Abstract
Background.
In the Po Valley aflatoxins play a relevant role: the local food economy is heavily based on cereal cultivations for animal feed and human nutrition. Aims of this project are the identification of new compounds that inhibit Aspergillus proliferation, the development of new inhibitors of aflatoxins production, and the set-up a practical screening procedure to identify the most effective and safe compounds.
Design and Methods.
New compounds will be synthetized with natural origin molecules as ligands and endogenous metal ions to increase their bioavailability for the fungi as metal complexes. A biotechnological high-throughput screening will be set up to identify efficiently the most powerful substances. The newly synthesized compounds with effective antifungal activities, will be evaluated with battery of tests with different end-points to assess the toxic potential risk for environmental and human health.
Expected impact of the study for public health.
The fundamental step in the project will be the synthesis of new compounds and the study of their capability to inhibit aflatoxin biosynthesis. A new, simple, inexpensive and high-throughput method to screen the anti-fungine and anti-mycotoxin activity of the new synthesised compounds will be applied. The evaluation of possible risks for humans due to toxic and genotoxic activities of the molecules will be made with a new approach using different types of cells (bacteria, plants and human cells).
Significance for public health.
Aflatoxins contamination constitutes a health emergency because aflatoxins and mycotoxins, besides being toxic, are among the most carcinogenic substances known. Even if Aspergillus are dominant in tropical regions, recently are becoming a serious problem also in Europe and in Italy, especially in area as the Po Valley in which this problem play a particularly important role, because the local food economy is heavily based not only on cereal cultivations aimed at animal feed but also on the production of derivatives to human nutrition. The aims of this research are the development of new bioactive molecules, obtained by natural molecules and metal ions, that are able to reduce the risk of food contamination by aflatoxin, but are harmless for environmental and health and the evaluation of the newly synthesized compounds using a battery of tests with different end-points to assess the toxic potential risk for environmental and human health.
Key words: Aflatoxins, human health, metal complexes, toxicity, genotoxicity
Background
Aflatoxins contamination constitutes a social emergency and this can also be inferred from the call for proposals launched in 2009 by the European Food Safety Authority to study the increase in aflatoxin B1 in cereals as a result of climate change.1
Aflatoxins are a class of mycotoxins produced principally by two species of Aspergillus, namely A. flavus and A. parasiticus. In particular, in environmental conditions, such as hot and humid climates, these fungi proliferate and can produce aflatoxins, mycotoxins that, besides being toxic, are among the most carcinogenic substances known.2
A. flavus and parasiticus are dominant in tropical regions, but are becoming a serious problem also in Europe and in Italy. The Po Valley is a territory in which all these problems play a particularly important role, because the local food economy is heavily based not only on cereal cultivations aimed at animal feed, but also on the production of derivatives directly intended to human and animal nutrition. The proliferation of these moulds has a dramatic influence on the bio-economy because they grow on carbon-rich substrates, as polysaccharides, and are commonly found on starch-rich substrates, such as cereals. Moreover, residues of aflatoxin and their metabolites can enter the food chain and can also be found in the meat of animals fed with aflatoxin contaminated fodder, offal and eggs.2
The ability of A. flavus to colonise crops and to synthesize aflatoxin is multifactorial and it is intimately connected with development, secondary metabolism regulation, adaptability to environmental conditions, and sensing host-signalling defence molecules.3,4
Fungal biocontrol appears promising in mitigating Aspergillus infection and aflatoxin contamination. However, this single strategy will not probably be the ultimate answer: the integration of different approaches in a cost-effective manner may be the greatest challenge we will face in providing safer food and feed production in the 21st century. To control the production of aflatoxin it is possible to intervene pre-harvest or post-harvest. At the pre-harvest stage, prevention of aflatoxin can be achieved by adopting good agronomical practices, by looking for breeding resistant varieties, or by using genetic engineering. Post-harvest treatments can be divided in three different groups: natural, physical and chemical methods, which are focused on destroying, modifying or adsorbing aflatoxin.3
Among good agronomical practices, the direct control of mycotoxin-producing fungi by using synthetic fungicides is still the most effective way to intervene. Even if synthetic fungicides can induce some concerns, the evaluation of this opportunity should not be discarded, particularly in situations which require a prompt answer to an emergency.
