Abstract
Background:
Fungal infections of the central nervous system, especially cerebral mucormycosis or brain abscess are very rare.Cerebral mucormycosis is a rare disease. It is not an independent disease, but a secondary opportunistic infectious disease.
Materials and methods:
This study has collected the data of 81 cases of intracranial mucormycosis from 28 Chinese hospitals, within 37 years, as well as reviewed the literatures and retrospectively analyzed and summarized this disease's background, clinical classifications, risk factors, pathology, clinical manifestations, diagnosis, treatment, and prognosis.
Results:
The 81 IM cases were aged between 15 days (the youngest) and 79 years (oldest), with a mean age of 41.6 years. Among them, 12 cases were <1 year old (the infant group), six cases were within one to 13 years old (the children group), and 63 cases were >14 years old (the adult group ). 45 cases were male and 36 were female, with a male/female ratio of 1.25:1.0. The shortest duration of the disease was three days, and the longest was 248 days.
Conclusions:
This study helped to realize an early diagnosis and treatment, improve the cure rate, and reduce mortality.
Keywords: Brain, intracalvarium, mucor
INTRODUCTION
Fungal infections of the central nervous system, especially cerebral mucormycosis or brain abscess are very rare.[1,2,3,4,5,6,7,8,9] Although mucormycosis has been discovered since nearly two centuries,[9,10,11,12,13] a complete understanding and consensus about this disease cannot be reached yet and needs further exploration.[3,6,7,8,9,10] Over the last few years, with an increase in malignant cancers, diabetes, leukemia, and acquired immune deficiency disease, as well as a rise in organ transplantation, and a wide application of appropriate antibiotics, hormones, anticancer drugs, and immunosuppressants, the incidence of this disease has exhibited an increasing trend.[3,6,7,9,10,11,12,13,14,15,16,17,18,19] As people lack knowledge about this disease, an early diagnosis will be difficult. Therefore, treatment is always delayed, resulting in high mortality and poor prognosis.[2,3,6,9,10]
This study summed the data of 81 cases of intracranial mucormycosis (IM) from 28 hospitals, within 37 years, reviewed the relative literature, and analyzed this disease's historical background, classification, risk factors, pathology, clinical manifestations, diagnosis, treatment, and prognosis. By means of this analysis, it was concluded that IM was not an independent disease, but a secondary opportunistic infectious disease. When the body suffered from serious diseases such as malignant cancers, leukemia, and diabetes, which caused low immunity for a long period, it would be very easy for the patient to be accompanied with the infection of nasal and orbital mucormycosis. Meanwhile, it could spread into the intracalvarium through local invasion or blood circulation, and become an intracranial infection-based systemic disease. Hence, the doctors must understand and comprehend this disease, and should perform positive and effective treatment, including timely and thorough surgical treatment. Otherwise, it would bring a disastrous and poor prognosis to the patients, even death. As Anna et al. emphasized in the Guide of the Third Europe Leukemia Conference, in 2012,[18] when a patient with leukemia was infected by mucormycosis, besides adequate broad-spectrum antibiotics and amphotericin B, timely surgery must be performed to thoroughly remove the lesion. Otherwise, the patient would have no chance of survival.
MATERIALS AND METHODS
This study collected 81 IM cases from 28 hospitals, from 1975 to 2013, and retrospectively analyzed the clinical manifestations, symptoms and signs, imaging, risk factors, clinical classification, pathology, treatment, and prognosis.
RESULTS
General information: The 81 IM cases were aged betweem 15 days (the youngest) and 79 years (oldest), with a mean age of 41.6 years. Among them, 12 cases were <1 year old (the infant group), six cases were within one to 13 years old (the children group), and 63 cases were >14 years old (the adult group ). Forty-five cases were male and 36 were female, with a male/female ratio of 1.25 : 1.0. The shortest duration of the disease was three days, and the longest was 248 days. This study was conducted in accordance with the declaration of Helsinki, and conducted with approval from the Ethics Committee of the Tianjin First Center Hospital. Written informed consent was obtained from all the participants. Clinical manifestations: Seven cases were fulminant, 26 cases were acute, 35 cases were subacute, and 13 cases were chronic. The first symptoms included 20 cases of nasal pain, 19 cases of eye pain, 16 cases of headache, seven cases of high fever, five cases of vomiting and vision loss each, four cases of cough, chest pain, weakness, and paralysis (cerebral infarction) each, two cases of facial skin infection, cirrhosis, diarrhea, convulsion, periodontitis, otitis media and coma each, one case of post-facial trauma infection, and one case of sepsis. There were only 14/81 cases (17.3%) that were diagnosed before surgery or death in the whole group, among which the diagnosis rate before the twenty-first century was 4/53 (7.5%) and after the twenty-first century, it was 10/28 cases (35.7%). Fifty-eight of the 81 cases (71.6%) were not correctly diagnosed, and most were misdiagnosed as meningitis, encephalitis, brain abscess, intracranial space-occupying, cerebral infarction, nasal sinusitis, and so on. Nine of the 81 cases (11.1%) were not mentioned in the diagnosis. The endoscopy of 28/81 cases (34.5%) revealed a black eschar inside the oral or nasal cavity [Figure 1]. The biopsy reported 39/81 cases (48.1%) of mucormycosis. The fungal culture (30/81 cases, 37%) revealed that 22 cases were positive (73.3%), including 18/30 cases (60%) of Mucor, three cases of Rhizopus, and one case of Aspergillus.
