Abstract
Background:
To prevent pre-eclampsia in populations with insufficient dietary calcium (Ca) intake, the World Health Organisation (WHO) recommends routine Ca supplementation during antenatal care (ANC). WHO guidelines suggest a complex dosing regimen, requiring as many as 5 pill-taking events per day when combined with iron and folic acid (IFA) supplements. Poor adherence may undermine public health effectiveness, so simpler regimens may be preferable. This trial will compare the effect of the WHO-recommended (higher-dose) regimen vs. a simpler, lower-dose regimen on supplement consumption and pill-taking behaviours in Kenyan ANC clients.
Design and methods:
This is a parallel, non-inferiority, cluster-randomized trial; we examined 16 primary care health facilities in Kenya, 1047 pregnant women between 16-30 weeks gestational age. Higher-dose regimen: 1.5 g elemental calcium in 3 separate doses (500 mg Ca/pill) and IFA (60 mg Fe + 400 µg folic acid) taken with evening dose. Lower-dose regimen: 1.0 g calcium in 2 separate doses (500 mg Ca/pill) with IFA taken as above. Measurements: Primary outcome is Ca pills consumed per day, measured by pill counts. Secondary outcomes include IFA pills consumed per day, client knowledge, motivation, social support, and satisfaction, measured at 4 to 10 weeks post-enrolment. Statistical analyses: Unit of randomization is the healthcare facility; unit of analysis is individual client. Intent-to-treat analysis will be implemented with multi-level models to account for clustering.
Expected public health impact:
If pregnant women prescribed lower doses of Ca ingest as many pills as women prescribed the WHO-recommended regimen, developing a lower-dose recommendation for antenatal Ca and IFA supplementation programs could save resources.
Significance for public health.
Pre-eclampsia is a leading cause of maternal mortality. Based on clinical evidence of significant reduction in risk of pre-eclampsia, the WHO recommends including calcium (Ca) supplementation in antenatal care services in settings with inadequate dietary Ca intakes. A high daily amount of Ca administered in a complex dosing regimen is recommended to maximize efficacy and bioavailability. Factors such as client adherence, motivation, cost and logistical complexities may undermine effectiveness when implemented in public health programs. This cluster-randomized trial will compare Ca supplement consumption between higher and lower-dose regimens delivered through antenatal care in Kenya, integrated with iron-folic acid supplementation. If a lower-dose regimen improves adherence, women’s Ca supplement consumption may be comparable to that achieved under a complex, higher-dose regimen. Evidence gained from this trial will guide public health planning for antenatal calcium supplementation programs to maximize benefits through reducing logistical, cost and adherence barriers.
Key words: Preeclampsia, anaemia, calcium supplements, iron-folic acid, adherence
Background
Hypertensive disorders in pregnancy, including pre-eclampsia, are major contributing factors to maternal mortality. While the pathogenesis of these disorders has not been fully elucidated,1 populations with inadequate dietary calcium (Ca) intake have been shown to be at greater risk.1,2 Two systematic reviews of randomized controlled efficacy trials found Ca supplementation significantly decreases preeclampsia, reducing the risk of developing the condition by half.3,4
Based on this evidence, the World Health Organisation (WHO) strongly recommends the introduction of Ca supplementation in populations with low dietary intake as part of existing and routine antenatal care (ANC) programs to prevent preeclampsia.5 Many ANC programs are already implementing daily iron-folic acid (IFA) supplementation to combat pregnancy-related anaemia, therefore addition of Ca will require an integrated prescribing and counselling protocol.6 To optimize bioavailability and efficacy, the WHO recommends 1.5-2.0 g of elemental Ca in 3-4 divided doses, preferably taken with food. This regimen should begin at 20 weeks of gestation and continue through delivery. Furthermore, to limit potential negative iron-calcium interaction, separation in the administration of Ca and IFA supplements is suggested.5,6 However, the evidence of an interaction is inconclusive, with some evidence that the interaction has a transient and clinically non-significant impact.7
Integrating Ca and IFA supplementation while taking the two types of supplements at different times adds up to 4-5 separate pill-taking events daily (if the client is anaemic, twice daily administration of iron supplements is the recommended therapy). This complex regimen, while consistent with approaches tested in existing efficacy trials, does not account for such factors as client adherence, dietary patterns, motivation and satisfaction, which were not examined in the trials in the systematic reviews. Previous research has shown that client medication adherence decreases with an increase in regimen complexity.8 Furthermore, delivery of a regimen with high numbers of doses faces high cost and potential logistic complexities. Other studies, while not conclusive, have suggested that lower-dose Ca regimens, in particular 1.0 g daily, may be efficacious.9 This would support use of a simpler, lower-dose regimen. In addition, removing the recommendation to separate Ca from IFA administration, given the lack of evidence of significant clinical effects of interaction, would be a reasonable approach to reduce the regimen complexity and its potential effects on adherence and supplement consumption.
