat-map representation of differential DNA methylation between murine LH AMLs with over expression of Evi1 (red) vs. LG AMLs without Evi1 over expression (Blue) as measured by the ERRBS assay). Each rows represents a differentially methylated CpG site, while columns represent individual leukemia specimens, numbers underneath represent specimen ID.
Table 1.
Correlation of selected gene signatures with clinical outcome in cytogenetically defined AML.
| Data set | LG-signature, gene symbols |
LH-signature, probsets |
LH-signature, gene symbols |
|---|---|---|---|
| St.Jude MLL-AML (n=21) | p=0.760 | NES=1.55; p<0.013 |
NES=1.43; p<0.037 |
| Erasmus MLL-AML (n=15) | p=0.802 | NES=1.43; p<0.040 |
NES=1.43; p<0.037 |
| t(8;21) AML (n=14) | p=0.608 | ||
| M7-AML (n=7) | p=0.839 | ||
| Other-AML (M1-M5) (n=43) | p=0.201 |
Composition of the LH signature was further analyzed using leading edge overlap in GSEA (Subramanian et al., 2005). Out of 121 genes in the LH-signature (converted to human HG-U133A_2), the leading edge in Erasmus data set consisted of 52 genes, while in the St. Jude CRH dataset leading edge was 37 genes. 20 genes (p<0.002) were in common between St. Jude CRH and Erasmus UMC leading edge datasets (Supplemental table 1, Supplemental figure 5C). This procedure shows that the LH signature contains a sub-signature that is particularly strongly associated with poor clinical outcome in MLL-AML. To test the significance of the 20 genes (LE20), we assessed for enrichment of LE20 signature in the poor prognosis group from our dataset (n=35) and again found significant enrichment in poor clinical outcome group (Supplemental figure 6E). qPCR analysis demonstrated higher expression of 15 out of LE20 genes in LGMPHSC over LGMPGMP (n=3 for each) (Supplemental figure 6F). These data demonstrate that in a mouse model of MLL-AF9 AML an HSC cell-of-origin associated signature correlates with poor clinical outcome in human AML.
LGMPGMP and LGMPHSC demonstrate different 5-methyl cytosine levels
Previous reports have demonstrated that patterns of cytosine methylation in DNA (5’-mC) can be used to classify types of AML, and correlate with leukemia patients’ clinical outcomes irrespective of gene expression patterns (Figueroa et al., 2010). Recently we demonstrated that EVI1-positive and EVI1-negative MLL-rearranged AML differ morphologically and phenotypically (Bindels et al., 2012), moreover expression of EVI1 in human AML correlated with poor outcome (Lugthart et al., 2008). Furthermore, loss of one allele of DNMT1 leads to a reduction of 5-mC levels and subsequently a less aggressive MLL-AF9 mediated AML (Broske et al., 2009; Trowbridge et al., 2012). We therefore wanted to assess DNA methylation to see if differences might be associated with good vs. poor outcome in human leukemias and with murine leukemias derived from different cells of origin.
Direct comparison of 5’-mC profiles in EVI1+ and EVI1− MLL-rearranged AML samples (from GSE18700) analyzed using the HELP assay (Figueroa et al., 2009a) revealed 260 differentially methylated regions annotated to 296 genes. Globally, EVI1+ MLL-AMLs demonstrated higher 5’-mC methylation as compared to EVI1− MLL-AMLs (Figure 4A). This shows within the molecularly defined MLL-AML group of leukemias there is a poor prognosis group (EVI1+) that have relatively high levels of 5’-mC and there is a better prognosis group (EVI1−) that have relative low levels of 5’mC. Next we assessed 5’-mC levels in LGMPHSC and LGMPGMP using the enhanced reduced representation bisulfite sequencing assay (ERRBS) (Akalin et al., 2012a) and we identified 4088 differentially methylated sites. Similar to the human EVI1+ MLL-AMLs, LGMPGMP were hypo-methylated as compared to LGMPHSC (Figure 4B), thus demonstrating that relative high levels of 5’-mC are found in poor prognosis human MLL-AML and murine MLL-AF9 AML derived from HSC. These data show that using AML samples originated from HSC and committed progenitors we were able to reproduce the diversity of global 5’-mC levels observed in primary human MLL-AMLs.