Figure 1.
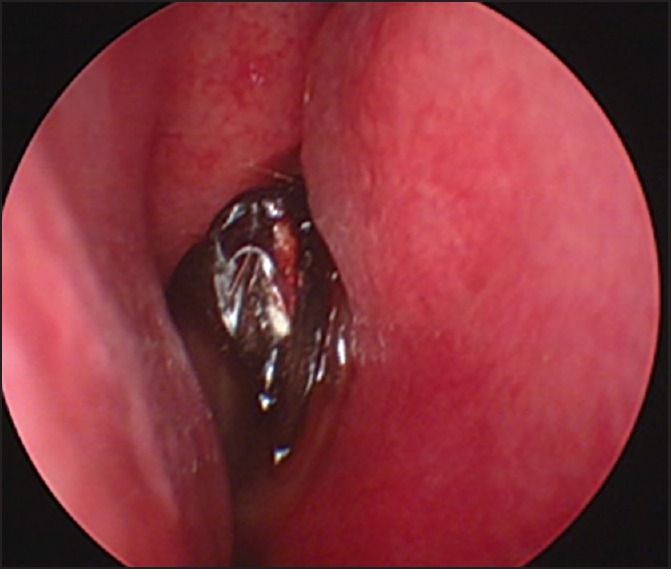
Endoscopy results. The bilateral middle concha and nasal septum had a scattered, dark purple or black scab and necrosis (white arrow). The suspected fungal infection was histologically confirmed as a Mucor
Clinical classification: The cases could be divided into 11 types:
Rhinocerebral type;
Rhino-orbital cerebral type;
Ear-cerebral type;
Oral-cerebral type;
Meningoencephalitis type;
Encephalitis type;
Pulmonary type;
Gastrointestinal type;
Cutaneous type;
Focus type;
Disseminated type also known as sepsis type.
Imaging: Computed tomography (CT) scanning was performed for 55 of the 81 cases (68%) and magnetic resonance imaging (MRI) scanning was performed for 41/81 cases (51.3%). Involvement of the nasal cavity and paranasal sinuses was exhibited in 36/55 cases (65.5%) and it appeared like thickened mucosa on the CT. The soft tissues of the sinal cavity exhibited high density [Figure 2a]. Bone destruction and thinning of the sinal wall were exhibited in 30/55 cases (54.5%). Orbital invasion was exhibited in 23/55 cases (42%), which appeared like an enlarged extraocular muscle and discontinuous orbital bone [Figure 2b]. The cranial CT and MRI showed that the intracranial brain tissue invasion mainly occurred in the frontal lobe (especially the basal pinacoid of the frontal lobe), followed by the basal ganglia, temporal lobe, parietal lobe, occipital lobe, and cerebellum. The low-density area [Figure 2c] or T1-weighted images appeared as low signals and the T2-weighted images showed a high signal in the acute phase. The boundary was not clear [Figure 2d and e]. Approximately four weeks later, enhanced scanning exhibited that the lesion was significantly annularly enhanced, with edema and space-occupying effects around [Figure 2f and g].
Figure 2.
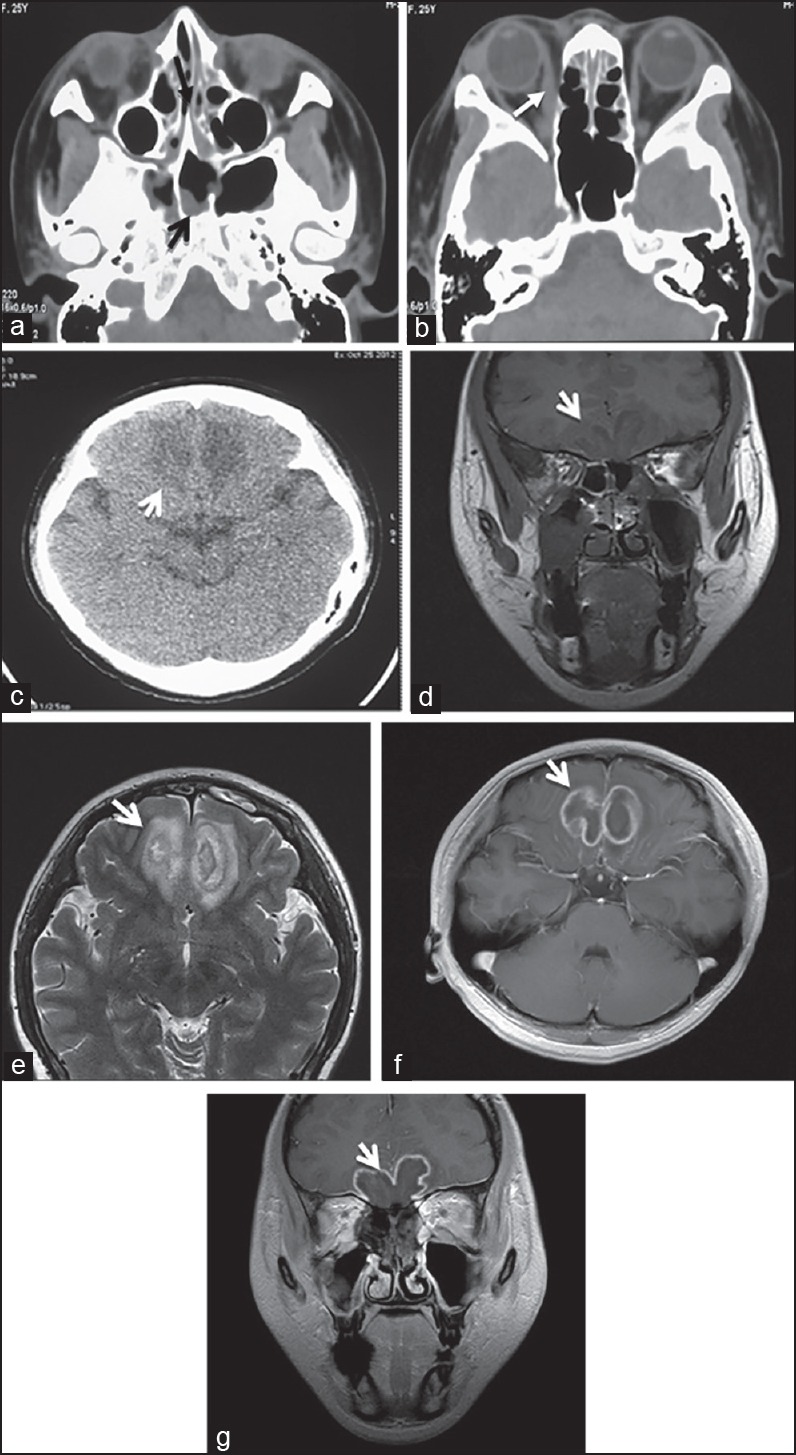