Objectives
This trial aims to compare the effect of a higher Ca and IFA dosing regimen with a lower-dose regimen on supplement consumption and pill taking behaviours among ANC clients in rural Kenya. Specifically, we will compare a higher-dose regimen that includes 3 Ca pills and 1 IFA pill daily to a lower-dose regimen that includes 2 Ca pills and1 IFA pill daily. We seek to determine whether the two regimens will lead to comparable numbers of pills consumed, due to differences in adherence to regimen.
Primary outcome
We assessed supplement consumption at follow-up visits approximately 4-10 weeks. Average number of Ca supplements consumed per day will be compared between women assigned the higher-dose and lower-dose regimens.
Secondary objectives
Consumption of IFA supplements is a secondary outcome, to ascertain whether complexity of Ca regimen has any impact on adherence to IFA. Data were collected on maternal, health worker, and intervention delivery characteristics that theories predict may also influence adherence to both supplements. We will assess changes in pregnant women’s knowledge, motivation, self-efficacy, and general and adherence-specific social support, and the attitudes and satisfaction of health care workers delivering the two regimens. These intermediate outcomes will be used to assess the fidelity, appropriateness, and acceptability of the Ca and IFA intervention, and to examine contextual factors that may modify uptake and adherence.
Hypothesis
We will test the following hypothesis: the lower-dose regimen will lead to Ca supplement consumption rates that are not inferior to (by a margin of 0.25 pill/day) the rate of Ca supplement consumption in the higher-dose regimen.
Design and Methods
Study design
This study is a parallel, cluster-randomized, non-inferiority study comparing two Ca and IFA dosing regimens: higher dose vs. lower dose. The higher-dose regimen, consistent with current WHO recommendations, involves 1.5 g elemental Ca (as calcium carbonate) in 3 pill-taking events (500 mg Ca/pill) and IFA (60 mg Fe + 400 µg folic acid) taken with the evening dose. The lower dose regimen involves 1.0 g elemental Ca (as calcium carbonate) in 2 pill-taking events and IFA (60 mg Fe + 400 µg folic acid) taken with the evening dose. Total supplements consumed was assessed during up to two follow-up visits from 4 to 10 weeks after enrolment. Secondary outcomes were also measured at baseline and at follow-up.
Timeline
Recruitment of study participants commenced on September 22nd 2014. Respondent follow-up was completed on June 12th 2015. Data analysis will commence in September 2015.
Setting
This study was conducted in Malava sub-county, Kakamega County in Western Kenya. The target population consists of pregnant women receiving antenatal care services and healthcare workers providing such services in healthcare facilities. The location was selected in consultation with Micronutrient Initiative Kenya and Ministry of Health personnel. The county headquarters, Kakamega town, is located 52 km north of Kisumu. Malava sub-county, located northeast of Kakamega with a population of over 220,000, is primarily rural. The sub-county headquarters is located in the largest town, Malava. ANC services are provided by one sub-county referral facility in Malava, 3 health centres providing ambulatory preventive and curative services, and 15 smaller dispensaries providing preventive health services.
