Abstract
Background:
Cutaneous cytology has long been shown to be useful in the diagnosis of several erosive, vesicular, and bullous skin lesions. The Tzanck smear although an old tool, still remains a simple, rapid, easily applied, and inexpensive test for these skin lesions.
Aims and Objectives:
The aim of this study was to evaluate the diagnostic value of Tzanck smear by determining its sensitivity and specificity in various erosive, vesicular, and bullous skin lesions.
Materials and Methods:
One hundred and forty-two patients with erosive, vesicular, and/or bullous skin lesions were included in the study. Four groups of disorders were identified: infections, immunologic disorders, genodermatosis, and spongiotic dermatitis. All the study cases were evaluated by Tzanck smear. Definitive diagnosis was established by standard diagnostic techniques (including when appropriate, viral serology, bacterial culture, histopathology, direct immunoflourescence).
Results:
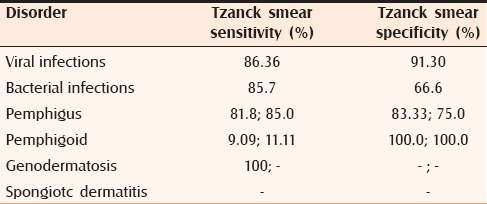
The sensitivity and specificity of cytologic findings was respectively 86.36% and 91.30% for viral infections; for bacterial infections, it was 85.7% and 66.6%. The sensitivity and specificity of Tzanck smear was respectively 85.0% and 83.33% for pemphigus; for bullous pemhigoid it was 11.11% and 100.0%. Tzanck smear sensitivity in genodermatoses was 100%. The sensitivity and specificity of the test in spongiotic dermatitis could not be calculated due to an insufficient number of patients.
Conclusion:
The Tzanck smear is a quick and reliable tool for the evaluation of various erosive and vesiculobullous skin lesions.
Keywords: Sensitivity, specificity, Tzanck smear
INTRODUCTION
Cytodiagnosis has been used as an aid to the rapid diagnosis of numerous skin conditions (Greek word: Kytos = hollow vessel).[1,2,3] George Papanicolaou is considered the father of exfoliative cytology. For dermatologic diseases, cytology was first used by the Frenchman, Arnault Tzanck in 1947 in herpetic infections and pemphigus.[4,5,6] Since then, cytology has been used in the diagnosis of various vesiculobullous, erosive, tumoral, and granulomatous diseases, and the method has been termed the “Tzanck smear.”[3,7,8]
A typical Tzanck cell is a large, rounded keratinocyte with a hypertrophic nucleus, hazy or absent nucleoli and abundant basophilic cytoplasm, which is deeper peripherally on the cell membrane due to cytoplasmic condensation at the periphery, leading to a perinuclear halo.
Studies reporting the diagnostic value of Tzanck smear in various erosive and vesiculobullous lesions are limited, especially in the Indian literature.[8] Although Tzanck smear cannot substitute the standard diagnostic methods, in the hands of an experienced dermatologist, it can aid in establishing the clinical diagnosis with ease and rapidity and can serve as an adjunct to the diagnostic methods.[2,9,10,11]
AIMS AND OBJECTIVES
To establish the diagnostic value of Tzanck smear in various erosive, vesicular, and bullous skin lesions in relation to the standard diagnostic methods used for these disorders, thereby illustrating the sensitivity and specificity of this test.
MATERIALS AND METHODS
The study was a cross-sectional hospital-based study, conducted from April, 2012 to March, 2013. Patients with intact blisters or erosions, not on treatment, of any age and from both the gender groups were included in the study.