CT and MRI performance of IM (a) Cranial CT scanning showed: The nasal cavity and sinus showed a broader inflammation (black arrow). (b) Normal cranial CT scanning showed: The medial rectus and lacrimal gland of the right eye, as well as the swollen right periorbital soft tissue (white arrow). (c) Cranial CT showed the slabby low density area of the frontal lobe's underside in the acute phase (white arrow). (d) Normal cranial MRI scanning showed T1-weighted images (coronal) during the third week of onset. (e) The T2-weighted image showed (axial): That the underside of the bilateral frontal lobes had uneven long T1 and T2 signals, the boundary was unclear, the structures had been destroyed, the sulcus and gyrus were not clear, and the peri-lesion area had edema (white arrows). (f) Cranial MRI (sagittal) in the fifth week of hospitalization. (g) Coronal enhanced scanning showed: That the lesion appeared as a uniform annular enhancement (white arrow)
Risk factors: Most cases were based on primary diseases, and the most common primary disease was diabetic ketoacidosis (38/81 cases, 47%), followed by rhinitis, sinusitis, and nasal tumors (23/81 cases, 28.4%), intracranial tumors and cirrhosis (7/81 cases each, 8.6%), blood diseases (6/81 cases, 7.4%), cerebral infarction (four cases), facial infection (two cases), lung disease, otitis media, and facial trauma (1 case each), unexplained reasons (4/81 cases, 4.9%), and the total was 94 cases. The onset of most cases in this study was related to the antibiotic (47/81 cases, 58%) and hormone (28/81 cases, 34.6%), and the application of chemotherapy drugs had 6/81 cases (7.4%).
Diseased regions and pathology: According to the imaging and autopsy, it was found that most cases invaded the cerebral lobe, including 32 cases of frontal lobe, 25 cases of basal ganglia, 16 cases of temporal lobe, nine cases of cerebellum, six cases of parietal lobe, five cases of occipital lobe, four cases of brain stem, three cases of brain ventricle and orbital apex (each), two cases of cavernous sinus, 24 cases of meninges, and five cases of unclear records, and the total was 134 cases. Pathological appearance: The brain tissue structures were destructed and exhibited inflammatory infiltration characteristics of fungus, mucormycosis, a lot of pus balls, neutrophils, multinucleated giant cells, and phagocytes. Periodic acid-Schiff (PAS) staining was positive and the mucormycosis emboli could be seen inside the vessels (100×), (HE 200×) [Figure 3].
Figure 3.