Randomization, treatment allocation, and masking
Treatment was randomly allocated at the level of cluster, considering each primary healthcare facility in Malava sub-county as a potential cluster. There were 19 healthcare facilities providing antenatal primary care services in the sub-county at the time of cluster selection. All healthcare facilities with at least 60 first ANC visits according to the 2013 sub-county administrative data were selected to participate in the study. Based on this criterion, 16 healthcare facilities were selected. Prior to treatment allocation, we received commitment from the county and sub-county health management teams that all selected facilities will participate in the study and none of the selected facilities opted out. To allocate treatment, consultants from the Cornell Statistical Consulting Unit (CSCU) generated a roster of 16 numbered units randomly allocated to two different groups A and B. Group A had been pre-designated as higher-dose and group B as lower-dose. A member of the collaborating team in Kenya was requested to assign serial codes 01-16 to the selected participating facilities, prior to receipt of the randomization roster from CSCU. The randomization roster was matched to the serial numbers to determine facility treatment allocation by the project coordinator.
This study is open-label. Research assistants, healthcare providers and participants are all aware of the allocated prescription regimen. They are however unaware of the specific hypothesis of the study.
Study participants
Study participants were recruited from women attending regularly scheduled ANC visitations, on the days that have been designated as recruitment dates at each participating primary care facility. Women 16-30 weeks gestational age were considered for inclusion. Gestational age was assessed primarily by self-reported last menstrual period (LMP) but where self-reported LMP differed from clinician assessment during the ANC consultation, clinician assessment was accepted. Exclusion criteria included age <15 years old, adequate dietary or medicinal consumption of Ca (assessed with a dietary and medicinal Ca consumption screening tool developed for the study), and intention to leave the study community before 8 weeks from date of recruitment interview.
Intervention
As part of this cluster-randomized trial, the study team worked with the sub-county health management team to integrate Ca supplementation into ANC services at all facilities in Malava sub-county. Aside from the regimen assignment, all other interventions were similarly delivered across all healthcare facilities in the sub-county, irrespective of participation in the cluster-randomized trial or the study arm within this trial. These blanket interventions were: i) Direct provision of Ca supplements to all facilities to avoid stock-outs; ii) direct stop-gap provision of iron-folic acid supplements to all facilities to avoid stock-outs; iii) community mobilization of pregnant women to attend ANC clinics through training and motivation of community health workers; iv) Training ANC providers on Ca supplementation and counselling techniques; v) Development and distribution of appropriate counselling guides and job aids to all facilities; and vi) development and distribution of take-home behaviour change communication materials (i.e., calendar and poster) to healthcare facilities to facilitate pregnant women’s adherence to their recommended regimen and encourage familial support.
Health care workers from the study facilities were trained in the randomly allocated treatment protocol during 4 training sessions that took place on 4 different days at the same venue. All training sessions contained modules focused on purpose, rationale, prescription regimen, benefits and side-effects of Ca and IFA supplementation as well as training on counselling techniques. The 4 training sessions were similar in content except for dosing regimen for Ca supplementation. Almost all (40/42) healthcare workers who provide ANC in the sub-county attended a session in which the prescription regimen reflected the treatment allocation of their cluster; the remaining did not attend any initial session. The same set of facilitators, which included 2 members of the sub-county health management team and 2 members of the investigating team, delivered all training sessions. Make-up training sessions were facilitated for 13 healthcare workers who were newly recruited by the government during the course of the study and one of the two healthcare workers who missed the original training. The make-up training sessions were also consistent with the treatment allocation for the health workers’ facilities.
