Abstract
Background:
This study was conducted to evaluate in vitro antibacterial potential of ethanolic extract of Calotropis gigentica.
Materials and Methods:
The inhibitory effect of the ethanolic extract was tested against Streptococcus mutans and Lactobacilli casei by using disc diffusion method.
Results:
Ethanolic extract of Calotropis gigentica showed 16 mm and 14 mm of minimum inhibition zone at 1.25% concentration for S. mutans and lactobacilli, respectively.
Conclusion:
Calotropis gigentica was found to effective against S. mutans and lactobacilli.
Keywords: Antibacterial, Calotropis gigentica, lactobacilli, Streptococcus mutans
INTRODUCTION
Caries is the most prevalent chronic disease affecting the human race. In many ways, it can be considered as a disease of modern times as the occurrence of the caries seems to be much higher in the last few generations. It is an irreversible microbial disease of the teeth affecting the hard tissues of the tooth, characterized by demineralization of the inorganic substances and destruction of the organic substance of the tooth, often leading to cavitation. There are virtually no geographic areas in the world whose inhabitants do not exhibit some evidence of dental caries.[1]
Caries is a complex and vibrant process where a multitude of factors influence and initiate the progression of disease. It is caused by a certain type of acid producing bacteria, in particular, mutans group of Streptococcus and Lactobacillus which cause damage in the presence of fermentable carbohydrates such as sucrose, fructose, and glucose.[1] Hence, Streptococcus mutans and Lactobacilli are the chief implicating microorganisms responsible for dental caries. For prevention of dental caries, their level should be reduced in the oral cavity.
As the light of prevention has dawned on the dental profession, it has been possible to direct focused efforts toward this major dental diseases tormenting mankind. Newer methods and methodologies have been experimented to combat caries in a wider aspect. One such is the usage of herbs and plants as a therapeutic compound. Since the evolution of mankind, these were in use as a traditional medicinal system. In this day and age due to the emergence of drug resistance, the need for the development of newer antibacterials is on.[2,3] In recent times, plants are at the great attention of researchers for the development of alternative drugs with less side effects.[4]
Calotropis gigentica which is normally identified as weed plant is a wasteland plant. It is natural habitat of Asian countries and is used by tribal people for many diseases such as a toothache, sprain, ear ache, anxiety, pain, diarrhea, and mental disorders. Its various actions such as cytotoxicity, wound healing, antipyretic, and anticandidal activity have been documented.[5,6,7,8] Latex from the plant has many medicinal uses, it is used to treat boils, scabies, bruises, burns, cuts, sores, boils, wounds, and to stop bleeding. It contains triterpenes, cysteine proteinase, and galactin and is used for cold and heart condition also.[9,10,11]
The current study is intended to evaluate and compare in vitro, the efficacy of Calotropis gigentica as anticariogenic agent on the basis of its folk uses.
MATERIALS AND METHODS
Preparation of Calotropis gigentica extract
Fresh Calotropis gigentica leaves were collected from the wasteland of Jaipur and after washing shade dried at room temperature for 3-4 days. Three hundred grams of powdered calotropis was separately macerated with 100% ethanol. The extracts were filtered with Whatman filter paper to obtain a clear filtrate. The filtrates were reduced at 60°C to obtain a solid residue.
Preparation of five different concentrations of extracts
One gram of all the extracts were taken and added to 4 ml of sterile saline. This will give 25% dilution of the extract. One milliliter of this solution was added to another 1 ml of sterile saline to create 12.5% dilution of the extract. In this solution, another 1 ml of sterile saline was added to make it 6.25% to which 1 ml of sterile saline solution was added to make it 3.125% dilution. Then 1 ml of 3.125% solution was added into another 1 ml of sterile solution this gave us 1.6% dilution.
For ethanol extract, 5 g of dried leaves were dissolved in organic solvent ethanol. Along with solvent dimethyl sulfoxide was added according to the concentration since it acts as an inert solution. These solutions were used for further antimicrobial evaluation.
