Abstract
Background:
Cutaneous leishmaniasis (CL) is an infectious disease of tropical and semitropical areas of the world. The cold and harsh winter conditions of the Kashmir Valley do not favor the survival and growth of the Leishmania parasite or its vector, the sand fly, and the disease was until now practically unheard of in the Kashmir Valley.
Aims:
There has been a recent rise in the number of cases of CL in the Kashmir Valley. Against this background, the present study was taken up to describe the epidemiology, clinical features, and management outcomes of CL in the Kashmir Valley, where it represents a new phenomenon.
Materials and Methods:
Patients with direct smear-confirmed CL were evaluated. For each patient, we noted age, gender, geographical origin, stays in endemic areas, clinical aspects, number, site and size of lesions, treatment, and outcome. All the infected patients were treated with sodium stibogluconate. The dose, route of administration, adverse effects, and the clinical response in each patient was noted down.
Results:
Eighteen patients, 11 males (61.12%) and 7 females (38.88%) were studied. The age of the patients ranged from 3 to 60 years (mean age 29.8). The majority of our patients (16, 88.9%) belonged to two hilly areas, Uri and Karnah. Duration of the disease ranged from a minimum of 1 month to a maximum of 18 months (mean duration 4.6 months). Lesions in most of our patients (16, 88.9%) were located on the face including the lip and nose. The size of lesions varied from 4 to about 50 mm (average 2-3 cm). Most of our patients (13, 73.3%) had only a single lesion and a few (5, 26.7%) had two or three lesions. The clinical type of lesion in most of our patients (16, 88.9%) was noduloulcerative, only two (11.1%) had nodular (nonulcerative) lesions. Sixteen patients; all with facial lesions were treated with intravenous sodium stibogluconate. A complete response was seen in 14 (87%), without any major adverse effect. Two adult patients with extrafacial lesions were treated with four doses of weekly intralesional injections of sodium stibogluconate. A complete response was seen in both, without any major adverse effect.
Conclusion:
The emergence of CL in this nonendemic area is of great epidemiological importance. Because no parasite isolation and characterization was carried out, further epidemiological studies and taxonomic differentiation of the species are required.
Keywords: Clinical study, cutaneous leishmaniasis, Kashmir Valley
INTRODUCTION
Cutaneous leishmaniasis (CL) is an infectious disease caused by various species of Leishmania protozoa, mainly transmitted by the bite of Phlebotomine sandflies. It occurs worldwide throughout Africa, Asia, South America, the Middle East, and the Mediterranean region.[1]
In India, the cold and harsh winter conditions of the the Kashmir Valley do not favor the survival and growth of the Leishmania parasite or its vector, the sand fly, and the disease is practically unheard in The the Kashmir Valley. Although three cases of CL in native Kashmir have been reported, all the three belonged to the Uri belt in Southwest Kashmir.[2] Leishmaniasis is one of the so-called neglected tropical diseases. Although not fatal, CL can lead to significant morbidity as well as social stigma. CL can arise at any age and it often causes cosmetic disfiguration.[3]
There has been a recent rise in the number of cases of CL in the Kashmir Valley. Keeping this background, the present study was taken up to describe the epidemiology, clinical features, and therapeutic outcomes of CL in the Kashmir Valley, where the disease represents a new phenomenon.
MATERIALS AND METHODS
We conducted a hospital-based study covering a 2-year period (2011–2012) in the Department of Dermatology, Venereology and Leprosy, SKIMS Medical College Hospital, which is a tertiary care center located in the central district of the Kashmir Valley. Print and electronic media was used to bring awareness among people and field doctors about the clinical presentation of CL. They were advised to refer doubtful cases to our department for further management. Patients studied had CL confirmed by direct smear of the lesions and demonstration of Leishmania amastigotes within the macrophages. The smear was taken from the edge of the lesion, fixed in methanol, stained with Giemsa stain, and microscopically evaluated for Leishmania donovani (LD) bodies. A total of 18 patients with CL were enrolled. For each patient, we noted age, gender, geographical origin, stay in endemic areas, clinical aspects, number, site and size of lesions, treatment, and outcome. Patients were followed-up for a period of 6–12 months.