The Ca supplements used in the trial were Ostocal Calcium and Vitamin D3 film-coated tablets manufactured by Eskayef Bangladesh Limited and purchased through Madawa Pharmaceuticals, Nairobi, Kenya. All calcium products contained 200 IU of vitamin D per pill.
Sample size
The primary outcome of this study is supplement consumption expressed as the number of Ca pills taken per day. The inferiority margin is a mean difference of 0.25 pill consumption per day i.e the null hypothesis of inferiority of the lower-dose regimen will be rejected if the upper border of the confidence interval around the mean difference in daily calcium consumption between the two study arms is below 0.25 pill. Based on preliminary data, the standard deviation of daily supplement consumption is expected to be 0.9 pill/day. A sample size of 194 women per group will allow rejection of the null hypothesis that the lower-dose regimen results in reduced average daily consumption of Ca (by >0.25 pill) than the higher-dose regimen, assuming a standard deviation of 0.9 pill/day, for a one-sided test with alpha of 0.05 and power of over 0.80. Women were sampled from the 16 facilities in the study area that reported more than 60 first ANC visits in 2013. Assuming an intra-cluster correlation of 0.02 and an average cluster size of 60 participants from each of the 16 eligible facilities, the design effect equals 2.18. Therefore, 423 women/group are needed in this clustered sample to achieve the same power as 194 women/group in a randomized design in which all observations are independent.
Measurements
A team of 6 trained interviewers recruited participants and administered the survey questionnaires. All survey questionnaires were translated into Kiswahili and translated back into English to verify translation quality and content. The contextual appropriateness and understanding of the questions were assessed during pre-testing of the instruments.
Data were collected from each participant at up to three time points, as shown in Table 1. Recruitment days for each participating facility were determined and communicated to staff and communities in advance. At the enrolment visit, all ANC clients attending the facility on the designated day were screened and consent was sought from those found eligible for the study, prior to their ANC consultations. Consenting clients were enrolled, a demographic survey was administered, and they were asked to participate in an exit interview after their ANC consultations. After the exit interview, enrolled participants were requested to return for follow-up visits approximately 4 and 8 weeks later (acceptable window for follow-up is 4-10 weeks). At follow-up visits, participants were interviewed prior to their ANC consultations and their remaining pills were counted. During an exit interview after the ANC consultation, newly-received pills were counted. After the first follow-up interview, participants were requested to return for a second follow-up visit (with similar data collection activities, as shown in Figure 1) approximately 4-6 weeks later, the final visit before exit from the study. To determine supplement consumption, supplement counts were measured 1-2 times between 4 and 10 weeks after enrolment. Participants that missed follow-up visits were tracked to their homes for supplement counts to be conducted, when traceable. Details of adherence behaviours were assessed by self-report using a 3-day recall record of timing of doses taken, supplemented by a 30-day recall measure of frequency of taking Ca and IFA supplements, using a 4-point scale ranging from almost never to almost always. Self-report measures of 3-day and 30-day recall were developed for optimal patient understanding and administration of the measures in the study setting. Such self-report measures have demonstrated high correlation to an objective pill count measure in other populations.10
Table 1.
Measures used at each interview visit with antenatal care clients in Kenya participating in a cluster randomized trial comparing consumption of calcium supplements with higher- vs. lower-dose regimens.
| Measure | Interview visit* | ||
|---|---|---|---|
| Enrollment | Follow up 1 | Follow up 2 | |
| Informed consent | x | ||
| Inclusion/exclusion criteria | x | ||
| Allocation of participant ID | x | ||
| Demographic data | x | ||
| Knowledge transfer | x | ||
| Knowledge retention | x | x | |
| Perceived adherence barriers/facilitators | x | ||
| Experienced adherence barriers/facilitators | x | x | |
| Adverse events | x | x | |
| Motivation and appropriateness | x | x | x |
| Self-efficacy | x | x | x |
| General social support | x | x | |
| Adherence specific social support | x | x | |
| Health worker fidelity | x | x | x |
| Client satisfaction | x | x | x |
| 3-day/30-day self-report adherence measure | x | x | |
| Supplement counts: Dispensed/Remaining | D | D/R | D/R |
*Each interview is conducted before an antenatal care consultation, with an exit interview portion completed after the consultation. Follow-up 1 occurs 4 weeks after the enrollment interview and follow-up 2 occurs 8 weeks after enrollment.