Reagent and materials
Brain heart infusion agar (Hi-Media Laboratories, Mumbai, India), Rogosa agar (Hi-Media Laboratories, Mumbai, India), S. mutans culture (IMTECH, Chandigarh, India), Lactobacilli casei culture (IMTECH, Chandigarh, India), sterile glass petri dishes, Calotropis gigentica were used.
Method
Brain heart infusion agar and Rogosa agar media were prepared according to manufacturer's instructions. Twenty milliliters of the medium was poured into sterile glass petri dishes.
After the medium was set, 5 holes of 8 mm diameter were punched out in the medium, and agar plugs were removed. S. mutans and L. casei cultures (IMTECH, Chandigarh, India), removed from stock and concentration adjusted to 105 organism/ml were used for inoculation of the medium. BHI agar (Hi-Media Laboratories, Mumbai, India) was used for S. mutans and Rogosa agar (Hi-Media Laboratories, Mumbai, India) for L. casei.
Once the organisms were inoculated and spread on the surface of the medium, plates were inoculated with calotropis. One plate was used for one particular concentration of the extract. In each plate, five different holes were filled with a different volume of an extract (75 μl, 50 μl, 25 μl, 10 μl, 5 μl) of one particular concentration of extract. The concentration used was 1.25%, 2.5%, 5%, 10%, and 20%. The plates were incubated in CO2 for 48 h, and the zone of inhibition was noted, measured, and recorded[12] [Figure 1].
Figure 1.
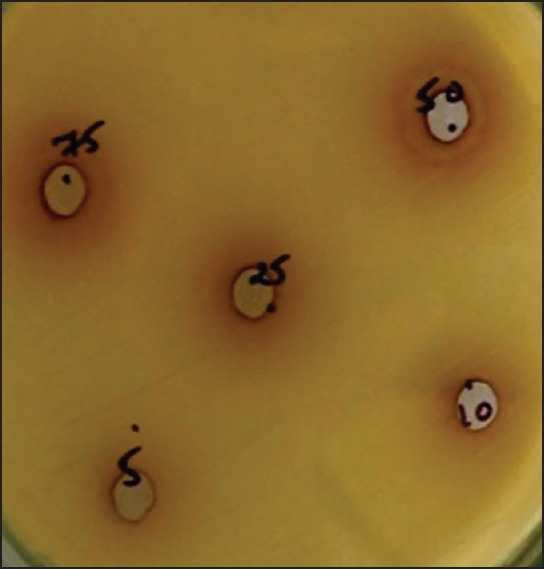
Zone of inhibition
RESULTS
In the present study, antibacterial assay of Calotropis gigentica against cariogenic bacteria was done. The results were assessed by measuring presence or absence of inhibition zone at various concentrations.
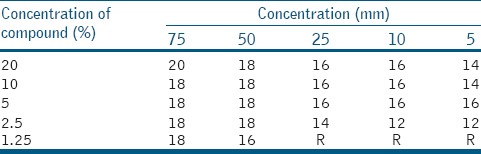
In an ethanolic extract of Calotropis gigentica for S. mutans, 16 mm of minimum inhibition zone was calculated at 50 μl volume and 1.25% concentration. Maximum inhibition zone of 20 mm was observed at 20% concentration and 75 μl volume [Table 1].
Table 1.
Ethanolic extract of Calotropis gigentica for Streptococcus mutans
In an aqueous extract of Calotropis gigentica for S. mutans, 12 mm of minimum inhibition zone was calculated at 75 μl volume and 1.25% concentration. Maximum inhibition zone of 14 mm was observed at 20% concentration and 75 μl volume [Table 2].
Table 2.
Aqueous extract of Calotropis gigentica for Streptococcus mutans
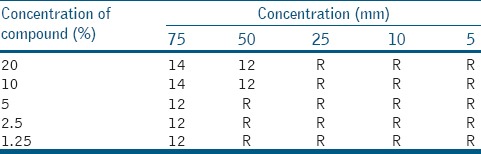
Table 3 shows that for L. casei minimum zone of inhibition was found at 12 mm minimum zone of inhibition was found at 2.5% concentration at 5 μl and at 50 μl concentration 16 mm of inhibition found at a concentration of as low as 1.25%. For lactobacilli also maximum inhibition zone of 20 mm was observed at 20% concentration and 75 μl volume [Table 3].