After obtaining a written consent, all the patients were subjected to the Tzanck smear, which was obtained from an intact blister. The youngest vesicle or bulla, preferably less than three days old, was preferred for sampling since older lesions may get crusted or secondarily infected and the characteristic cytomorphology may no longer be present. In case of a vesicle or bulla, the intact roof of the lesion was incised along one side with a scalpel and folded back. The fluid contents were then carefully swabbed. The floor of the lesion was then scraped with the sharp edge of a scalpel. In case of an erosion, scrapings were taken from its advancing border. The cellular material obtained was then spread in a thin layer onto a clean microscopic slide, following which it was fixed in methanol for 2–3 min and was then stained with 2–3 drops of a stock solution of May–Grunwald–Giemsa stain (prepared by diluting 1 part of stain with 3 parts of distilled water) for another 5–10 min. The stain–water mixture was then poured off and the slide was quickly washed off and allowed to dry. The slide was then examined under a light microscope (×10 and ×40 magnification, and ×100 magnifications with immersion oil).
In lesions suspected to be due to herpetic infections, blood samples were analyzed for IgM class antibodies to varicella zoster virus and herpes simplex virus 1 and 2 using disposable kits (manufactured by DIESSE diagnostica Senese S.p.A. Italy) applied on a CHORUS instrument.
In lesions suggestive of bullous impetigo, sterile swabs used for aseptically collecting the exudate or pus from the lesions were subjected to bacterial culture.
In cases of suspected immunobullous disorders and genodermatosis, skin biopsy specimens were sent for histopathology (in 10% formalin) and direct immunoflourescent studies (in normal saline). For suspected cases of spongiotic dermatoses, skin biopsy specimens were sent for histopathological examination.
The result of each standard diagnostic technique and that of the Tzanck smear performed in various disorders was recorded, in order to determine the sensitivity and specificity of the latter.
RESULTS
The study included 142 patients, divided into four groups: infections, immunologic disorders, genodermatosis, and spongiotic dermatitis [Table 1].
Table 1.
Serology and Tzanck smear findings in different viral infections
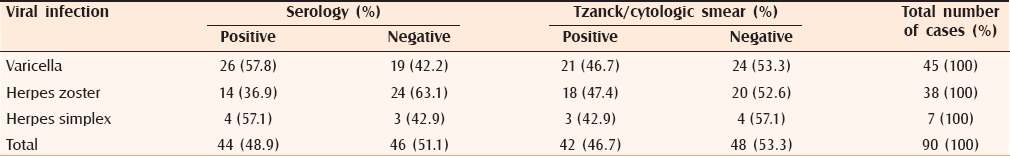
Two types of infections were noted—viral in 90 patients and bacterial in 10 patients. Among viral group, varicella was the most common, seen in 45 (50%) patients, followed by herpes zoster in 38 (42.2%) patients and herpes simplex in 7 (7.8%) patients. In the herpes simplex group, there was one case of herpes genitalis and the remaining six cases were those of herpes labialis. Cases of bacterial infection included 10 cases of bullous impetigo.
The second group of immunologic disorders was seen in 40 (28.2%) patients. This group consisted of two classes of disorders—pemphigus and its variants [Table 2], diagnosed in 28 (70%) patients and pemphigoid in 12 (30%) cases. Eleven cases of bullous pemphigoid were seen and there was one case of pemphigoid gestationis.
Table 2.
Organisms grown on bacterial culture

In addition, a single case of genodermatosis, diagnosed to have Hailey–Hailey disease, and one case of spongiotic dermatitis found to have irritant contact dermatitis with a vesiculobullous presentation were also included in the study.
Three main types of lesions were identified in the study with vesicles (122) being the most common, followed by erosions (90) and bullae (25).
Results obtained from standard diagnostic techniques
Infections
Although culture remains the gold standard for diagnosing viral infections, but due to non-availability of the procedure in our setup viral serology was used to establish the diagnosis. Serology performed in 90 cases of viral infections was positive in 44 [Figure 1 and Table 2]. Bacterial culture [Figure 2] to diagnose bullous impetigo was positive in seven out of 10 cases of bullous impetigo [Table 3].
Figure 1.
Positive viral serology in herpetic infections
Figure 2.