HSC-derived AML are less responsive to Cytarabine and doxorubicin than GMP-derived AML
We have established that AMLs derived from different cells of origin possess differences in the kinetics of disease development, inherit gene expression differences that define the cell of origin, and differences in 5-mC levels. Taken together with several previous reports that found a correlation between poor clinical outcome of AML patients and elevated expression of HSC specific genes (Gentles et al., 2010; Mullighan et al., 2009; Valk et al., 2004), we hypothesized that AML originated from HSC and GMP may respond differently to therapeutics. To test this hypothesis we assessed the sensitivity of AMLHSC and AMLGMP in vitro to a commonly used chemotherapeutic drug doxorubicin. Total bone marrow cells (>90% GFP+ cells) from primary recipient mice that had succumbed to AML were exposed to serial dilutions of doxorubicin for 2 days followed by assessment of live cells using an MTT assay. The cell survival assay established IC50 concentrations for AMLHSC and AMLGMP approximately 80 and 20 ng/ml respectively (Figure 5A). We further assessed response of AMLHSC and AMLGMP to one cycle of conventional chemotherapy, similarly to a previously reported protocol (Zuber et al., 2009). Secondary (2°) recipient mice were transplanted with 2×103 total BM cells from primary (1°) mice that had succumbed to either AMLHSC (n=8) or AMLGMP (n=11). Starting on day 10 the mice were bled from the tail vein twice weekly followed by FACS for GFP+ leukemia cells. When the percentage of GFP+ cells in peripheral blood reached 20–40% levels, the mice were split into 2 groups; one group was treated for 5 days with Ara-C (100 mg/kg) and 3 days with doxorubicin (3 mg/kg) according to (Zuber et al., 2009), and the other group was not treated. The AML burden was assessed 12 days after the initial treatment in BM, PB, and SP (Figure 5B). One cycle of chemotherapy significantly reduced the percentage of AMLGMP cells in PB from 81.9±1.5% to 53.1±17.3% (p<0.01), while reduction of AMLHSC cells from 77.1±9.9% % to 53.3±9.2% in was less significant (p=0.07). The treatment did not significantly affect AMLHSC cells in BM or SP, while it clearly reduced the percentage of AMLGMP from 93.5±2% to 70.2±9.7% (p<0.02) in BM and from 71.4±3.3% to 38.3±6.2% in SP (p<0.02) (Figure 5C). Similarly the treatment did not significantly reduce splenomegaly in mice developing AMLHSC while SP weights in mice with AMLGMP cells were reduced from 510±29 to 375±60 mg (p<0.01) (Figure 5D). These data suggest that resistance to chemotherapy may be in part governed by cellular machinery active in HSC that is maintained in LSC derived from HSC, and help explain why patients that have leukemias with high level expression of HSC gene expression programs do poorly.
Figure 5. HSC-originated AMLs are less sensitive to chemotherapy.
A. HSC and GMP-derived AML samples (n=2 each) we plated at 1×105 cells/well in 96-well plates with serial dilutions of doxorubicin. After 2-day incubation MTT was added for 4 hours followed by cell lysis and colorimetric analysis of incorporated dye. Cell viability is expressed as percentage of not treated control. Bars represent standard deviation between technical triplicates. B. Graphical presentation of experimental timeline assessing AML sensitivity to chemotherapy treatment. C. Therapeutic sensitivity of secondary AML originated from HSC or GMP. Treatment with one cycle of Ara-C and Dox (AD) relived AML burden of mice with AMLGMP but not AMLHSC. Not treated (NT) mice displayed similar AML burden between groups. Bars represent standard deviation between replicates; two-tailed t-test was used to determine p-value. D. Similarly, the treatment did not reduce splenomegaly of mice with AMLHSC while significantly reduced spleen mass in mice with AMLGMP. Brackets represent standard deviation between replicates.