All the infected patients were treated with sodium stibogluconate. The dose, route of administration, and adverse effects were noted down. The clinical responses were categorized as follows: complete cure was defined as 100% re-epithelialization of the ulcer (or for nonulcerative lesions, involution of the lesion with or without scar formation); partial response was defined as at least 50% reduction in size of lesion and/or re-epithelialization of at least 50% of lesion; failure was defined as any outcome worse than 50% re-epithelialization of the ulcer or a partial initial response that, on follow-up, continued to be active.
RESULTS
Eighteen patients, 11 males (61.12%) and 7 females (38.88%) were studied. The results are summarized in table 1. The age of the patients ranged from 3 to 60 years (mean age 29.8 years). The majority of our patients (16, 88.9%) belonged to two hilly areas Uri and Karnah. Only two (11.1%) patients belonged to some other districts, both had visited Uri in the recent past. Duration of the disease ranged from a minimum of 1 month to a maximum of 18 months with a mean duration of 4.6 months. Lesions in most of our patients (16, 88.9%) were located on the face, including the lip and nose [Figures 1–5]. One patient had a lesion over the neck and the other over forearm. Only one patient had involvement of the face as well as the forearm. Mucosal involvement in the form of the lip lesion extending into the mucocutaneous junction was seen in one patient [Figure 4]. The size of lesions varied from 4 mm to about 50 mm but the average size was about 2–3 cm. Most of our patients (13, 73.3%) had a single lesion and a few (5, 26.7%) had two or three lesions. The clinical type of lesion in most of our patients (16, 88.9%) was noduloulcerative, only two (11.1%) had nodular (nonulcerative) lesions [Table 1]. One of our patients had shown unusually large, persistent tumorous growths over the nose [Figure 2]; the biopsy taken from the growth was consistent with CL [Figure 6]. All the patients were confirmed as CL by smear showing LD bodies [Figure 7]. None of our patients had human immunodeficiency virus infection (routine screening was performed), and none had any significant comorbid diseases.
Table 1.
Clinical, therapeutic, and outcome features of our patients
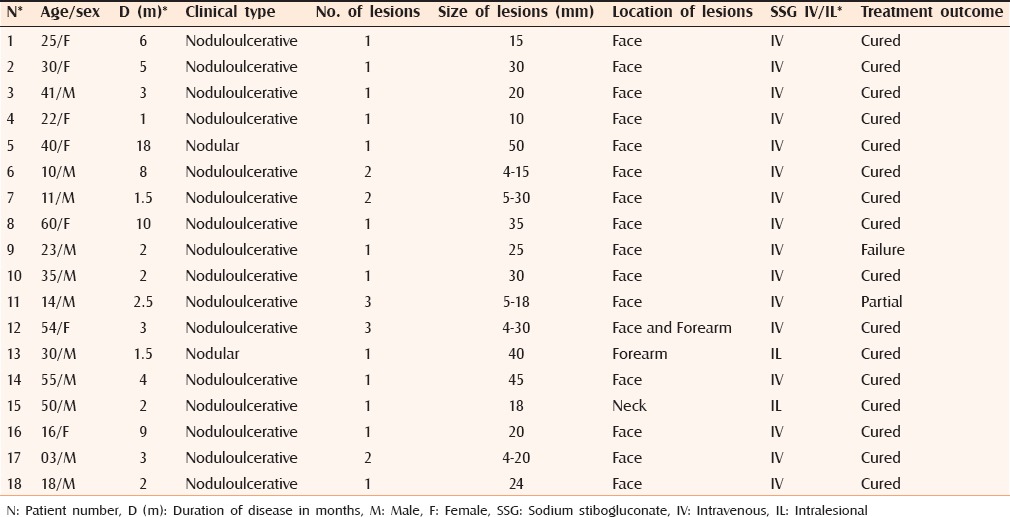
Figure 1.
(a) Cutaneous leishmaniasis showing typical infiltrated erythematous plaque with crusting and a satellite lesion. (b) Complete clearance of the lesions 3 weeks after treatment
Figure 5.