Table 3.
Ethanolic extract of Calotropis gigentica for Lactobacilli casei
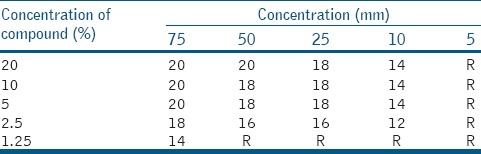
Table 4 shows that there is no antibacterial activity of aqueous extract against L. casei.
Table 4.
Aqueous extract of Calotropis gigentica for Lactobacilli casei
DISCUSSION
Plants are an imperative source of potentially precious structures for the development of novel chemotherapeutic agents. Extracts from these have been in use as a tradition to treat a large number of infectious diseases together with those caused by bacteria, fungi, protozoa, and viruses. Calotropis gigantea is one such plant found widespread in most of the agricultural and nonagricultural fields, and the practice of this plant for medicinal purpose has been documented by various researchers.[8]
Flavonoids, triterpenoids, alkaloids, steroids, glycosides, saponins, terpenes, enzymes, alcohol, resin, fatty acids and esters of calotropeols, volatile long chain fatty acids, and glycosides and proteases have been isolated from the various parts of the plant C. gigantea.[13]
Previous studies on the antimicrobial activity of C. gigantea extracts have revealed its antibacterial potential against numerous microorganisms such as Sarcina lutea, Bacillus megaterium, B. subtilis, Shigella sonnei, Escherichia coli, and Pseudomonas aeruginosa. Hence, in the present study, we analyzed the antibacterial assay of Calotropis gigentica against cariogenic bacteria namely S. mutans and L. casei. The anti-cariogenic potency of Calotropis gigentica was evaluated by the presence or absence of inhibition zones and zone diameters (mm). It is evident from the result that, ethanolic extract of Calotropis gigentica showed a maximum inhibitory zone against S. mutans and L. casei in a dose-dependent manner.
In our study, ethanolic extract of C. gigantea was used, and it was found to be more efficient than other extracts. This might be due to the polar nature of the solvent, that is, ethanol, which resulted in leaching of more active ingredients during extraction for antimicrobial active substance from calotropis compared to other solvents.[14,15]
Phytochemical screening of bioactive ingredients of ethanolic extracts of latex indicates the presence of alkaloids, flavonoids, glycosides, saponins, tannins, steroids, triterpenoids, and phenols [Table 5].[16] Earlier studies have reported the presence of phytochemicals like cardenolides, flavonoids, terpenes, pregnanes, nonprotein amino acid, and cardiac glycoside as major constituents in C. gigantea may possibly acknowledge the medicinal property of this plant. Flavonoids and other constituents does exhibit a wide range of biological activities such as antimicrobial, anti-inflammatory, analgesic, anti-allergic, cytostatic, and antioxidant properties.
Table 5.
Qualitative analysis of the phytochemicals of ethanolic extract Calotropis

In ethanolic extract, the presence of these phytochemical could be responsible for anticariogenic activity. These phytochemical constituents are secondary metabolites of plants that provide the defense mechanism against herbivorous, insects, and microorganisms.[17]
Since only one more study is there on anticariogenicity of Calotropis gigentica, the results of our study cannot be compared with others. In this study, chloroform extract was found to be effective[16] and in dentistry ours is the first study of its kind. Spectrometric analysis showed the presence of nonanoate, a saturated fatty acid which may be accountable for anticariogenicity.[18]
Extracts of plants and phytochemicals are identified to have antimicrobial properties; these can be of immense significance in therapeutic treatments. Over the last decade, a number of studies have been conducted all around the world to demonstrate such efficiency. Various plants have been tried because of their antimicrobial traits, which are due to compounds synthesized in the secondary metabolism of the plant. Plant extracts are found to have a great potential as antimicrobial compounds against numerous microorganisms.