Figure 1.
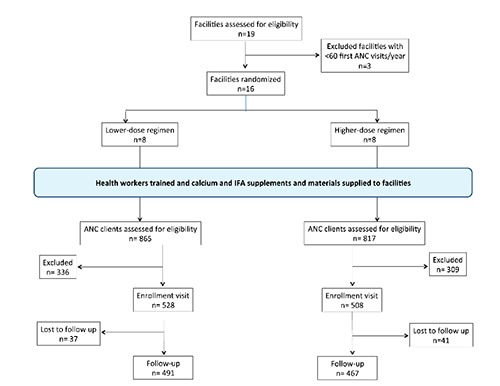
Participant flow diagram through the phases of enrolment, intervention allocation, follow-up, and data analysis in a cluster-randomized non-inferiority trial of two antenatal calcium regimens delivered to pregnant women in Kenya.
Several secondary outcomes were assessed during each interview. The interviews cover a range of psycho-social constructs, including a series of questions designed to evaluate factors most important to pill taking behavior. Additional outcomes include pregnant women’s knowledge, self-efficacy, motivation, and general and adherence-specific social support.
Exit interviews at each visit were used to assess health care provider fidelity to the intended protocol. These interviews sought information about whether or not ANC clients received an adequate number of supplements, as well as accurate and complete information about their dosing regimen, the purpose, benefits, and side-effects of Ca and IFA supplementation. Interviews also covered perceptions of the feasibility, appropriateness, and acceptability of the intervention, and motivations for adherence.
The experience of the health care providers was measured with a healthcare workers’ survey on professional characteristics; knowledge of Ca, IFA, anaemia and pre-eclampsia; and perceptions of the feasibility, appropriateness, and acceptability of delivering Ca supplements through ANC. Data were collected through surveys administered before and after the training sessions, and through healthcare worker interviews at the midline and end of the study.
Statistical analysis
This is a cluster randomized trial and outcomes will be analysed using statistical methods that account for the hierarchical structure of the data. Supplement consumption assessed as average number of pills consumed/day (averaged across follow-up visits) will be the primary dependent variable. This continuous outcome will be modelled using a 2-level linear mixed effects model to account for participants nested within health facilities. The fixed effect will be group (higher vs. lower dosing regimen) and the random effect will be cluster (health facility). The null hypothesis of inferiority will be rejected if the upper bound of the confidence interval of the fixed effect coefficient is <0.25 pills. Analysis will be done on both intent-to-treat and per protocol bases. Statistical analysis will be done using Stata Statistical Software: Release 14 (StataCorp. 2013).
Ethical aspects
This study was reviewed and approved by the Institutional Review Board at Cornell University and Kenyatta National Hospital and University of Nairobi Ethics and Research Review Committee. All respondents were given detailed information about the objectives and purpose of the study and written informed consent was obtained from each respondent before enrolment. This trial is registered as NCT02238704.