Discussion
In this study we have experimentally addressed the question as to whether the cell of origin can influence the phenotype of the resulting leukemia and LSC. We demonstrate that MLL-AF9 transforms HSC and GMP at a single cell level. In vitro experiments demonstrated similar efficiency of immortalization, while in vivo, the kinetics and frequency of leukemia development is greater for clones initiated from HSC. We also show that the immunophenotype of the cellular population enriched for LSC activity in both HSC and GMP derived leukemias is most consistent with a mid-myeloid cell (LGMP). However, leukemias that originate from HSC retain enhanced expression of a set of “stem cell associated” genes (LH-signature). Patients whose leukemia samples express higher levels of the LH-signature have a shorter relapse free survival as compared to patients whose samples express less of the LH-signature. Moreover, leukemias derived from HSC demonstrate less of a response to doxorubicin and Cytarabine than do leukemias that arise from GMP. These data show that the cell origin influences the ultimate biology of the leukemia that arises and establishes clinically relevant gene expression differences that are determined by the cell of origin.
Longstanding questions in cancer biology include the cell of origin of specific cancers and whether differences in the cells of origin from one cancer to the next might influence the clinical behavior of the resultant cancer (Visvader, 2011). Recent studies have utilized mouse models of leukemia and other cancers to demonstrate that cancer can arise from multiple cell types within a tissue (Chen et al., 2004; Wang et al., 2008) reviewed in (Krivtsov and Armstrong, 2007). In AML it is clear that certain fusion oncoproteins such as BCR-ABL are only capable of initiating disease when expressed in HSC whereas other oncogenes such as MLL-AF9 and MOZ-TIF2 can transform both HSC and more committed progenitor cells (Cozzio et al., 2003; Huntly et al., 2004; Krivtsov et al., 2006). However, it has been unclear whether expression of an oncogene in different developmental cell types within a tissue can influence the phenotype of the resulting leukemia and LSC. Our data directly addresses this question, and demonstrate that the cell in which MLL-AF9 is expressed influences the resultant behavior genetic and epigenetic programs of the LSC even if the immunophenotype of the leukemia and LSC is similar.
Globally the LSC derived from either HSC or GMP are most like a mid-myeloid progenitor cell, likely representing a cell just downstream of the normal GMP. Even though the immunophenotype is similar for LSC derived from different cells of origin, there are reproducible differences in gene expression. For example, Evi1, c-jun, Fos1, Bcl6 (Parekh et al., 2008), Gsr1, Nedd9 and Zfp36 (Selmi et al., 2012) are highly expressed in HSC-derived LSC but not GMP derived LSC (Supplemental Figure 6F). Therefore we demonstrate that the cell in which the oncogene is initially expressed can influence the final gene expression program of the LSC even if the LSC represents a stage of hematopoietic development further downstream of the HSC. Thus it appears that expression of MLL-AF9 in GMP activates a stem-cell associated program that is sufficient to induce leukemia, but that expression of MLL-AF9 in HSC allows maintenance of a more extensive stem cell-derived program that influences the LSC behavior.
The findings presented here provide insight into the diversity of gene expression in AML. It appears that we have modeled high-risk MLL-rearranged AML arising from chromosomal translocation arising in hematopoietic stem cell. Our data suggests that it is likely that other good-risk MLL-rearranged AML arise from more differentiated myeloid progenitors that likely include but may not be limited to granulocyte-macrophage progenitors. Gene expression analyses performed on human leukemias have demonstrated that differences in gene expression between various leukemias can in part be described by the presence of recurrent mutations including chromosomal translocations (Ross et al., 2004; Valk et al., 2004). Emerging genomic-scale assessment of DNA methylation profiles suggests a second level of heterogeneity (Bullinger et al., 2009; Figueroa et al., 2009b). However, the biological mechanisms that influence gene expression and epigenetic programs beyond recurrent mutations are largely unknown. Our data suggest that the epigenetic state of the cell in which the oncogene is initially expressed (in this case HSC vs. GMP) contributes to the final gene expression and epigenetic program of the leukemia in a fashion that impacts the overall behavior and drug response. If, at the time of acquisition of an oncogenic event, the epigenetic state of a cell determines what genes/programs can be activated, then one can predict that the combination of the cell of origin or overall epigenetic state combined with genetic changes will determine the final gene expression profile.