A few molecules are able, to a major or minor extent, to inhibit aflatoxin biosynthesis, but the modes of action of these inhibitors are still poorly understood.5 It is well known that the production of mycotoxins by the moulds is strictly connected to the redox equilibrium in the cell. Even if the molecular details of this correlation are still not clear, evidence is emerging that the production of reactive oxygen species (ROS) by the mould and by the host during the mould/plant interactions is able to affect the biosynthesis of aflatoxins.5 Inorganic substances have been long used for their capacity of inhibiting the development of moulds and bacteria: metal ions are known to play an essential role in growth-inhibitory effects. In this perspective, our bio-inorganic approach will allow us to individuate chemicals that interfere with one or more pathways involved in fungal growth.
A fluorimetric procedure to detect the effect of ROS scavengers on aflatoxin biosynthesis in A. flavus has been recently validated by some of us,6 and the effect of various plant extracts for their efficacy in preventing A. flavus germination and aflatoxin production has been already studied.6
Aim of the study
The aim of this project is to develop new typologies of inhibitors of Aspergillus proliferation and particularly of aflatoxins production, harmless to the environment and to human health.
In this study, substances such as thiosemicarbazones and hydrazones will be used. These substances are known for their significant biological activity,7 and in particular their metal complexes have often shown improved biological properties.
We will also aim at developing a practical screening procedure to identify the most effective and safe compounds.
The main objectives of the project are: i) the synthesis of new bioactive molecules, obtained by complexing metal ions with molecules of natural origin, in order to reduce the risk of food contamination by aflatoxin; ii) the synthesis of new bioactive molecules with antifungal activity and harmless for environment and human health; iii) the development of simple, inexpensive and high-throughput methods for detecting mycelia growth inhibition and mycotoxin production; iv) the development of approach to assess toxicity, genotoxicity and epi-genotoxicity of the new compounds, before their diffusion in environment; v) a database correlating chemical structures and biological/toxicological activities.
Design and Methods
The scheme of the project is reported in Figure 1.
Figure 1.
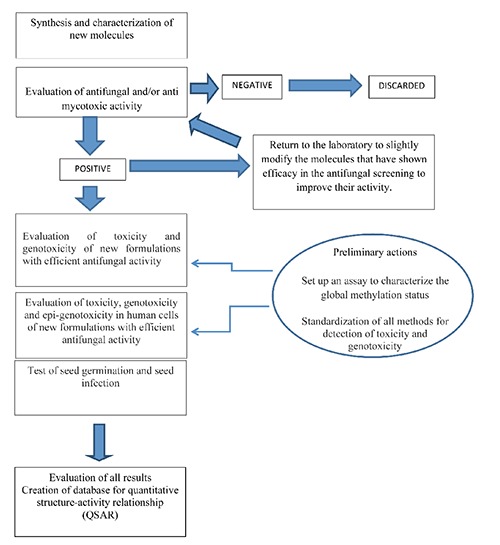
Scheme of the research.
Synthesis and characterization of new active molecules
At the beginning, the main activity will be the synthesis of a wide variety of new compounds, obtained starting from molecules of natural origin, that then will be used as ligands for metal ions (in particular, Cu2+).7 The molecules of natural origin will be functionalized in order to modulate their main properties (solubility, complexing ability, lipophilicity,…). Once the ligands are synthesized and properly characterized, first-transition-series metal ions will be used to obtain the relative metal complexes. Endogenous metal ions (i.e. manganese, iron, copper and zinc) will be used preferentially.
Characterisation of the metal complexes will be conducted both in the solid state, when possible by means of X-ray diffraction on single crystal, and in solution by spectrophotometric and potentiometric titration, ESI-MS and NMR measurements. The study of the stability of the metal complexes in solution is necessary, to relate the biological activity to the chemical species effectively present at physiological pH. Cyclic voltammetry will be performed to study the electrochemical properties of the metal complexes in order to evaluate their redox activity. These studies would provide information on the stability of the metal complexes in presence of a physiological media, and provide hints to the ligands structural modifications conferring a greater complex stability, to be correlated with their biological activity.
Evaluation of antifungal activity of the new compounds
The new compounds will be tested to determine their effects on fungal germination and growth and aflatoxin biosynthesis. To assess the effect on germination and mycelium development, conidia of different strains of A. flavus will be inoculated on synthetic culture media in the presence of serial dilutions of the relevant compound. Both toxigenic (afla+) and non-toxigenic (afla-) strains of A. flavus will be considered since both types of strains are present in a natural population colonizing the crop fields. Germination and biomass increase will be determined spectrophotometrically by the use of 96 well micro-plates.