Bacterial culture performed on blood agar
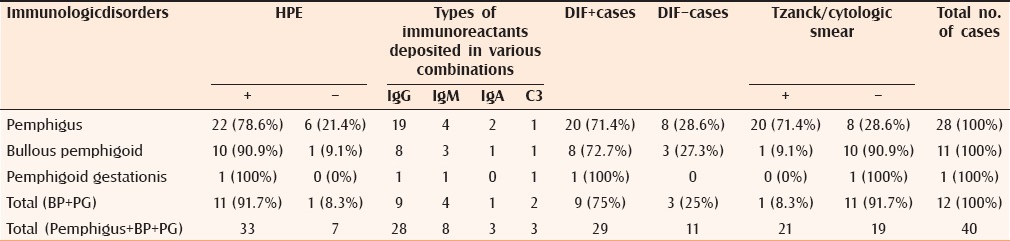
Table 3.
Results of histopathological examination, DIF, and cytology in various immunobullous disorders
Immunologic disorders
Histopathologic and direct immunoflourescence (DIF) examination was performed in all the 40 cases of immunologic disorders (DIF being the gold standard), giving a 78.6% and 71.4% positivity in pemphigus group, respectively. In bullous pemphigoid, the values were 91.7% and 75.0%, respectively [Figure 3 and Tables 4a and b].
Figure 3.
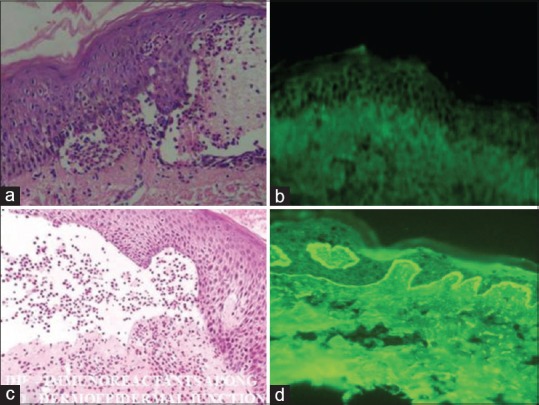
(a) Hematoxylin and eosin of pemphigus vulgaris showing intraepidermal cleft [×400] and (b) direct immunoflourescence showing “Fish net appearance” in pemphigus vulgaris (c) Hematoxylin and eosin showing subepidermal cleft in bullous pemphigoid [×400] and (d) direct immunoflourescence showing deposition of immunoreactants along the DEJ in bullous pemphigoid
Table 4a.
Tzanck smear findings in various subtypes of pemphigus
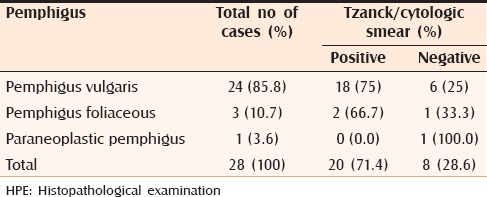
Table 4b.
Tzanck smear sensitivity and specificity in various groups of disorders studied
Genodermatosis
Histopathological examination in one case of Hailey–Hailey disease in the study was suggestive of the diagnosis [Figure 4].
Figure 4.
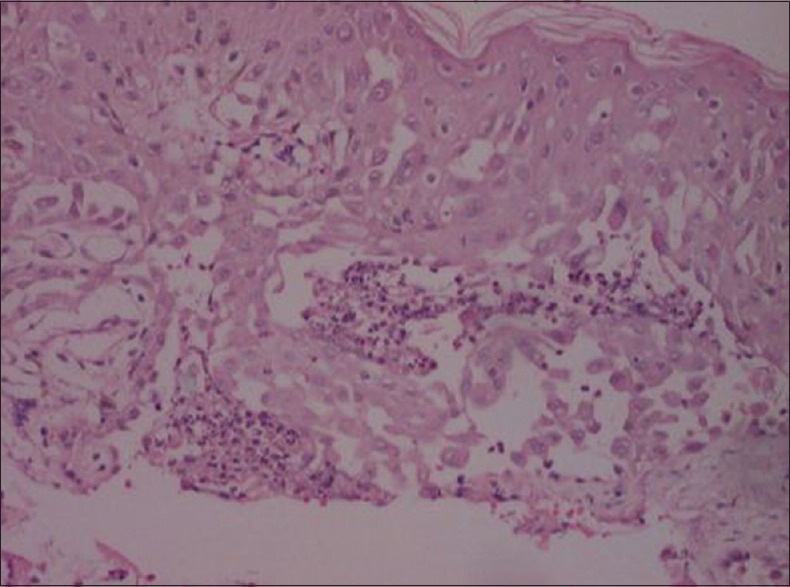
Hematoxylin and eosin showing delepilated brick wall appearance in Hailey–Hailey disease (×400)
Spongiotic dermatitis
Histopathologic examination was used for establishing the diagnosis in a single case of irritant contact dermatitis.