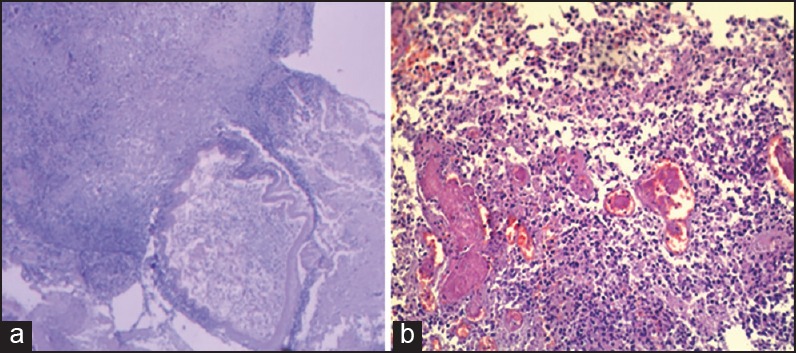
Pathological changes of IM (a) Pathological observation: showed an abscess, accompanied with multinucleated giant cell reaction, the Mucor was visible. PAS staining was positive (100×). (b) Pathology: The brain tissues were damaged and accompanied with the inflammatory infiltration characteristics of fungal groups, a great number of pus balls, neutrophils, multinucleated giant cells, and phagocytes. Mycotic emboli could be seen inside the blood vessels (HE 200×)
Treatment and prognosis: Most cases applied antifungal drugs when the efficacy was poor or pathologically diagnosed, with amphotericin B (1.0-1.5 mg•kg•d) as the preferred drug, followed by the fluconazole, caspofungin 50 mg for intravenous drip l/d. Efficacy: Nineteen out of 81 cases (23.5%) survived (5/53 cases (9.4%) before 2000, and 14/28 cases (50%) after 2000), among which 11 cured cases were diagnosed early, early surgical resection of intracranial lesions was performed, and they were treated early, with enough antifungal drugs. Eight cases improved. Sixty-two of 81 cases (76.5%) died, among whom 24/81 cases (29.6%) died within two weeks and 10/81 cases (12.3%) were newborns. The mortality rate before 2000 was 88.7% (47/53) and 53.6% (15/28) after 2000.
DISCUSSION
This research considered that IM was not an independent disease, but a secondary opportunistic infectious disease. When the body suffered from such serious diseases as malignant cancers, leukemia, and diabetes, which caused low immunity for a long period, it would be very likely that the patient was accompanied with the infection of nasal and orbital mucormycosis. Meanwhile, it could spread into the intracalvarium through local invasion or blood circulation, and become an intracranial infection-based systemic disease.[2,3,4,5,6,7,8,9,10,11,15,16,17,18,19] At present, this disease still needs to be deeply studied. Therefore, the author collected 81 IM cases from 28 hospitals, within 37 years, and reviewed the related literatures for the retrospective analysis of this disease's historical background, classification, risk factors, pathology, clinical manifestations, diagnosis, treatment, and prognosis, with the aim of allowing physicians in all areas to understand and comprehend this disease. Therefore, the patient could be diagnosed and treated early, thus improving the cure rate and reducing mortality. The results are summarized as the following:
Historical background and classification: According to the report of Upender et al.,[10] IM was first found by Meyer, about two centuries ago, in 1815, to be a pathogen. In 1855, Kurchenmeister et al.[11] reported the first case of IM-infected lung cancer. In 1876, it was confirmed that this bacteria could make people sick. In 1943, the entity of mucormycosis was clear, that is, this mold was the saprophytic or parasitic fungus of Zygomycotina → Zygomycetes → Mucorales → Mucoraceae, and it was also known as zygomycosis.[12] It could cause nasal, orbital or intracranial inflammations. In 1955, rhino-orbito-cerebral mucormycosis (ROCM) was first named, and it was found that the Mucor would first attack and parasitize the inside of the nasal mucosa, and then spread to the orbit, eventually invading the brain, or it could directly invade the brain from the nasal cavity (i.e., Rhinocerebral mucormycosis (RCM).[10,11,12] The incidence of IM was low, as reported by Talmi et al.[13] Most reported cases were individual cases or re-retrospective analyses of small samples from these two centuries.
As for the clinical classification of IM, according to the primary sites and invaded organs, as also those combined with pathological changes, IM could be divided into:
RCM: The lesions were involved with the nasal mucosa, which caused mucosal degeneration and necrosis through parasitism and reproduction. It would then continue to expand to various nasal sinuses, especially the ethmoid, sphenoid, and frontal sinuses. Once it eroded the bone wall, it could cause IM.
ROCM: Upender et al.,[10] named it cerebrorhino-orbital mucormycosis. After the lesions invaded the orbit directly from the nasal cavity, it would then infect the intracranium. However, the Mucor of few cases originated orbitally and then infected the intracranium.
Ear–cerebral mucormycosis: Long-term ear infections, such as otitis media, would easily combine the mucormycosis-caused encephalitis or abscess. When the Mucor invaded the petrous bone, it could lead to basicranial osteomyelitis. The basicranial type was very rare.
Oral cerebral mucormycosis: The combination of acute or chronic tonsillitis or periodontitis and mucormycosis could enter the intracranium through the facial blood vessels. This type was very rare.
Meningoencephalitis mucormycosis: The patient would show increased intracranial pressure and meningeal irritation symptoms, such as, high fever, unconsciousness, headache, and vomiting.
Encephalitis mucormycosis: The patient would show the symptoms of cerebral parenchymal damages, such as unconsciousness, hemiplegia, aphasia, seizures, and increasing intracranial pressure or intracranial pressure accompanied with the meningeal irritation.
Pulmonary mucormycosis: The pathogenic bacteria entered the lungs through the respiratory tract and then caused bronchitis and/or pneumonia. Few cases would die of acute pulmonary infarction caused by the vascular embolism.
Gastrointestinal mucormycosis: The patient often had the clinical manifestations of gastrointestinal disease, and the severe cases would exhibit an ulcer, intestinal cramp, even intestinal obstruction, and perforation associated with acute peritonitis, caused by the mesenteric vein thrombosis.