Recent studies in breast cancer cell lines and mouse models of pancreatic cancer have demonstrated that the epigenetic state of cells prior to full transformation can influence which cells give rise to cancer (Gidekel Friedlander et al., 2009; Iliopoulos et al., 2009). These reports suggest that an aberrant signal or injury can induce epigenetic changes that enhance transformation potential. Studies of mouse models of brain tumors established that glioblastoma might arise from genetic lesions in neural stem cells as well as in glial progenitors reviewed in (Chen et al., 2012) and the cell of glioma origin influenced developmental characteristics of the tumors (Lei et al., 2011). Genomic correlation studies of human glioblastoma samples established that subtypes of gliomas often share molecular signatures with normal neural cell types (Verhaak et al., 2010). However, due to the lack of a detailed understanding of neural development as compared to hematopoietic development, the direct relationship between cell of origin and subtypes of Gliomas has remained unclear. Recently it has been documented in detail how the cell of origin influences the phenotype of iPSC (Kim et al., 2010; Polo et al., 2010). Our data are in line with these concepts in that cells at different stages of development can be transformed with the same oncogene and produce LSC that, while similar, have reproducible differences in gene expression that are clinically relevant and show that these cell of origin based differences recapitulate what is seen in clinical samples. Indeed it appears that LSC express specific genes and carry levels of DNA methylation that are determined by the cell of origin and that the unique gene expression profiles might serve as a cellular “memory” of the cell in which the oncogene was first expressed. Together, these data contribute to growing evidence that the process of cancer development is driven by genetic mutations in oncogenes and tumor suppressors, which interact with the epigenetic landscape to give rise to a tumor with unique biological and clinical behavior (Feinberg et al., 2006; Jones and Baylin, 2007). These data predict that a combination of therapies that target genetic changes and epigenetic programs will be synergistic in their ability to eradicate tumors. A major focus is to determine how to appropriately combine modulators of epigenetic programs and genetically targeted therapies for clinical benefit.
Materials
Mice, cells, antibodies, and sorting of hematopoietic progenitors
8 to 12 week C57BL/6 mice (Charles River Laboratories) were used as bone marrow donors and recipients. KLS were isolated from mouse bone marrow as Lin− (CD3; CD4; CD8a; CD19; B220; Gr1; TER119; IL-7R) c-Kit+ Sca1+ and LT-HSC as Lin− c-Kit+ Sca1+ CD34− (Osawa et al., 1996). Myeloid progenitors were sorted as previously described (Akashi et al., 2000) CMP Lin− Sca1− c-Kit+ Fc-RγII/IIIlow CD34+; GMP Lin− Sca1− c-Kit+ Fc-RgII/IIIhigh CD34+; MEP Lin− Sca1− c-Kit+ Fc-RγII/III− CD34− using FACSAria high-speed cell sorter equipped with 407nm, 488nm, and 540nm lasers (BD Biosciences).
Retroviruses and Culture of hematopoietic progenitors. Retroviral supernatants were produced by co-transfection of 293T cells (Krivtsov et al., 2006). 1–15×104 LT-HSC; KLS or GMP were incubated with retroviral supernatant supplemented with 10ng/ml each IL-3; IL-6 and 20ng/ml SCF, 40–48 hours after infection GFP+ 7AAD− cells were re-sorted and either injected into sublethally (600Rads) irradiated recipient mice, collected for RNA, or plated at 1 cell per well in 96-well plates.
Serial replating of colonies
Colonies were grown in 96-well plates in 100 µl per well of M3234 Methyl Cellulose media (Stem Cells Inc, Vancouver, BC, Canada) supplemented with 10 µg/ml both IL-3; and IL-6; and 20 µg/ml SCF (Peprotech, NJ). At the end of the 1st week 10–50 colonies were solubilised with 200 ul of IMDM, and 500 cells re-plated into a well in another 96-well plate with 100 ul of methylcellulose supplemented with IL-3, IL-6, and SCF. This process is repeated every 5–7 days for up to 9 weeks and re-plating efficiency between colonies initiated from GMP and KLS is compared.