Cytologic/Tzanck smear findings
Infections
Cytologic examination was performed in all the 100 cutaneous infectious lesions, showing acantholytic cells and multinucleated giant cells. Overall, Tzanck smear positivity in the 90 cases of viral infections was 46.7% [Table 2]. Other nonspecific features seen in the cytological smears of viral infections included squamous cells, ballooning degeneration, and inflammatory cells. Acantholytic cells and neutrophils were noted in seven (70%) specimens from patients with bullous impetigo [Figure 5].
Figure 5.
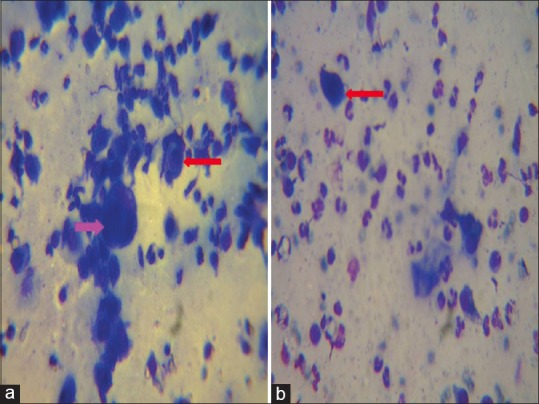
Tzanck smear showing Multi Nucleated Giant cells (MNGs) (pink arrow) and Tzanck cells (red arrow) in herpetic infections (a) and Tzanck cells with numerous neutrophils in bullous impetigo (b). The stain used for preparing all the Tzanck smears was May - Grunwald-Giemsa stain (stock solution is prepared by diluting 1 part of stain with 3 parts of distilled water). Magnification used is 100X
Immunologic disorders
Acantholytic cells admixed with superficial squames and few inflammatory cells were present in the cytologic examination of pemphigus lesions from 71.4% patients [Table 4a]. Morphological patterns in mucosal smears were identical to that of skin smears [Figure 6].
Figure 6.
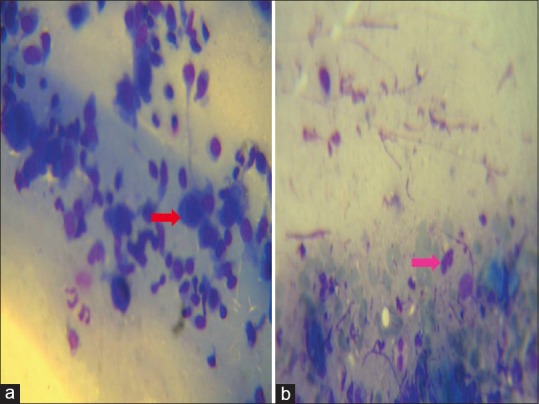
Tzanck smear showing Tzanck cells (red arrow) in pemphigus (a) and eosinophil (pink arrow) in bullous pemphigoid. The stain used for preparing all the Tzanck smears was May - Grunwald-Giemsa stain (stock solution is prepared by diluting 1 part of stain with 3 parts of distilled water). Magnification used is 100X
Cytologic examination in 10 of 11 bullous pemphigoid cases and 1 case of pemphigoid gestationis [Table 4a] showed numerous eosinophils without Tzanck cells [Figure 6].