Cutaneous mucormycosis: In very rare cases, the skin wounds would be infected by the Mucor, and these could be divided into the primary and secondary types. In the acute phase, the patient would exhibit inflammation and a severe case would have suppuration, necrosis, and even gangrene. The lesion could invade the skin, subcutaneous layer, muscle, and even bone.
Focus mucormycosis: The primary would be from the bone, heart, liver, kidney, bladder, uterus or thoracic and abdominal aorta, and other organs. The clinical manifestations varied according to the different involved organs; it was very rare, but the mortality rate was very high.
Disseminated mucormycosis: Also known as sepsis, the pathogenic bacteria invaded the blood vessels and formed acute inflammation and thrombotic lesions in multiple organs through the blood circulation. This type was very rare and the mortality rate was 100%.
In short, the Mucor could invade the intracranium by:
Directly eroding the madreporic plate or madreporic mesh and the frontal- and sphenoid-sinal bone wall,
Spreading into the intracranium through the nasal vein and basicranial vein,
Entering the orbital vessels or optic canal through the maxillary sinus and ethmoid sinal bone plate, and then spreading into the intracranium, and
Spreading into the intracranium through the blood circulation.[10,13,20,21,22]
Among the above types, RCM and ROCM were the most common types, accounting for about 75%.[1,2,3,6,7,10,16,18,19] Thus, ROCM was used as an example for discussion.
Etiology and risk factors: The Mucor was a saprophytic bacteria that largely presented in the soil and air.[10,18,19,20,21,22] ROCM had a strong invasion and the latency period was short, the onset was quick, and the disease progression was also rapid, thus the mortality rate was very high. It was reported that the most common fungus that caused mucormycosis was the Rhizopus, accounting for about 70% to 90% of the bacteria, followed by Mucor and Rhizomucor.[18,19,22] This study considered that ROCM was an opportunistic infectious disease caused by the mucor and low immunity was one of the major pathogenic factors. Therefore, improving the patient's immunity was one of the key measures for the prevention of mucormycosis.
The most common clinical causative factors and risk factors were diabetic ketoacidosis, accounting for about 70%.[16,18,19] This study found that 38 of 81 cases were of diabetic ketoacidosis (47%), and the reasons were:
The acidic serum could promote the growth of the mucor,[16] block and decrease the iron-binding capacity of serum transferrin, and increase the free iron, thus promoting the reproduction of the Mucor;
The ketoreductase inside the mucor could promote the mucor to use the host's ketones and increase the host's sensitivity toward the Mucor;[10,16,18,19]
High blood glucose and low pH could weaken the chemotaxis and adhesion of neutrophils toward the mycelium,
Weak inhibitory effects of the macrophage toward the spores and hyphae,[10,18,19] thus was more conducive to the growth of the Mucor.
Secondly, in this study, there existed 23/81 cases (28.4%) of nasal sinusitis and nasal tumors, seven of 81 cases (8, 6%) of intracranial tumors and cirrhosis each, and six of 81 cases (7.4%) of blood diseases, which were lower than those shown in literature. In literature, leukemia accounted for 35.5%,[10,18,19,21,22,23] as well as uremia, radiotherapy, and chemotherapy, severe malnutrition, large area burns or immunodeficiency diseases (such as AIDS), and patients with long-term application of antibiotics, hormones or immunosuppressive agents would be prone to have this disease.[1,2,3,4,5,6,7,8,9,10,16,18,19,21] Most of the cases in this study were related to the application of antibiotics (47/81 cases, 58%) and hormones (28/81 cases, 34.6%). Anna et al.[18] reported that the Guide of Third European Leukemia Conference (2012) stressed that the leukemia patients would be very susceptible to mucormycosis. Although this disease was rare, it did exhibit an increasing trend in the last decades. The French epidemiological study showed that the annual incidence rate of mucormycosis in patients suffering from blood diseases rose from 0.7 persons/per million in 1997 to 1.2 persons/per million in 2006.[23,24]
Histopathology: One of the greatest features of the Mucor was its affinity to the blood vessels, especially the arteries. It could reproduce in the internal elastic film layer, and thus, separate the elastic membrane and middle layer, and cause serious damage to the intima, and form thrombosis, infarction, and hemorrhage, as the Mucor could produce proteinase and elastase, so the mycelium could encroach along the surrounding blood vessels.[6,7,8,9,10,18,19,21] Furthermore, this blood vessel-addict just determined the pathological features of mucormycosis.[18,19,21,22,23,24] It might also form purulent arteritis and inflammatory emboli, and thus cause mycotic cerebral infarction or fungal aneurysm.[8] When it invaded the veins, the bleeding would be much easier. If the above lesions formed a vicious cycle, they would aggravate the local tissues' microenvironment ischemia much more, cause hypoxia and acidosis, as well as promote the infection and spread of the Mucor.[2,3,4,5,6,7,18,19,21] The pathological feature of ROCM was the acute fulminant lesion, mainly appearing as coagulative necrosis and fungal vasculitis, but it could also form pyogenic granuloma. The chronic invasion mainly appeared as chronic pyogenic granuloma, which was often accompanied by chronic non-specific inflammation,[2,5,6,7,10] as well as nerve fiber degenerative necrosis, inflammatory cell infiltration, and a great destruction of the hyphae and even the bone. In order to show the lesions more clearly, a special staining could also be performed, such as, PAS and Gomori's Methenamine Silver (GMS) staining, which could make it much easier to distinguish.[2,5,6,7,10,19,21,22]
Clinical manifestations: The data showed that ROCM could occur at any age, with the no gender difference, and could occur in various organs. It would exhibit a fulminant, acute, subacute or chronic onset, with the majority as subacute, while the progression was rapid and ranged from several days to several weeks. The chronic case was rare and the progression was slow. It ranged from a few months to years;[10] the acute or fulminant cases progressed faster, if not treated in time or appropriately, and the patient could die within several weeks.[7,10,18,19,21] The reasons for the significant reduction in mortality after 2000 were:
The level of healthcare had significantly increased and
the diagnosis and treatment levels had also gradually improved.