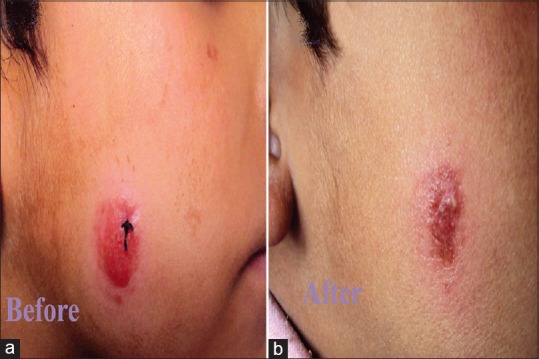
(a) Cutaneous leishmaniasis in a child showing nonulcerated nodular lesion. (b) Clearance of the lesion with atrophy and milia formation 3 weeks after treatment
Figure 4.
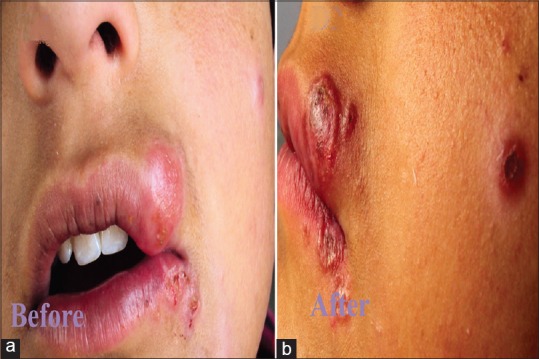
(a) Multiple lesions of cutaneous leishmaniasis in a child with mucosal involvement. (b) Regression of the lesions 5 weeks after treatment
Figure 2.
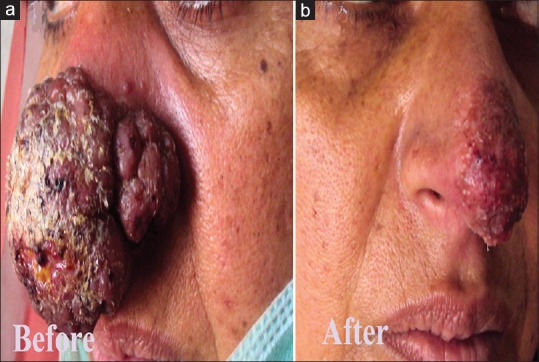
(a) Cutaneous leishmaniasis showing a large tumorous growth over the nose. (b) Marked improvement 3 months after treatment
Figure 6.
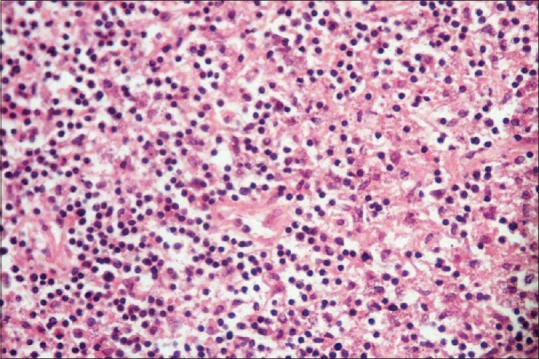
Photomicrograph showing foamy histiocytes with Leishmania donavani bodies (H and E stain, ×400)
Figure 7.
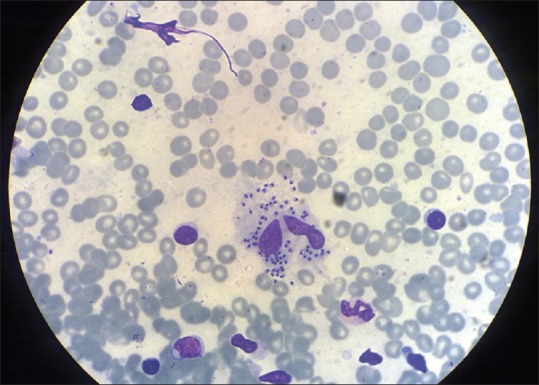
Smear taken from the lesion showing Leishmania donavani bodies (Giemsa, ×400)
Figure 3.