RNA amplification and qPCR
We routinely isolated RNA from 1–5×104 cells, and performed two rounds of in vitro transcription and labeling as described (Krivtsov et al., 2006) and http://www.broad.mit.edu/mpr/publications/projects/leukemia/protocol.html. qPCR was performed using premixed reaction solution with SyberGreen (Bioline, Taunton, MA) and gene specific primers, sequences are available upon request.
Statistical analysis
The Poisson distribution analysis of leukemia initiating cell frequencies in total bone marrow and sorted cellular fractions was calculated using L-Calc™ software (StemCell Technologies Vancouver, BC)
Data, analysis and statistical methods
RNA from human samples was hybridized to either a 40k cDNA microarray platform (n=15) or Human Genome U133plus2.0 Arrays (n=20; Affymetrix, Santa Clara). Data normalization and filtering was performed separately for the cDNA and Affymetrix datasets. Datasets were combined following the averaging of the expression of multiple clones/probesets measuring the same gene and the mean centering the cDNA and Affymetrix data in order to exclude platform specific effects. (Bullinger et al., 2008; Noordermeer et al., 2011). The raw data files will be available from Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/accession#GSE18483.FromGSE11889 (Bruns et al., 2009) we selected arrays for 5 normal HSC and GMP. GSE1159 (Valk et al., 2004); the clinical data was available for 15 patients. Microarray data from St.Jude hospital (Ross et al., 2004) was downloaded from http://www.stjuderesearch.org/data/AML1/ and used according to outcome data provided in supplemental tables 16 and 17. Two arrays (JD0026 and JD0027) were not used because of strong chromatic aberrations. Microarray data were analyzed with GenePattern 3.2 software package http://www.broadinstitute.org/cancer/software/genepattern/ raw expression data was normalized using RMA algorithm with quantile normalization and background correction. Hierarchical clustering was performed and visualized using the HierarchicalClustering and HierarchicalClusteringViewer modules. The data were preprocessed (max/min≥1.25 and max-min≥25, thresholds: mix≥10, max≤20000. Principal component and differential gene expression analyses were performed on the preprocessed data set (max/min≥2, max-min≥50, thresholds: min≥10, max≤20000). T-test with asymptotic p<0.05 was used for differential gene expression analysis between LG and LH populations. Release 31 of Affymetrix conversion tables was used for conversion of 430A2 probesets to gene symbols, HG-U133A probesets or gene symbols.
Patients
35 adult AML patient samples harboring a t(11q23) including 25 t(9;11) treated on AML HD93, HD98A, HD98B, or SG 07-04 were provided by the German-Austrian AML Study Group (AMLSG) with patient informed consent and institutional review board approval from all participating centers(Schlenk et al., 2009). 12 patients received an allogeneic BMT in first remission. Conventional chromosome banding and fluorescence-in-situ-hybridization (FISH) were performed at the central reference laboratory of the German-Austrian AMLSG. All samples included into the study contained at least 80% of leukemic cells following Ficoll-density gradient centrifugation based enrichment of samples.
Enhanced Reduced Representation Bisulfite Sequencing (ERRBS)
High-molecular weight DNA was extracted from 400,000–1,000,000 cells from 3–4 biological replicates from LGMPHSC (LH) and LGMPGMP (LG). ERRBS libraries were prepared as previously described (Akalin et al., 2012a). The libraries were sequenced on an Illumina HiSeq2000 sequencer per manufacturer’s recommended protocol for 50 bp single-end read runs. Image capture, analysis and base calling were performed using Illumina’s CASAVA 1.7. Alignment of sequenced reads was performed using the Bismark aligner (Krueger and Andrews, 2011) and downstream analysis was performed using R 2.15 and the MethylKit R package (Akalin et al., 2012b). Differentially methylated CpGs between LGMPHSC and LGMPGMP were determined using logistic regression and the likelihood ratio test. Observed p-values were adjusted with the SLIM method (Wang et al., 2011). Significant differentially methylated CpGs were selected at q-value < 0.01 and a minimum methylation difference of 25%.