Talmi et al.,[13] thought that ROCM occurred easily in fall and winter, and stressed that the vulnerable population, such as, immunocompromised patients, and those with diabetic ketoacidosis, malignant cancer, and leukemia, had a high incidence, normally with nasal and eye symptoms being the first symptoms. The black ulcer and eschar inside the nasal cavity was one of the most important signs of highly-suspicious mucormycosis.[5,6,7,10,16,18,19,21] When the craniocerebrum was involved, the patient would have headaches, vomiting, paralysis or seizures, even consciousness disorders. Talmi et al.,[13] divided ROCM into four phases: Phase I: The lesions were confined to the nasal mucosa; Phase II: The lesions were confined to the nasal cavity, nasal sinuses, and orbit; Phase III: The lesions invaded the intracranial structures, causing cognitive disorders; Phase IV: The lesions severely invaded the intracranial structure, causing unconsciousness, paralysis or aphasia. The initial symptoms of this study were mainly nasal pain, eye pain, headache, fever, vomiting, and vision loss. However, there also appeared one clinical manifestation that was easily overlooked, namely, eight out of 81 cases (10%) had limb weakness and numbness, just like a stroke. The cranial CT and MRI showed that the frontal lobe, parietal lobe, and/or basal ganglia exhibited low-density, the T1-weighted images showed low signals, and T2-weighted images showed high signals. Gollard et al.[25] also found this phenomenon and considered that the cerebrovascular violation, after IM infection, was one of the reasons of cerebrovascular disease in young patients who could not be ignored. Calli[26] reported one case of IM-caused pontine infarction. Eucker et al.,[27] reported that cerebral angiography had confirmed that mycotic cerebral vasculitis and vascular occlusion might cause cerebral infarction. Therefore, the author believed that patients from the clinic, who had unexplained and conventional treatment with not-easily-controlled fever, accompanied by stroke, should be highly suspected for IM.
Diagnosis: In the past, the diagnosis rate of mucormycosis was low, only an autopsy could confirm it.[23,24] Wang[20] reported that the misdiagnosis rate of 27 IM cases in the last century was 89%. In this study, the misdiagnosis rate was 71.6% and most of the cases were misdiagnosed as meningitis, encephalitis, brain abscess, intracranial space-occupying lesion cerebral infarction, and sinusitis. Therefore, the most critical key of improving the cure rate and reducing mortality was early diagnosis.[24]
At present, the early diagnosis of ROCM is still very difficult, which is mainly because there are no specific early symptoms of this disease and the symptoms of most cases such as nasal and eye symptoms that appear during the treatment of primary diseases, such as, diabetes and leukemia, are not obvious, so they are more easily overlooked.[18,19,21,22,23,24] Generally, when the symptoms are obvious, it indicates that the disease has entered Phase III or IV.[13] Therefore, the author has proposed the following diagnostic ideas:[5,6,7,8,10,18,19,21,22,23,24]
For clinical manifestations of serious systemic diseases, such as, diabetes, leukemia, and malignant cancers, a large number of antibiotics, hormones or immunosuppressive agents must be given. When the patients have high fever, nasal or ocular symptoms or headaches, especially if a black eschar is found in the nasal cavity, it must be suspected that the patients have this disease. During the treatment, each abnormal sign and symptom that appears must be closely observed, so that misdiagnosis may be reduced.
Imaging: CT and MRI reveal that the nasal and orbital soft tissues exhibit abnormal densities and signals. The cranial CT manifestations show that irregular low-density areas exist inside the cerebral parenchyma. An MRI shows the low T1-weighted imaging signals and high T2-weighted imaging signals. Enhanced MRI scanning shows that the lesions are annularly enhanced. Although a CT and MRI can diagnose the intracranial inflammation and brain abscess, it is still difficult to make a diagnosis of the cause.
Fungal microscopy and culture: The nasal black eschar tissue is taken under the endoscope for a biopsy. If fungal hyphae are found, the patient can then be confirmed with mucormycosis. One of the gold standards of mucormycosis diagnosis is the fungal culture; a positive result can confirm the disease; however, a negative result cannot exclude the disease.[18,19,21,22,23] The authors have used l0% potassium hydroxide to process the samples, which helps in mycelium detection.[10,18,19]
Histopathology: A combination of craniotomy and resection of the brain abscess or a pathological autopsy can firmly diagnose the disease.