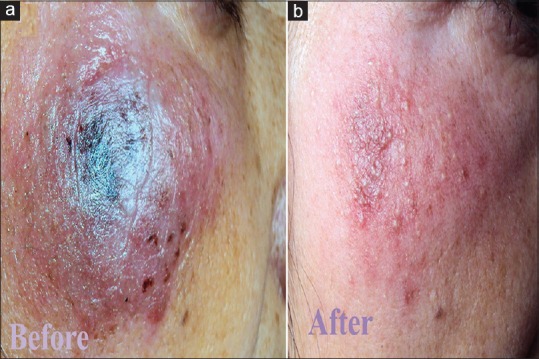
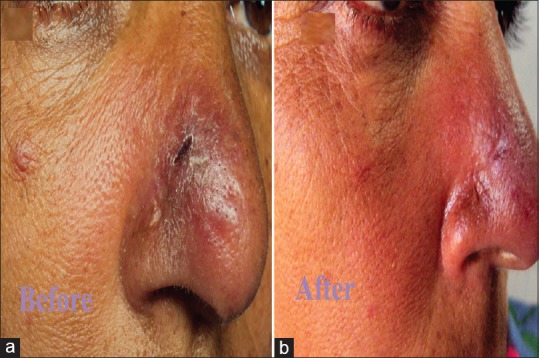
(a) Erythematous and edematous plaque of cutaneous leishmaniasis with crusting. (b) Clearance of the lesion with little atrophy and milia formation 3 weeks after treatment
Sixteen patients, all with facial lesions were treated with intravenous sodium stibogluconate, 20 mg/kg per day for 15–20 days. No significant adverse effects were noted in any except in one who developed abdominal pain and chemical pancreatitis after the sixth dose. In the latter case, the treatment was temporarily stopped for a few days (until the serum amylase level came down to near normal), and restarted at a lower dose of 12 mg/kg of intravenous sodium stibogluconate, which she tolerated well. A complete response was reported in 14 (including the one in whom we had given a lower dose), a partial response in one and a failure in one patient.
Two adult patients with extrafacial lesions (having lesions over the neck and forearm) were treated with four doses of weekly intralesional injections of sodium stibogluconate (total amount, approximately 150 mg in each). A complete response was seen in both without any major adverse effect. Figures 1–5 depict the clinical presentation (A) and treatment outcome (B) of some of our patients.
DISCUSSION
CL usually occurs in areas with a hot and dry climate and in India, the desert areas of Rajasthan, Gujarat, and the plains of the northwestern frontier are endemic for this disorder. Some indigenously acquired cases have also been described in Assam, Kerala, Haryana, and Himachal Pradesh.[4,5] Cutaneous and systemic leishmaniasis has been unknown in the Kashmir Valley and to the best of our knowledge, only a few cases have been reported so far, all belonging to the Uri belt in southwest Kashmir.[2] This is not unexpected in view of temperate climatic conditions found in this part of the country. In our study, all the patients except two belonged to two hilly areas, namely, Uri and Karnah, the other two had also visited the said area. Unlike rest of the the Kashmir Valley, the hot and arid climate of these areas is quite conducive to the growth and development of Leishmania and the sand fly.[6] Epidemics of CL have sometimes been associated with construction work and formation of new townships as these activities lead to an intrusion into the habitat of the vector.[7] Also factors such as migration of people from villages to small townships coupled with climatic changes over the last few years could be another contributing factor for the emergence of this new phenomenon in the Kashmir Valley. The patients evaluated in the current study were mostly native to the area who gave no history of travel to other endemic areas during the past one year.