Genodermatosis
The group included one case of Hailey–Hailey disease wherein Tzanck smear disclosed acantholytic cells [Figure 7(a)].
Figure 7.
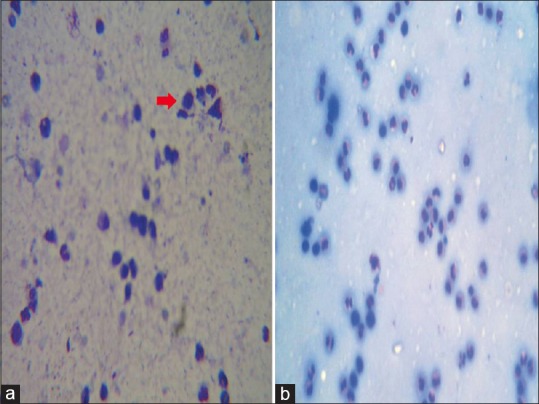
Numerous acantholytic cells (red arrow) seen on a Tzanck smear in Hailey–Hailey disease (a) and negative Tzanck smear in irritant contact dermatitis (b). The stain used for preparing all the Tzanck smears was May - Grunwald-Giemsa stain (stock solution is prepared by diluting 1 part of stain with 3 parts of distilled water). Magnification used is 10X
Spongiotic dermatitis
In case of irritant contact dermatitis, Tzanck smear showed numerous polymorphonuclear leukocytes. However, no tadpole cells were seen [Figure 7(b)].
Calculations of the value of Tzanck smear findings
The diagnostic value of Tzanck smear was calculated in different disorders by substituting the parameters obtained above in the formulas (true positives/[true positives + false negatives]) for estimating the sensitivity, and (true negatives/[true negatives + false positives]) for the specificity [Table 5].
Table 5.
Various disorders in the study

DISCUSSION
Tzanck smear relies on the pathogenetic mechanism of acantholysis (Greek akantha meaning a thorn or prickle, and lysis is loosening).[12] In this process, the coherence between epidermal cells is lost due to breakdown of their intercellular bridges.[8] The cells remain intact but are no longer attached to each other; they tend to become rounded, resulting in intraepidermal clefts, vesicles, and bullae.
For herpetic infections, the sensitivity and specificity of the Tzanck smear, when measured against serology, were 86.33% and 91.30%, respectively, in our study. However, cytology could not differentiate the various types of herpetic infections and this distinction was made purely on clinical grounds. The diagnostic value of Tzanck smear when compared with viral serology was investigated by Durdu et al. who noted a 84.7% sensitivity and 100% specificity of the test.[2] A number of other studies in the literature have also determined the sensitivity and specificity of Tzanck smear in herpetic infections using viral culture, polymerase chain reaction (PCR), and indirect immunoflourescence as the standard diagnostic methods.[13,14,15,16] Oranje et al. reported a greater than 80% sensitivity of the test in herpetic infections.[12] Ozcan et al. reported a Tzanck smear sensitivity and specificity of 76.9% and 100%, respectively in herpetic infections in comparison with PCR in their study.[13] Solomon et al. studied the results of Tzanck smear and viral cultures in 30 patients and reported the sensitivity of Tzanck smear as 53%.[16] Our results for the sensitivity and specificity of Tzanck smear in viral infections were higher than those reported in the literature previously, suggesting that Tzanck preparation offers a more immediate answer than does serology, as antibodies appear 2–5 days after the appearance of rash and their levels peak during the second and third weeks.
Cytologic examination in bullous impetigo revealed dyskeratotic acantholytic cells and numerous neutrophils in 70% cases, giving a sensitivity of 85.7% and 66.6% specificity, with culture positivity in 70% cases in our study. The values were lower than those reported by Durdu et al. (sensitivity = 92% specificity = 99%)[2] This difference can be explained on the basis of insufficient number of cases.