A standard growth assay based on the measurement, on solid medium, of the time dependent variation of mycelium colony diameter will be performed. Aflatoxin accumulation in the culture medium will be detected and quantified by the use of a micro-plate based fluorimetric procedure that was recently developed and validated.6,8
Once the chemical-physical properties and the molecular structures of the new complexes have been elucidated and their antifungal and antimycotoxigenic activity determined, the next step will be the evaluation of their toxicity and genotoxicity using different organisms (bacteria, plant cells and human cells). This approach will be exploited for the design of new potential antifungal molecules without toxic side effects on humans.
Mutagenicity tests on new molecules with efficient antifungal activity
Mutagenicity tests (below described) used in this projects will be validated and standardized using for comparison molecules commonly used as biocides in agriculture.
Then, to assess the potential toxic and genotoxic risks for environmental and human health of the new molecules, a battery of tests with different genetic end-points will be carried out: the Salmonella/microsome test to detect point mutations on bacteria and the micronucleus Allium cepa test in root cells to detect chromosomal damage.
Salmonella/microsome test
The samples, dissolved in a compatible solvent (DMSO), will undergo the Salmonella/microsome test (Ames test) at increasing doses, with S. typhimurium TA98 and TA100 strains, which are usually utilized for environmental studies,9 with and without metabolic activation (S9 mix) to highlight the presence of indirect and direct mutagenic substances. TA98 strain allows to detect frame-shift mutagens and TA100 strain responds to base-pair substitution mutations. Plates will be incubated at 37°C in the dark for 72 hours, after which revertant colonies will be counted and a dose-response curve will be construct; the net amount of revertants for mg of substance will be evaluated through a linear regression model.
Allium cepa test
In a toxicity assay, equal-sized young onion bulbs will be exposed for 96 hours in the dark to different dilutions of pure compounds dissolved in DMSO. Root length will be used to calculate the EC50 value of each compound and to identify the concentration to adopt in the A. cepa genotoxicity assay.10 Other macroscopic parameters (turgescence, consistency, change in colour, root tip shape) will be used as toxicity indexes.10
The Allium cepa micronucleus test will be performed using 6 equalsized young bulbs per sample.11 After 72-hour pre-germination in mineral water, the bulbs will be exposed to samples for 24 hours; after that, they will be replaced in mineral water for 44 hours of recovery time, fixed in acetic acid and ethanol (1:3) for 24 hours and finally stored in 70% ethanol. For microscopic analysis, five roots of each sample will be considered: at least 1000 cells/slide will be scored for mitotic index (as a measure of cellular division and therefore of sample toxicity) and 2000 cells/slide will be scored for micronucleus frequency. The results will be expressed as number of micronuclei per 1000 cells and the data will be analysed using Dunnett’s tests.
Toxicity, genotoxicity and epi-genotoxicity evaluation in human cells of the new molecules with efficient antifungal activity
The biological activity of the new active molecules will be assessed on normal cells deriving from human districts related to possible xenobiotic ways of interaction with the human body, to investigate the risks linked to specific exposition. We will perform toxicological assays on: human fibroblasts, human lung epithelial, human gastrointestinal cell lines and human leukocytes.
On these cells we will determine the following.
Direct toxicity: a screening of the direct toxicity of the newly synthesized molecules over a panel of human normal cell lines will be performed. Determination of Growth Inhibition (GI) will be performed by a colorimetric method for determining the number of viable cells in proliferation or chemosensitivity assays (MTS assay).12 MTS is bioreduced by cells into a formazan product that is soluble in tissue culture medium. The conversion of MTS into aqueous, soluble formazan is accomplished by dehydrogenase enzymes found in metabolically active cells. On the basis of their GI50 (50% growth inhibition), we will choose the compounds with the lowest antiproliferative effect on cells to be further analyzed for their genotoxic effects.
ROS induction: ROS production is measured by a fluorescence assay, using 2’,7’-dichlorfluorescein-diacetate (DCFH-DA). DCFH-DA is a non-fluorescent compound that can pass through cell membranes. Once it reaches the cytoplasm, esterases remove the acetates to produce 2’, 7’-dichlorodihydrofluorescein (DCFH), which is trapped in the cell because of its polarity. DCFH is easily oxidized to 2’, 7’-dichlorofluorescein (DCF), a highly fluorescent compound.
The Comet Assay or Single-Cell Gel Electrophoresis (SCGE) assay is a useful approach for assessing DNA damage, evaluating the presence, after electrophoresis, of fragmented DNA outside the core of the nucleus.13 Relaxed and/or broken DNA fragments, negatively charged, migrate towards the anode and the resulting image has the appearance of a comet. The amount of DNA migrated from the head of the comet indicates the extent of the DNA damage. This quantity is dependent on the size of DNA fragments and the number of broken ends in the strands. The Comet assay is usually performed at pH>13 to detect, in addition to single and double strand breaks, alkali-labile sites such as adducts, a purinic and apyrimidinic sites, oxidation of the nitrogenous bases, etc.