Primary AML methylation by HELP
DNA methylation data using the HELP assay was obtained from a previously published cohort of patients (Figueroa et al., 2010). 11 patients corresponding to the MLL-rearranged cases from clusters 8 and 11 from that cohort was selected for direct comparison using a T-test followed by adjustment of p-values for multiple testing using the Benjamini-Hochberg procedure. Significant probe sets were selected on the basis of an FDR < 0.05 and a minimum methylation delta of log2 > 2, which corresponds approximately to at least a 25% methylation difference.
Supplementary Material
Figure 3. Correlation of LH- and LG-signatures expression with clinical outcome.
Kaplan-Meier analysis for relapse free survival comparing the cluster-defined subset of samples with high- and low-levels of LH-signature gene expression (A) and high and low LG-signature expression (B). p-value was calculated using log-rank test.
Highlights.
LSCs that originate from either stem or progenitor cells are similar to myeloid progenitors.
LSCs retain cell of origin specific gene expression profiles.
LSCs derived from different cells of origin recapitulate epigenetic differences in human AML.
Cell of origin influences drug/therapy sensitivity of resultant AML.
Acknowledgements
We would like to thank Renee Wright for technical help and Megan Smith for administrative assistance. This work was supported in part by the National Cancer Institute (5P01CA66996), the Leukemia and Lymphoma Society and the Harvard Stem Cell Institute.
References
- Akalin A, Garrett-Bakelman FE, Kormaksson M, Busuttil J, Zhang L, Khrebtukova I, Milne TA, Huang Y, Biswas D, Hess JL, et al. Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukemia. PLoS Genet. 2012a;8:e1002781. doi: 10.1371/journal.pgen.1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, Mason CE. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 2012b;13:R87. doi: 10.1186/gb-2012-13-10-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Arai S, Yoshimi A, Shimabe M, Ichikawa M, Nakagawa M, Imai Y, Goyama S, Kurokawa M. Evi-1 is a transcriptional target of MLL oncoproteins in hematopoietic stem cells. Blood. 2010 doi: 10.1182/blood-2009-07-234310. [DOI] [PubMed] [Google Scholar]
- Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, Beverloo HB, Chang M, Creutzig U, Dworzak MN, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114:2489–2496. doi: 10.1182/blood-2009-04-215152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels EM, Havermans M, Lugthart S, Erpelinck C, Wocjtowicz E, Krivtsov AV, Rombouts E, Armstrong SA, Taskesen E, Haanstra JR, et al. EVI1 is critical for the pathogenesis of a subset of MLL-AF9-rearranged AMLs. Blood. 2012;119:5838–5849. doi: 10.1182/blood-2011-11-393827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broske AM, Vockentanz L, Kharazi S, Huska MR, Mancini E, Scheller M, Kuhl C, Enns A, Prinz M, Jaenisch R, et al. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet. 2009;41:1207–1215. doi: 10.1038/ng.463. [DOI] [PubMed] [Google Scholar]
- Bruns I, Czibere A, Fischer JC, Roels F, Cadeddu RP, Buest S, Bruennert D, Huenerlituerkoglu AN, Stoecklein NH, Singh R, et al. The hematopoietic stem cell in chronic phase CML is characterized by a transcriptional profile resembling normal myeloid progenitor cells and reflecting loss of quiescence. Leukemia. 2009;23:892–899. doi: 10.1038/leu.2008.392. [DOI] [PubMed] [Google Scholar]
- Bullinger L, Dohner K, Kranz R, Stirner C, Frohling S, Scholl C, Kim YH, Schlenk RF, Tibshirani R, Dohner H, Pollack JR. An FLT3 gene-expression signature predicts clinical outcome in normal karyotype AML. Blood. 2008;111:4490–4495. doi: 10.1182/blood-2007-09-115055. [DOI] [PubMed] [Google Scholar]
- Bullinger L, Ehrich M, Dohner K, Schlenk RF, Dohner H, Nelson MR, van den Boom D. Quantitative DNA methylation predicts survival in adult acute myeloid leukemia. Blood. 2009;115:636–642. doi: 10.1182/blood-2009-03-211003. [DOI] [PubMed] [Google Scholar]