All the 28 cases of pemphigus in our study were subjected to Tzanck test, histopathological examination, and direct immunoflourescence, the positivity rate of each being 71.4%, 78.6%, and 71.4%, respectively. Positivity of acantholytic cells in Tzanck preparations of pemphigus has been reported between 93.3% and 100%, by Blank et al.[17] and Ruocco et al.[9] Similar findings were reported by Shaheen et al. in their study on 37 cases of active pemphigus.[18]
In our study, Tzanck smear was 81.8% sensitive when compared with histopathology and 85.0% when using direct immunoflourescence. The corresponding values for specificity were 83.33% and 75.0%, respectively. Durdu et al., reported a Tzanck smear sensitivity of 100% and specificity of 43.4% in pemphigus.[2] Shaheen et al. reported the overall sensitivity of Tzanck smear in pemphigus to be 73% with a smear positivity rate of 75%.[18] Mokhtari et al. in their study on 15 patients concluded that pemphigus vulgaris can be diagnosed by cytology.[19] Coscia-Porazi et al. studied 40 cases with oral pemphigus and reported positive Tzanck smears in 37.[4]
Our findings indicate that cytomorphologic studies may be useful in screening suspected cases of pemphigus, whereas histopathologic and immunologic tests provide a reliable definitive diagnosis.
Tzanck smear only serves to readily rule out pemphigus. In a study by Durdu et al., seven of nine bullous pemphigoid patients showed numerous eosinophils without acantholytic cells on Tzanck preparation.[2] The diagnosis of bullous pemphigoid was confirmed using histopathology and direct immunoflourescence in our study, the positivity rates of each being 91.7% and 83.33%, respectively. Tzanck smear gave 100% specificity in bullous pemphigoid in our study, thereby providing a reliable means of ruling out pemphigus.
Durdu et al. have also reported Tzanck smear positivity in a single case of Hailey–Hailey disease in their study.[2] In our study, the sensitivity of Tzanck smear was determined to be 100%, which, however, cannot be relied upon due to an insufficient number of cases.
In our study, there was a single case of spongiotic vesicular dermatitis in the form of irritant contact dermatits, in whom cytology revealed numerous polymorphonuclear leukocytes.
However, tadpole cells were not demonstrable, leading to a negative Tzanck smear. Pariser reported Tzanck smear to be 83% sensitive and 100% specific in spongiotic vesicular dermatitis.[20] Durdu et al. also reported similar findings with a cytological sensitivity and specificity of 85.1% and 99.3%, respectively. However, due to paucity of cases, the diagnostic value of Tzanck smear in spongiotic vesicular dermatitis could not be evaluated in our study.
The limitation of the current study was that due to an insufficient number of patients the sensitivity and specificity of Tzanck smear findings in certain diseases such as genodermatosis and spongiotic dermatitis could not be calculated. Larger studies over a longer period of time are required to establish the diagnostic value of Tzanck smear in these groups of disorders.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Durdu M, Seçkin D, Baba M. The Tzanck smear test: Rediscovery of a practical diagnostic tool. Skinmed. 2011;9:23–32. [PubMed] [Google Scholar]
- 2.Durdu M, Baba M, Seçkin D. Value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;5(9):958–64. doi: 10.1016/j.jaad.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Eryilmaz A, Durdu M, Baba M, Yildirim FE. Diagnostic reliability of the Tzanck smear in dermatologic diseases. Int J Dermatol. 2014;5(3):178–86. doi: 10.1111/j.1365-4632.2012.05662.x. [DOI] [PubMed] [Google Scholar]