Characterization of global methylation status
Epigenotoxicology is a new branch of toxicology that studies how exposures to environmental pollutants could result in changes of DNA-methylation and expression that can impact human health. To characterise if the new drugs could alter the global DNA methylation status, a new modified version of the Comet assay will performed using normal human cell lines deriving from the districts (i.e. gastrointestinal tract, pulmonary epithelium and epidermis) that can be potentially exposed to environmental pollutants. We will use Methy-sens Comet Assay, based on the use of different methylation sensitive restriction enzymes, to study the influence of the newly synthesized chemicals on the human epigenome. Furthermore, we will analyse also the epigenetic effects of currently used anti-aflatoxin drugs.
Our goals will be to characterize cell models and to find new methods to elucidate the relation between epigenetic modification and environmental factors.
After the first screening, the most promising compounds could be slightly modified in order to understand which structural parameter can improve the molecular activity.
Test of seed germination
The molecules will be tested to assess possible effects on seed germination. To assess possible negative effects on seed viability, the compounds that have passed the selection step described above (and here called good candidates) will be tested on corn seeds. Different concentrations of the good candidates will be used and assayed according to the Warm Test Germination in paper method provided by ISTA.14 Good candidates will then be tested for their efficacy in preventing corn seed infection using a laboratory assay that we have recently developed.15 Aflatoxin accumulation in the seed derived meal will be detected either by using a commercial immune-assay (ELISA) or a validated HPLC based assay.15
Evaluation of the results and quantitative structure-activity relationship models
At the end, a database for quantitative structure - activity relationship models (QSAR) for each compound with all data obtained in all tests will be created.
Communication and dissemination
Communication and dissemination activities are important issues of the project. For successful dissemination of results, multiple target audiences will be identified (researchers, students, farmers, seed and pesticide companies, public health agencies…); each of them needs to be addressed in a different manner, using different media and with different messages. A website will be created describing the laboratory methods and the main results obtained, allowing greater dissemination of the project and debate among researchers on the topic (www.aflatox.it).
Expected impact of the study for public health
Several studies concerning thiosemicarbazones, their metal complexes and their antifungal properties, but there are no systematic studies that correlate the properties and the structures of these compounds and, in particular, no studies are available about their mechanism of action in fungi growth inhibition.
Developing new molecules displaying fungicidal and/or anti-mycotoxin activity can result to be a challenging and frustrating activity also for the labour intensive and costly procedures required to screen a large panel of compounds: in fact, blind testing (i.e without any preliminary evidence of efficacy) even for a few tenths of compounds in the field would not be reasonably affordable from an economic point of view.
Development of simple, inexpensive and high-throughput methods for detecting mycelia growth inhibition and aflatoxin production in controlled laboratory conditions is thus highly desirable.
In our study, a new method, recently developed by some members of the team, will be applied to screen the anti-mycotoxin activity of the newly synthesized compounds.6,15 The devised procedure has the potential to cope with the high demand of a multi-factorial assay: different compounds, different concentrations, different A. flavus strains, since it relies on a multi-well microplate assay and fluorimetric determinations by the use of a microplate reader. In addition, kinetic of aflatoxin accumulation in the growth medium may be easily assessed, providing information on the possible developmental targets (germination, hyphae elongation, etc) of the relevant molecule (inhibitor). Moreover time scheduled addition of the molecules may be performed: this may give additional hints on the possible targets of the inhibitor. The overall data collected may provide the knowledge basis for new formulations of the inhibitors or of a mix of two or more molecules.
One of the greatest problems that the world is facing today is that the environmental contamination is increasing with every passing year and is causing heavy and irreparable damage to our environment and directly or indirectly to the human health. In 2012, the Regional Bureau of Lombardia (Italy), presented a complete vademecum to contrast the soil, subsoil and groundwater pollution, in particular for remediation activities. Among the actions to be set up in case of contamination, the risk assessment for human health is obviously a priority.
To understand the impact on environment and human health of the new compounds based on functionalized natural molecules and metal ions, we will adopt a set of biological assays able to define both environmental and human toxicological risks. In this context, the complete toxicological analyses of the newly synthesized compounds could prevent both soil and food, direct or indirect, contamination and save soil operators health.