- Chen D, Livne-bar I, Vanderluit JL, Slack RS, Agochiya M, Bremner R. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell. 2004;5:539–551. doi: 10.1016/j.ccr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Chen J, McKay RM, Parada LF. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 2012;149:36–47. doi: 10.1016/j.cell.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzio A, Passegue E, Ayton PM, Karsunky H, Cleary ML, Weissman IL. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17:3029–3035. doi: 10.1101/gad.1143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- Figueroa ME, Lugthart S, Li Y, Erpelinck-Verschueren C, Deng X, Christos PJ, Schifano E, Booth J, van Putten W, Skrabanek L, et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell. 2010;17:13–27. doi: 10.1016/j.ccr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa ME, Melnick A, Greally JM. Genome-wide determination of DNA methylation by Hpa II tiny fragment enrichment by ligation-mediated PCR (HELP) for the study of acute leukemias. Methods Mol Biol. 2009a;538:395–407. doi: 10.1007/978-1-59745-418-6_20. [DOI] [PubMed] [Google Scholar]
- Figueroa ME, Skrabanek L, Li Y, Jiemjit A, Fandy TE, Paietta E, Fernandez H, Tallman MS, Greally JM, Carraway H, et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood. 2009b;114:3448–3458. doi: 10.1182/blood-2009-01-200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentles AJ, Plevritis SK, Majeti R, Alizadeh AA. Association of a leukemic stem cell gene expression signature with clinical outcomes in acute myeloid leukemia. JAMA. 2010;304:2706–2715. doi: 10.1001/jama.2010.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidekel Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D, Vasile E, DePinho RA, Jacks T. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell. 2009;16:379–389. doi: 10.1016/j.ccr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschel S, Schlenk RF, Engelmann J, Rockova V, Teleanu V, Kuhn MW, Eiwen K, Erpelinck C, Havermans M, Lubbert M, et al. Deregulated Expression of EVI1 Defines a Poor Prognostic Subset of MLL-Rearranged Acute Myeloid Leukemias: A Study of the German-Austrian Acute Myeloid Leukemia Study Group and the Dutch-Belgian-Swiss HOVON/SAKK Cooperative Group. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.41.5505. [DOI] [PubMed] [Google Scholar]
- Haas K, Kundi M, Sperr WR, Esterbauer H, Ludwig WD, Ratei R, Koller E, Gruener H, Sauerland C, Fonatsch C, et al. Expression and prognostic significance of different mRNA 5'-end variants of the oncogene EVI1 in 266 patients with de novo AML: EVI1 and MDS1/EVI1 overexpression both predict short remission duration. Genes Chromosomes Cancer. 2008;47:288–298. doi: 10.1002/gcc.20532. [DOI] [PubMed] [Google Scholar]
- Huntly BJ, Shigematsu H, Deguchi K, Lee BH, Mizuno S, Duclos N, Rowan R, Amaral S, Curley D, Williams IR, et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6:587–596. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauter J, Wagner K, Schafer I, Marschalek R, Meyer C, Heil G, Schaich M, Ehninger G, Niederwieser D, Krahl R, et al. Prognostic factors in adult patients up to 60 years old with acute myeloid leukemia and translocations of chromosome band 11q23: individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2009;27:3000–3006. doi: 10.1200/JCO.2008.16.7981. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Sonabend AM, Guarnieri P, Soderquist C, Ludwig T, Rosenfeld S, Bruce JN, Canoll P. Glioblastoma models reveal the connection between adult glial progenitors and the proneural phenotype. PLoS One. 2011;6:e20041. doi: 10.1371/journal.pone.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenberg B. Acute myeloid leukemia: the challenge of capturing disease variety. Hematology Am Soc Hematol Educ Program. 2008:1–11. doi: 10.1182/asheducation-2008.1.1. [DOI] [PubMed] [Google Scholar]