There was a significant delay between clinical presentation and diagnosis of CL. The mean duration from the appearance of the lesion to diagnosis was 2.5 months. Most of the patients were already treated by primary physicians without considering the diagnosis of CL, resulting in a delay in the diagnosis. The clinical presentation of CL in our patient population was more or less uniform. The lesions were persistent, slowly growing, painless erythematous nodules and plaques showing superficial crusting, or ulceration (noduloulcerative type). These findings are consistent with published data on CL due to Leishmania tropica.[8,9] Only in two patients, the lesions were without crusting and/or ulceration (nodular type). Mucosal involvement as extension of an upper lip lesion was seen in one patient. Mucosal lesions are rarely caused by L. tropica and they occur usually on or around mucocutaneous junctions such as lips and nostrils.[5] One patient with an unusual tumorous growth over the nose [Figure 2A] was suspected in view of the area (she belonged to Uri), and unresponsiveness to other treatments. A biopsy was done in this case to further rule out other possibilities such as tuberculosis, rhinosporidiosis, sporotrichosis, and other deep mycoses, and malignancy such as squamous cell carcinoma. The biopsy showed LD bodies and granulomas consistent with leishmaniasis. Atypical clinical presentations of CL are increasingly seen these days. It is recommended that CL should be included in the differential diagnosis of common dermatological diseases such as erysipelas, chronic eczema, herpes zoster, paronychia; and uncommon disorders such as lupus vulgaris, squamous cell carcinoma, sporotrichosis, mycetoma, and other deep mycoses.[10] CL presents with a wide spectrum of expression, both clinically and histologically, and may mimic other inflammatory and neoplastic diseases. The diagnosis of CL relies on the identification of LD bodies in either a direct smear of the lesion or in a tissue section.[11] We were not able to know the actual species of leishmania involved, because of the lack of diagnostic facilities such as culture and polymerase chain reaction (PCR) in our setup. Even the specific vector species involved in the transmission remains unknown at present. However, we have notified the area and the matter has been discussed with local administration. Efforts are on to identify all the patients in the area and to effectively contain this new infection.
Many treatment modalities for CL are available: physical, topical, or systemic treatments. However, intralesional or systemic administration of antimonial compounds is still considered the standard treatment of CL.[12] Decisions about whether and how to treat CL should be individualized. The treatment approach depends in part on the Leishmania species/strain and the geographic area in which the infection was acquired; the natural history of infection, the risk for mucosal dissemination/disease, and the drug susceptibilities in the pertinent setting; and the number, size, location, evolution, and other clinical characteristics of the patient's skin lesions.[13] We decided to give intravenous antimony to patients presenting with facial lesions. Sixteen patients, all with facial lesions were treated with intravenous systemic sodium stibogluconate with a cure rate of more than 87%. One patient with and unusual growth over her nose had shown and exceptionally good result [Figure 2B]. The other two patients with extrafacial lesions were treated with intralesional sodium stibogluconate, and both were cured. There is a wide range of reported cure rates of this treatment (7%–97%). Because most of our patients had facial lesions and the Leishmania species were un-known, systemic treatment was justified.
Systemic administration of antimony may result in a wide spectrum of adverse effects, including cardiotoxicity, myalgias, pancreatitis, and pancytopenia. The most common adverse effect of intralesional injection ofantimony is pain at the site of injection.[14,15] In our patients, the treatment was overall well tolerated without major side effects (increase in serum amylase level in one patient).
In most of our patients, the response to treatment started in first week itself (decrease in induration and flattening of lesions), with significant reepithelialization seen on completion of treatment (third week). The healing process for a number of lesions continued after termination of therapy. After successful treatment, most recurrences occur within approximately 6 months; therefore longer follow-up may not be required, provided patients are advised to report any change in their scar.[16] None of our patients had a recurrence over a follow-up period of 6–12 months.
Limitations of the study
The study population was small and we could not succeed in the identifying the species of Leishmania. Furthermore, only smear-positive patients were enrolled in the study; as smear may not be positive in all patients with CL, we could have missed some patients.