Treatment: According to the data and literature, the author has proposed these basic principles for ROCM treatment:[2,3,4,5,6,7,8,9,10,16,18,19,21,22,23]
Treat the primary disease, such as, diabetes mellitus accompanied with ketoacidosis, leukemia, and so on, normally and systematically.
During the treatment, once mucormycosis is diagnosed, the patient must be immediately given an adequate systemic antifungal therapy, with the preferred drugs being amphotericin B (1.0~1.5 mg•kg•d and caspofungin 50 mg, intravenous drip l/d). Meanwhile, the primary tumor must be completely removed, surgically, to block the dissemination of mucormycosis, which is a very important factor.
Once ROCM is diagnosed, craniotomy must be immediately performed to remove the abscess. The patients cured in this study fully prove this point. As Anna Skiada has emphasized in the Guide of the Third Europe Leukemia Conference, in 2012,[18] when the patient with leukemia is infected with mucormycosis, besides the adequate broad-spectrum antibiotics and amphotericin B, timely surgery must be performed to thoroughly remove the lesion, otherwise the patient will have no chance of survival. The experience of this study indicates that the best surgical resection must reach the depth and breadth, such that, no mold can be found in the tissue sections again, which is essential for ROCM to be cured thoroughly. Yeung et al.,[28] reviewed nine cases of ROCM, among which surgery was carried out in the three cases that survived. Nithyanandam et al.,[29] reported 11 cases of ROCM, where debridement was performed in all cases and 10 cases were successful. In short, timely, urgent, and thorough surgical treatment was the only effective measure, and it was also the consensus of most scholars.[10,16,18,19,28,29]
It was suggested that the nasal surgical area could be topically rinsed using amphotericin B solution, which could play a role in killing and suppressing the Mucor. It was simple and without side effects.[10,11,12,13]
A general supportive therapy could be applied to increase the immunity.
Prognosis: The prognosis of ROCM was related to a variety of factors.[1,2,3,4,6,7,8,10,11] As most cases could not be diagnosed early, even misdiagnosed, the effect would not be ideal, and this was directly related to the prognosis. Some people thought that the nasal and orbital involvement, as well as the symptoms of nervous system deficiency suggested a poor prognosis and emphasized that when serious systemic diseases such as diabetic ketoacidosis, leukemia, malignant cancers or immune deficiency disease existed, even with the application of a large number of immunosuppressants, antibiotics or hormones, the prognosis would be poor.[13,18,19,21,30] Generally, if the lesions had invaded the intracranial regions, the prognosis would be poor.[9,10,16,17,18,19] Some scholars reviewed 294 cases of ROCM, and found that the total mortality was 48%.[13] Wang[20] reported that the mortality of IM was 100% in the 1980s. Before the twenty-first century, ROCM had almost no effective treatment, and the survival rate was only l2%. Later, after the therapy of surgery and amphotericin B was combined, the survival rate gradually increased to 85%.[10,13,18,19,20,21,30] The mortality rate had reduced from 88% in 1961 to the current 15~34%.[30]
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES
- 1.Abu El-Naaj I, Leiser Y, Wolff A, Peled M. The surgical management of rhinocerebral mucormycosis. J Craniomaxillofac Surg. 2013;41:291–5. doi: 10.1016/j.jcms.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Takao O, Kumiko T, Ichiro T, Masayuki S, Hideaki K, Mitsuaki I, et al. Successful treatment of rhino-orbital mucormycosis by a new combination therapy with liposomal amphotericin B and micafungin. Auris Nasus Larynx. 2012;39:224–8. doi: 10.1016/j.anl.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Imad AE, Leiser YA, Wolff MP. The surgical management of rhino-cerebral mucormycosis. J Cranio-Maxillo-Fac Surg. 2013;41:291–5. doi: 10.1016/j.jcms.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Suzan S, Reza A, Siamak AM. Rhino-Orbitocerebral Mucormycosis in a Patient with Idiopathic Crescentic Glomerulonephritis. Saudi J Kidney Dis Transpl. 2013;24:768–72. doi: 10.4103/1319-2442.113878. [DOI] [PubMed] [Google Scholar]
- 5.Ami P, Eliahu B, Sandhya N. Mucormycosis in an HIV-infected renal transplant patient: A case report and review of the literature. Am J Case Rep. 2014;15:74–8. doi: 10.12659/AJCR.890026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munir N, Jones NS. Rhinocerebral mucormycosis with orbital and intracranial extension: A case report and review of op timum management. Laryngol Otol. 2007;121:192–5. doi: 10.1017/S0022215106003409. [DOI] [PubMed] [Google Scholar]