- Lugthart S, van Drunen E, van Norden Y, van Hoven A, Erpelinck CA, Valk PJ, Beverloo HB, Lowenberg B, Delwel R. High EVI1 levels predict adverse outcome in acute myeloid leukemia: prevalence of EVI1 overexpression and chromosome 3q26 abnormalities underestimated. Blood. 2008;111:4329–4337. doi: 10.1182/blood-2007-10-119230. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, Ma J, Liu W, Cheng C, Schulman BA, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360:470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer SM, Sanders MA, Gilissen C, Tonnissen E, van der Heijden A, Dohner K, Bullinger L, Jansen JH, Valk PJ, van der Reijden BA. High BRE expression predicts favorable outcome in adult acute myeloid leukemia, in particular among MLL-AF9-positive patients. Blood. 2011;118:5613–5621. doi: 10.1182/blood-2011-06-359182. [DOI] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Parekh S, Prive G, Melnick A. Therapeutic targeting of the BCL6 oncogene for diffuse large B-cell lymphomas. Leuk Lymphoma. 2008;49:874–882. doi: 10.1080/10428190801895345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ME, Mahfouz R, Onciu M, Liu HC, Zhou X, Song G, Shurtleff SA, Pounds S, Cheng C, Ma J, et al. Gene expression profiling of pediatric acute myelogenous leukemia. Blood. 2004;104:3679–3687. doi: 10.1182/blood-2004-03-1154. [DOI] [PubMed] [Google Scholar]
- Schlenk RF, Dohner K. Impact of new prognostic markers in treatment decisions in acute myeloid leukemia. Curr Opin Hematol. 2009;16:98–104. doi: 10.1097/MOH.0b013e3283257adb. [DOI] [PubMed] [Google Scholar]
- Schlenk RF, Dohner K, Kneba M, Gotze K, Hartmann F, Del Valle F, Kirchen H, Koller E, Fischer JT, Bullinger L, et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia. Results from the AMLSG Trial AML HD98B. Haematologica. 2009;94:54–60. doi: 10.3324/haematol.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmi T, Martello A, Vignudelli T, Ferrari E, Grande A, Gemelli C, Salomoni P, Ferrari S, Zanocco-Marani T. ZFP36 expression impairs glioblastoma cell lines viability and invasiveness by targeting multiple signal transduction pathways. Cell Cycle. 2012;11:1977–1987. doi: 10.4161/cc.20309. [DOI] [PubMed] [Google Scholar]
- Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Stubbs MC, Kim YM, Krivtsov AV, Wright RD, Feng Z, Agarwal J, Kung AL, Armstrong SA. MLL-AF9 and FLT3 cooperation in acute myelogenous leukemia: development of a model for rapid therapeutic assessment. Leukemia. 2008;22:66–77. doi: 10.1038/sj.leu.2404951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge JJ, Sinha AU, Zhu N, Li M, Armstrong SA, Orkin SH. Haploinsufficiency of Dnmt1 impairs leukemia stem cell function through derepression of bivalent chromatin domains. Genes Dev. 2012;26:344–349. doi: 10.1101/gad.184341.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM, Beverloo HB, Moorhouse MJ, van der Spek PJ, Lowenberg B, Delwel R. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350:1617–1628. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Tuominen LK, Tsai CJ. SLIM: a sliding linear model for estimating the proportion of true null hypotheses in datasets with dependence structures. Bioinformatics. 2011;27:225–231. doi: 10.1093/bioinformatics/btq650. [DOI] [PubMed] [Google Scholar]
- Wang PY, Young F, Chen CY, Stevens BM, Neering SJ, Rossi RM, Bushnell T, Kuzin I, Heinrich D, Bottaro A, Jordan CT. The biologic properties of leukemias arising from BCR/ABL-mediated transformation vary as a function of developmental origin and activity of the p19ARF gene. Blood. 2008;112:4184–4192. doi: 10.1182/blood-2008-02-142190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA. The Wnt/β-catenin Pathway Is Required for the Development of Leukemia Stem Cells in AML. Science. 2010;327 doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Iwasaki M, Somervaille TC, So CW, Cleary ML. Meis1 is an essential and rate-limiting regulator of MLL leukemia stem cell potential. Genes Dev. 2007;21:2762–2774. doi: 10.1101/gad.1602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Radtke I, Pardee TS, Zhao Z, Rappaport AR, Luo W, McCurrach ME, Yang MM, Dolan ME, Kogan SC, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23:877–889. doi: 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.