Discussion
Pre-eclampsia is a leading cause of maternal mortality. Based on meta-analysis of randomized clinical trials of Ca supplementation showing significant reduction in risk of pre-eclampsia, the WHO recommends the inclusion of Ca supplementation in ANC services in settings with inadequate dietary Ca intake. The WHO guidelines recommend a high daily amount of Ca administered in divided doses, as well as a separate administration of IFA. Implementation of this recommendation would involve a complex dosing regimen, with as many as 5 separate pill-taking events per day. While this regimen is based on doses used in available efficacy trials and is optimized for bioavailability, factors such as client adherence, motivation and satisfaction, as well as cost and logistical complexities may undermine its effectiveness in public health programs.9 Ca supplements are bulky, and reducing the number of Ca pills needed would lead to significant savings in procurement, shipping, and storage costs. If a lower-dose regimen improves the feasibility of good adherence, women’s supplement consumption may be comparable to that achieved under a complex, higher-dose regimen. In this way, evidence gained from this trial could increase the benefits of antenatal calcium supplementation in public health contexts, through reducing logistical, cost and adherence barriers. This trial is not designed to measure clinical end-points. Research to directly assess impact of lower-dose Ca supplementation on clinical end-points such as pre-eclampsia will complement findings of this trial.
Beyond regimen complexity, there are implementation concerns of interest to policy makers and program planners within countries tasked with translating the WHO guidelines into effective programs. Our study is designed to shed light on issues of implementation regardless of regimen. ANC has been used as a service delivery platform for reaching pregnant women with proven interventions such as IFA supplements, insecticide-treated nets, malaria prophylaxis and maternal immunization. However, ANC programs have faced operational challenges and there is a need for implementation research to ensure that introducing Ca supplements using ANC as a platform does not displace existing programs. This cluster-randomized trial is supplemented with additional measures of acceptability, fidelity and feasibility of implementation that will provide preliminary insights on addressing these concerns. Providing program planners with evidence-based potential solutions to the integration of Ca supplementation into existing programs will accelerate policy adoption and the introduction of effective Ca supplementation programs. The cluster-randomized trial is a strong design for assessing the impact of regimen complexity on consumption of supplements. The strength of this study lies in the distinction between efficacy-optimized pill taking schemes, and effectiveness-optimized regimens for large-scale implementation. Prior research suggests overly complex pill-taking regimens result in lower adherence rates in patients.8 Total consumption of supplements may not be significantly reduced by a lower-dose regimen, if adherence rate to this simpler regimen exceeds that for the higher-dose regimen. By evaluating this hypothesis, this trial will provide evidence needed to facilitate translation of global policy guidance into large-scale practice.
Acknowledgments
The authors thank Lynnette Neufeld, Luz Maria de Regil, and Jean-Pierre Habicht for advice on research design.
Funding Statement
Funding: this research was supported by the Micronutrient Initiative (trial registration number NCT02238704).
References
- 1.Cheng MH, Wang PH. Placentation abnormalities in the pathophysiology of preeclampsia. Expert Rev Mol Diagn 2008;9:37-49. [DOI] [PubMed] [Google Scholar]
- 2.Cudihy D, Lee RV. The pathophysiology of pre-eclampsia: Current clinical concepts. J Obstet Gynaecol 2009;29:576-82. [DOI] [PubMed] [Google Scholar]
- 3.Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre-eclampsia and related problems: a systematic review and commentary. Int Gynaecol Obstet 2007;114:933-43. [DOI] [PubMed] [Google Scholar]
- 4.Buppasiri P, Lumbiganon P, Thinkhamrop J, et al. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. Cochrane Database Syst Rev 2011:CD007079. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Guideline: calcium supplementation in pregnant women. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 6.World Health Organization. Guideline: daily iron and folic acid supplementation in pregnant women. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 7.Lönnerdal B. Calcium and iron absorption: mechanisms and public health relevance. Int J Vitam Nutr Res 2010;80:293-9. [DOI] [PubMed] [Google Scholar]
- 8.Ingersoll K, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med 2008;31:213-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmeyr GJ, Belizán JM, von Dadelszen P, the C, Pre-eclampsia Study G. Low-dose calcium supplementation for preventing pre-eclampsia: a systematic review and commentary. Int Gynaecol Obstet 2014;121:951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simoni JM, Kurth AE, Pearson CR, et al. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav 2006;10:227-45. [DOI] [PMC free article] [PubMed] [Google Scholar]