- 7.Mohindra S, Mohindra S, Gupta R, Bakshi J, Gupta SK. Rhinocerebral mucormycosis: The disease spectrum in 27 patients. Mycoses. 2007;50:290–6. doi: 10.1111/j.1439-0507.2007.01364.x. [DOI] [PubMed] [Google Scholar]
- 8.Mathieu R, Pascale C, André K, Charles JM, Xavier P. Mucormycosis cerebral arteritis mimicking a flare in ANCA-associated vasculitis. Lancet Infect Dis. 2013;13:182–5. doi: 10.1016/S1473-3099(12)70143-3. [DOI] [PubMed] [Google Scholar]
- 9.Anne D, Thierry D, Sylvie VS. Fatal rhinocerebral mucormy-cosis with intracavernous carotid aneurysm and thrombosis: A late complication of transsphenoidal surgery? Acta Neurol Belg. 2013;113:179–84. doi: 10.1007/s13760-012-0151-9. [DOI] [PubMed] [Google Scholar]
- 10.Upender W, Abdullah B, Abdullah AM. Cerebro- rhino-orbital mucormycosis: An update: J Infect Public Health. 2012;5:116–26. doi: 10.1016/j.jiph.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Romero J, Bonifaz A, Sánchez C, Lagunas A. Rhinocerebral mucormycosis. Report of twelve cases. Rev Med Hosp Gen Mex. 2000;63:178–84. (in Spanish) [Google Scholar]
- 12.Schell WA. Histopathology of fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:251–76. doi: 10.1016/s0030-6665(00)80004-3. [DOI] [PubMed] [Google Scholar]
- 13.Talmi YP, Goldschmied RA, Bakon M, Barshack I, Wolf M, Horowitz Z, et al. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol Head Neck Surg. 2002;127:22–31. doi: 10.1067/mhn.2002.126587. [DOI] [PubMed] [Google Scholar]
- 14.Chan LL, Sanjay S, Dan J, Eduardo MD, Lawrence E. Ginsberg: Imaging of Mucormycosis Skull Base Osteomyelitis. AJNR Am J Neuroradiol. 2000;21:828–31. [PMC free article] [PubMed] [Google Scholar]
- 15.Virendra S, Bindu S, Rajeev S, Shalini A, Amrish B, Rishi B. Rhinocerebral Mucormycosis: A Diagnostic Challenge and Therapeutic Dilemma in Immunocompetent Host. J Oral Maxillofac Surg. 2012;70:1369–75. doi: 10.1016/j.joms.2011.06.209. [DOI] [PubMed] [Google Scholar]
- 16.David GB, Raúl GG, Carlos MG, Luis RL, Florencio MG. Mucormycosis of the head and neck: Report of five cases with different presentations. J Craniomaxillofacial Surg. 2012;40:584–91. doi: 10.1016/j.jcms.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Daniela M, Hartmut B, Marc Z, Tobias R, Alexander CK, Urs DA, et al. Mucormycosis of the head and neck. J Craniomaxillofacial Surg. 2012;40:321–7. [Google Scholar]
- 18.Anna S, Fanny L, Andreas HG, Livio P, Stephan Z, Raoul H, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: Guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3) Haematol. 2013;98:492–504. doi: 10.3324/haematol.2012.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palash KM, Santosh KM, Tanmoy KM. Mucormycosis of Pouch of Douglas in a Diabetic Woman. J Glob Infect Dis. 2012;3:172–4. doi: 10.4103/0974-777X.100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang LZ. Cerebral mucormycosis clinical analysis: 27 cases. Chin J Pract Intern Med. 1991;11:582–3. [Google Scholar]
- 21.Raquel A, Beatriz Á, Eduardo S, José IA, Héctor V. Rhinocerebral mucormycosis: Report on eight cases. Acta Otorrinolaringol Esp. 2010;61:301–5. doi: 10.1016/j.otorri.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 23.Bitar D, Van Cauteren D, Lanternier F, Dannaoui E, Che D, Dromer F, et al. Increasing incidence of zygomycosis (mucormycosis) Emerg Infect Dis. 2009;15:1395–401. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–9. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 25.Gollard R, Rabb C, Larsen R, Chandrasoma P. Isolated Cerebral Mucormycosis: Case Report and Therapeutic Considerations. Neurosurgery. 1994;34:174–7. doi: 10.1097/00006123-199401000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Calli C, Savas R, Parildar M, Pekindil G, Alper H, Yunten N. Isolated Pontine Infarction Due to Rhinocerebral Mucormycosis. Neuroradiology. 1999;41:179–81. doi: 10.1007/s002340050728. [DOI] [PubMed] [Google Scholar]
- 27.Eucker J, Sezer O, Lehmann R, Weber JR, Graf B, Denkert C, et al. Disseminated Mucormycosis Caused by Absidia Corymbifera Leading to Cerebral Vasculitis. Infection. 2000;28:246–50. doi: 10.1007/s150100070047. [DOI] [PubMed] [Google Scholar]
- 28.Yeung CK, Cheng VC, Lie AK, Yuen KY. Invasive disease due to Mucorales: A case report and review of the literature. Hong Kong Med J. 2001;7:180–8. [PubMed] [Google Scholar]
- 29.Nithyanandam S, Moire SJ, Ravindra RB, Reji KT, Majorie AC, D'Souza O. Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J Ophthalmol. 2003;51:231–6. [PubMed] [Google Scholar]
- 30.Cliff F, Timothy JS, Paul B, Tony A, Richard L. Survival after rhino-orbital-cere-bralmucor mycosis in an immunocompetent patient. Ophthalmology. 2000;107:555–8. doi: 10.1016/s0161-6420(99)00142-6. [DOI] [PubMed] [Google Scholar]