In particular, the evaluation of possible risks for humans due to epigenetic alterations induced by the tested molecules is a new approach. An ideal screen for epi-genotoxic compounds would be in vitro-based, medium- to high-throughput, and relevant to humans. In this context, we will set up a modified version of Comet assay to characterize the global methylation status on normal human cell lines, the use of these cells, to evidence human toxicological risks, is a new frontier in toxicology.
The approach proposed for studying the toxicity of the new compounds, based on a battery of test on human, plant and bacterial cells with different genetic end-points, will allow us to assess the potential risk for environment and human health. Moreover, this approach is in line with the requirements of European Directive 2010/63/EU on the use of alternative methods to animals models in toxicological studies.
Although considerable results have been reached in the control of aflatoxin production, there are still many questions unanswered. The information arising in the course of this research project may help to discover new molecules as effective inhibitors of toxin production.
Acknowledgements
We are grateful to Cariplo Foundation for funding to promote research in the Lombardy region and thank Dr. Andrea Festa for creating the project website.
Funding Statement
Funding: the study is funded by Cariplo Foundation, Lombardia, Italy, Grant N. 2014-0555.
References
- 1.EFSA. Call for proposals - CFP/EFSA/FEEDAP/2009/01: Review of mycotoxin detoxifying agents used as feed additives: mode of action, efficacy and feed/food safety. 2009. Available from: http://www.efsa.europa.eu/it/art36grants/article36/cfpfeedap200901.htm. Accessed on: June 2015. [Google Scholar]
- 2.Centre for Food Safety. Aflatoxins in foods: risk assessment studies, 2001. Available from: http://www.cfs.gov.hk. Accessed on: June 2015. [Google Scholar]
- 3.Reverberi M, Ricelli A, Zjalic S, et al. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl Microbiol Biotechnol 2010;87:899. [DOI] [PubMed] [Google Scholar]
- 4.Fox ME, Howlett BJ. Secondary metabolism: regulation and role in fungal biology. Curr Opin Microbiol 2008;11:481-7. [DOI] [PubMed] [Google Scholar]
- 5.Holmes R, Boston RS, Payne G. Diverse inhibitors of aflatoxin biosynthesis. Appl Microbiol Biotechnol 2008;78:559-72. [DOI] [PubMed] [Google Scholar]
- 6.Degola F, Dall’Asta C, Restivo FM. Development of a simple and high-throughput method for detecting aflatoxins production in culture media. Lett Appl Microbiol 2012;55:82-9. [DOI] [PubMed] [Google Scholar]
- 7.Pelosi G. Thiosemicarbazone metal complexes: from structure to activity. Open Crystall J 2010;3:16-28. [Google Scholar]
- 8.Degola F, Berni E, Restivo FM. Laboratory tests for assessing efficacy of atoxigenic Aspergillus flavus strains as biocontrol agents. Int J Food Microbiol 2011;146:235-43. [DOI] [PubMed] [Google Scholar]
- 9.Claxton LD, Matthews PP, Warren SH. The genotoxicity of ambient outdoor air, a review: salmonella mutagenicity. Mutat Res 2004;567:347-99. [DOI] [PubMed] [Google Scholar]
- 10.Fiskesjo G. The allium test: a potential standard for the assessment of environmental toxicity. Gorsuch JW, Dwyer FJ, Ingersoll CG, La Point TW, eds. Environmental toxicology and risk assessment. 2nd Volume, ASTM STP 1216. Philadelphia: American Society for Testing and Materials; 1993. pp 331-345. [Google Scholar]
- 11.Ma TH, Xu Z, Xu C, et al. The improved Allium/Vicia root tip micronucleus assay for clastogenicity of environmental pollutants. Mutat Res 1995;334:185-95. [DOI] [PubMed] [Google Scholar]
- 12.Malich G, Markovic B, Winder C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997;124:179-92. [DOI] [PubMed] [Google Scholar]
- 13.Singh NP, McCoy MT, Tice RR, et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988;175:184-91. [DOI] [PubMed] [Google Scholar]
- 14.International Rules for Seed Testing, ISTA Methods. 2007. Available from: http://www.seedtest.org/en/home.html. Accessed on: June 2015. [Google Scholar]
- 15.Degola F, Morcia C, Bisceglie F, et al. In vitro evaluation of the activity of thiosemicarbazone derivatives against mycotoxigenic fungi affecting cereals. Int J Food Microbiol 2015;200:104-11. [DOI] [PubMed] [Google Scholar]