CONCLUSION
The emergence of CL in this nonendemic area is of great epidemiological importance. Except an atypical case with tumorous growth over the nose, the clinical spectrum of our patients was more or less comparable to other endemic areas of the world. Any patient with nodular/nodulo-ulcerative lesion on exposed parts must be suspected for CL, especially if belonging to Uri and Karnah region of the Kashmir Valley. Besides, the response to treatment was also very good. Because no parasite isolation or characterization was carried out, further epidemiological studies and taxonomic differentiation of the species are required. Newer diagnostic modalities such as PCR may help in species identification of Leishmania and thereby confirming the presence of a new focus of CL. The public health authorities should make every effort to contain this new infection in the Kashmir Valley.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–96. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 2.Leherwal MA, Yasin SB, Ahmed SB. Diagnosis of cutaneous leishmaniasis by FNAC-Report of three cases. J Cytol. 2004;21:103–05. [Google Scholar]
- 3.Zaraa I, Ishak F, Kort R, El Euch D, Mokni M, Chaker E, et al. Childhood and adult cutaneous leishmaniasis in Tunisia. Int J Dermatol. 2010;49:790–3. doi: 10.1111/j.1365-4632.2010.04467.x. [DOI] [PubMed] [Google Scholar]
- 4.Muhammed K, Narayani K, Aravindan KP. Indigenous cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol. 1990;56:228–9. [Google Scholar]
- 5.Sharma NL, Mahajan VK, Kanga A, Sood A, Katoch VM, Mauricio I, et al. Localized cutaneous leishmaniasis due to Leishmania donovani and Leishmania tropica: Preliminary findings of the study of 161 new cases from a new endemic focus in Himachal Pradesh, India. Am J Trop Med Hyg. 2005;72:819–24. [PubMed] [Google Scholar]
- 6.Lysenko AJ. Distribution of leishmaniasis in the Old World. Bull World Health Organ. 1971;44:515–20. [PMC free article] [PubMed] [Google Scholar]
- 7.Kerdel-Vegas F, Harman R, Kerdel F, Dutta AK. Protozoan infections 11. In: Canizares O, Harman R, editors. Clinical Tropical Dermatology. 2nd ed. Massachusetts: Blackwell Scientific Publications; 1992. pp. 293–312. [Google Scholar]
- 8.Klaus S, Frankenburg S. Cutaneous leishmaniasis in the Middle East. Clin Dermatol. 1999;17:137. doi: 10.1016/s0738-081x(99)00006-1. [DOI] [PubMed] [Google Scholar]
- 9.Ashford RW. Cutaneous leishmaniasis: Strategies for prevention. Clin Dermatol. 1999;17:327–32. doi: 10.1016/s0738-081x(99)00051-6. [DOI] [PubMed] [Google Scholar]
- 10.Bari AU, Rahman SB. Many faces of cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol. 2008;74:23–7. doi: 10.4103/0378-6323.38402. [DOI] [PubMed] [Google Scholar]
- 11.Kocarslan S, Turan E, Ekinci T, Yesilova Y, Apari R. Clinical and histopathological characteristics of cutaneous leishmaniasis in Sanliurfa City of Turkey including Syrian refugees. Indian J Pathol Microbiol. 2013;56:211–5. doi: 10.4103/0377-4929.120367. [DOI] [PubMed] [Google Scholar]
- 12.Khatami A, Firooz A, Gorouhi F, Dowlati Y. Treatment of acute old world cutaneous leishmaniasis: A systematic review of the randomized controlled trials. J Am Acad Dermatol. 2007;57:335.e1–29. doi: 10.1016/j.jaad.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Masmoudi A, Hariz W, Marrekchi S, Amouri M, Turki H. Old World cutaneous leishmaniasis: Diagnosis and treatment. J Dermatol Case Rep. 2013;7:31–41. doi: 10.3315/jdcr.2013.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moskowitz PF, Kurban AK. Treatment of cutaneous leishmaniasis: Retrospectives and advances for the 21st century. Clin Dermatol. 1999;17:305–15. doi: 10.1016/s0738-081x(99)00049-8. [DOI] [PubMed] [Google Scholar]
- 15.Alkhawajah AM, Larbi E, al-Gindan Y, Abahussein A, Jain S. Treatment of cutaneous leishmaniasis with antimony: Intramuscular versus intralesional administration. Ann Trop Med Parasitol. 1997;91:899–905. doi: 10.1080/00034989760284. [DOI] [PubMed] [Google Scholar]
- 16.Palmer RA, Tran D, Hepburn NC, Ashton RE. The management of cutaneous leishmaniasis from Belize. Clin Exp Dermatol. 2001;26:16–20. doi: 10.1046/j.1365-2230.2001.00751.x. [DOI] [PubMed] [Google Scholar]