CONCLUSION
Calotropis gigentica has shown antimicrobial activity against S. mutans and lactobacilli. It is effective at as low as 1.25% concentration. This extract is a naturally available extract which can be used for several indications in dentistry for instance, as an antibacterial root canal obturating material or an antiseptic mouthwash. With currently available antibiotics facing an uncertain future in their efficacy, the emphasis is likely to be placed on naturally available resources/flora/fauna. Future in vivo studies are needed for deeper understanding about this herb.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rajendran R, Shivapatha Sundharam B. Shafer's Text Books of Oral Pathology. 5th ed. India: Elsevier; 2006. p. 567. [Google Scholar]
- 2.Lenski RE. Bacterial evolution and the cost of antibiotic resistance. Int Microbiol. 1998;1:265–70. [PubMed] [Google Scholar]
- 3.Raghunath D. Emerging antibiotic resistance in bacteria with special reference to India. J Biosci. 2008;33:593–603. doi: 10.1007/s12038-008-0077-9. [DOI] [PubMed] [Google Scholar]
- 4.Tulp M, Bohlin L. Unconventional natural sources for future drug discovery. Drug Discov Today. 2004;9:450–8. doi: 10.1016/S1359-6446(04)03066-1. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZN, Wang MY, Mei WL, Han Z, Dai HF. A new cytotoxic pregnanone from Calotropis gigantea. Molecules. 2008;13:3033–9. doi: 10.3390/molecules13123033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sartha V, Subramanian S, Sivakumar S. Evaluation of wound healing potential of Calotropis gigentica latex studied on excision wounds in experimental animals. Med Chem Res. 2009;10:9240–6. [Google Scholar]
- 7.Chitme HR, Chandra R, Kaushik S. Evaluation of antipyretic activity of Calotropis gigantea (Asclepiadaceae) in experimental animals. Phytother Res. 2005;19:454–6. doi: 10.1002/ptr.1672. [DOI] [PubMed] [Google Scholar]
- 8.Kumar G, Karthic L, Bhaskara Rao KV. In vitro anti candida activity of Calotropis gigentica against clinical isolates of Candida. J Pharm Res. 2010;3:539–42. [Google Scholar]
- 9.Lachman-White DA, Adams CD, Trotz UO. A Guide to the Medicinal Plants of Coastal Guyana. London: Common Wealth Science Council; 1987. p. 350. [Google Scholar]
- 10.Duke JA. CRC Hand book of Medicinal Herbs. Boca Raton, Florida: CRC Press; 1985. p. 677. [Google Scholar]
- 11.May AF. Paramaribo, Surinam: Vaco; and Zutphen. The Netherlands: De walburg Pers; 1982. Surinaams Kruidenboek (Sranan Oso Dresi) p. 80. [Google Scholar]
- 12.Sharma M, Dorwal R, Bhat KG, Kashyap N, Chandrashekhar P, Bagri S. Comparative evaluation of the antibacterial efficacy of the Aloe vera and Tulsi: An in vitro study. J Res Adv Dent. 2015;4(Suppl 1):170–5. [Google Scholar]
- 13.PS Kumar, E Suresh, S Kalavathy. Review on a potential herb Calotropis gigantea (L.) R. Br Sch Acad J Pharm. 2013;2:135–43. [Google Scholar]
- 14.Ahmad I, Mehmood Z, Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol. 1998;62:183–93. doi: 10.1016/s0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 15.Kareem SO, Akpan I, Ojo OP. Antimicrobial activities of Calotropis procera on selected pathogenic microorganisms. Afr J Biomed Res. 2008;11:105–10. [Google Scholar]
- 16.Saratha V, Subramanian SP. Evaluation of antifungal activity of Calotropis gigentica latex extract: An in vitro study. Int J Pharm Res. 2010;1:88–96. [Google Scholar]
- 17.Khan A, Ahmad A, Manzoor N, Khan LA. Antifungal activities of Ocimum sanctum essential oil and its lead molecules. Nat Prod Commun. 2010;5:345–9. [PubMed] [Google Scholar]
- 18.Ishnava KB, Chauhan JB, Garg AA, Thakkar AM. Antibacterial and phytochemical studies on Calotropis gigantia (L.) R. Br. latex against selected cariogenic bacteria. Saudi J Biol Sci. 2012;19:87–91. doi: 10.1016/j.sjbs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