- 4.Coscia-Porrazzi L, Maiello FM, Ruocco V, Pisani M. Cytodiagnosis of oral pemphigus vulgaris. Acta Cytol. 1985;29:746–9. [PubMed] [Google Scholar]
- 5.Cordero AA. The man behind the eponym. Arnault Tzanck, his work and times. Am J Dermatopathol. 1985;7:121–3. doi: 10.1097/00000372-198504000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Durdu M, Baba M, Seçkin D. More experiences with the Tzanck smear test: Cytologic findings in cutaneous granulomatous disorders. J Am Acad Dermatol. 2009;61:441–50. doi: 10.1016/j.jaad.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 7.Bindu B, Kurien A, Shenoi SD, Prabhu S. Role of slit skin smear examination in cutaneous T-cell lymphomas and other chronic dermatoses. Dermatol Online J. 2006;12:2. [PubMed] [Google Scholar]
- 8.Gupta LK, Singhi MK. Tzanck smear: A useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295–9. [PubMed] [Google Scholar]
- 9.Ruocco V, Coscia-Porrazzi L, Pisani M. Reliability of cytodiagnosis in oral pemphigus vulgaris. A study of 30 cases. J Dermatol. 1984;11:535–40. doi: 10.1111/j.1346-8138.1984.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 10.Motyl MR, Bottone EJ, Janda JM. Diagnosis of herpesvirus infections: Correlation of Tzanck preparation with viral isolation. DiagnMicrobiol Infect Dis. 1984;2:157–60. doi: 10.1016/0732-8893(84)90012-9. [DOI] [PubMed] [Google Scholar]
- 11.Calonje E, Brenn T, Lazar A, McKee PH. Chapter 4. Philadelphia: Elsevier Health Sciences; 2012. Acantholytic Disorders: McKee's Pathology of the Skin. [Google Scholar]
- 12.Oranje AP, Folker E, Choufoer-Habova J, Duivenvoorden JN. Diagnosticvalue of Tzanck smear in herpetic and non-herpetic vesicular and bullous skin disorders in paediatric practice. Acta Derm Venereol. 1986;66:127–33. doi: 10.2340/0001555566127133. [DOI] [PubMed] [Google Scholar]
- 13.Ozcan A, Senol M, Saglam H, Seyhan M, Durmaz R, Aktas E, et al. Comparison of theTzanck test and polymerase chain reaction in the diagnosis of cutaneous herpes simplex andvaricella zoster virus infections. Int J Dermatol. 2007;46:1177–9. doi: 10.1111/j.1365-4632.2007.03337.x. [DOI] [PubMed] [Google Scholar]
- 14.Sadick NS, Swenson PD, Kaufman RL, Kaplan MH. Comparison of detection of varicella-zoster virus by the Tzanck smear, direct immunofluorescence with a monoclonal antibody, and virus isolation. J Am Acad Dermatol. 1987;17:64–9. doi: 10.1016/s0190-9622(87)70172-8. [DOI] [PubMed] [Google Scholar]
- 15.Oranje AP, Folkers E. The Tzanck smear: Old, but still of inestimable value. Pediatr Dermatol. 1988;5:127–9. doi: 10.1111/j.1525-1470.1988.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 16.Solomon AR, Rasmussen JE, Varani J, Pierson CL. The Tzanck smear in diagnosis of cutaneous herpes simplex. JAMA. 1984;251:633–5. [PubMed] [Google Scholar]
- 17.Blank H, Burgoon CF. Abnormal cytology of epithelial cells in pemphigus vulgaris; a diagnostic aid. J Invest Dermatol. 1952;18:213–23. doi: 10.1038/jid.1952.25. [DOI] [PubMed] [Google Scholar]
- 18.Shaheen JA, Haroon TS, Mahmood T, Hussain I. Evaluation of sensitivity of Tzanck smear in pemphigus. J Pak Assoc Derma. 2003;13:175–8. [Google Scholar]
- 19.Mokhtari M, Rasolmali R, Kumar PV. Pemphigus vulgaris of skin: Cytological findings andpitfalls. Acta Cytol. 2012;56:310–4. doi: 10.1159/000333832. [DOI] [PubMed] [Google Scholar]
- 20.Pariser RJ. Diagnosis of spongiotic vesicular dermatitis by Tzanck smear: The “tadpole cell”. J Am Acad Dermatol. 1983;8:519–22. doi: 10.1016/s0190-9622(83)70058-7. [DOI] [PubMed] [Google Scholar]


