Abstract
Aspirin has been one of the oldest drugs in the field of medicine, with a wide range of applications. In dermatology, aspirin has shown benefit in a variety of disorders. Recently, reduction of melanoma risk with aspirin has been demonstrated. Although an analgesic to begin with, aspirin has come a long way; after cardiology, it is now found to be useful even in dermatology.
Keywords: Aspirin, dermatology, melanoma
INTRODUCTION
Salicylates have been used as an analgesic since the time of Hippocrates.[1] However, it was only in the 1890s that aspirin was introduced as an anti-inflammatory agent,[2] and the role of aspirin as an antiplatelet agent was realized only 70 years later.[3] Of late, the beneficial properties of aspirin have been utilized in treating a number of dermatologic disorders. This review discusses the role of aspirin in dermatology.
MECHANISM OF ACTION
Aspirin acts by irreversibly inhibiting both cyclooxygenase (COX) enzymes. Aspirin acetylates the serine 530 moiety of COX-1, and the serine 516 moiety of COX-2,[4,5] being 170 times more effective in inhibiting COX-1.[6] With the blockage of these enzymes there is a decreased production of prostaglandins and thromboxane-A2, responsible for its anti-inflammatory and anti-platelet effects, respectively. Aspirin also inhibits the neutrophilic activation of platelets by utilizing nitric oxide and cGMP.[7]
PHARMACOKINETICS
Taken orally aspirin is rapidly absorbed in the upper gastrointestinal tract.[8] The peak plasma level of aspirin is reached within 2 h of oral intake, following which it gradually declines. The plasma half-life of aspirin is 20 min.[9] Metabolism of aspirin occurs in the liver, with 75% of the drug getting metabolized to salicyluric acid, salicyl phenolic and acyl glucoronide, and gentesic acid.[10] The drug is excreted via the renal route. With increase in the urinary pH, elimination of aspirin is enhanced. The drug readily crosses the placenta and can be easily transferred via breast milk.
APPLICATIONS IN DERMATOLOGY
In dermatology all indications of aspirin are off label.
Necrobiosis lipoidica diabeticorum
Necrobiosis lipoidica diabeticorum (NLD) is an idiopathic dermatoses characterized by degenerative and granulomatous changes in the dermis.[11] The deposition of immune complexes in the walls of blood vessels, enhanced aggregation of platelets, and increased coagulation have been postulated as etiological factors for NLD development.[12] Aspirin has been found useful in NLD because of its antiplatelet property. By blocking aggregation of platelets, aspirin enhances cutaneous blood flow in NLD, and promotes ulcer healing.[13] An uncontrolled trial, with low doses of aspirin administered to seven NLD patients, showed considerable clinical improvement of lesions, in six out of seven patients.[14] Another uncontrolled trial using 80 mg of aspirin and 75 mg of dipyridamole, thrice daily in seven patients with ulcerative NLD heralded healing of ulcers in all patients over a 2 to 4 week period.[15] However studies by Beck et al.[16] and Statham et al.[17] did not agree with the therapeutic benefit of aspirin in NLD.
Malignant atrophic papulosis
Malignant atrophic papulosis (MAP) is a rare thrombo-obliterative disorder characterized by porcelain white skin lesions with surrounding telangiectasia.[18] Increased platelet aggregation has been proposed as a causative factor in MAP. In this setting, low doses of aspirin ranging from 81 to 325 mg per day is administered.[19,20] However, the therapeutic role of aspirin for MAP is limited, with the disease having a fatal outcome.
Erythromelalgia
Erythromelalgia is a clinical condition characterized by burning sensation and redness over the extremities.[21] Out of the three types of erythromelalgia, type I is associated with thrombocythemia, and occlusion of the vasculature of digital arteries and arterioles.[22] Aspirin's antiplatelet activity is responsible for the therapeutic benefit, seen in this condition. The dose of aspirin, ranges from 325 to 650 mg per day. The effect of a single 500 mg dose of aspirin lasts for 3 days. This long-lasting effect of aspirin can also be utilized as a diagnostic test for myeloproliferative disease-linked secondary erythromelalgia.[23,24,25] On the whole, however, it may be worthwhile using aspirin in all cases of erythromelalgia as considerable relief may be experienced after its usage.[26]
Raynaud's phenomenon
Aspirin has been found useful in the management of Raynaud's phenomenon (RP) secondary to vaso-occlusive pathology. Low doses of aspirin between 75 and 81 mg has been employed for this indication.[27] Increase in the blood viscosity and platelet aggregation along with altered fibrinolysis have been proposed as contributory mechanisms in RP involving the digital blood vessels.[28] Aspirin may therefore have a role in this setting, especially for patients with an acute ischemic crisis where aspirin is administered along with a short-acting calcium channel blocker-like nifedipine.[29] According to Akerkar and Bichile,[30] all patients with RP in a setting of scleroderma should receive a daily 150 mg of aspirin along with other medications for the same. Treatment may be given till the acute ischemic attack is tided or even for a long-term prophylaxis.
Erythema nodosum
Erythema nodosum (EN) is a septal panniculitis characterized by tender erythematous nodules involving both the shins in a symmetrical distribution. Aspirin is used here as an adjunct to the specific treatment regimen. The anti-inflammatory property of aspirin is employed in this setting to enhance analgesia and quicken resolution of cutaneous lesions.[31] The dose of aspirin for EN is 325 mg given at a 6 hourly interval.[32]
Vitiligo
Vitiligo is an acquired idiopathic pigmentary disorder characterized by depletion of epidermal melanocytes. Recently, it has been suggested that oxidative stress in vitiligo, mediated by hydrogen peroxide (H2O2) may significantly contribute toward melanocyte apoptosis.[33,34,35] It has also been noted that, a decrease in the levels of systemic catalase, glutathione peroxidase, and manganese superoxide dismutase in vitiligo patients, further enhances oxidative stress.[36,37,38] Because of oxidative stress, the inducible inflammatory COX-2 mRNA is increasingly expressed, with release of numerous inflammatory mediators and cytokines, prompting melanocyte apoptosis.[39,40] Aspirin acts by inhibiting COX-2 irreversibly, reducing the oxidative stress, increasing the release of leukotriene C4, a potent melanocyte mitogen, and reducing leukotriene B4 release.[41] Apart from this aspirin also possesses antioxidant properties, reduces the activity of antimelanocyte antibodies and soluble interleukin-2 receptors (SIL-2R), therefore decreasing immune-mediated melanocyte damage.[42] Aspirin also significantly improves the oxidative status of peripheral blood mononuclear cells and epidermal melanocytes. The salicylic acid moiety of aspirin, by an unknown mechanism can itself trigger the de novo biosynthesis of reduced glutathione, a potent antioxidant. Furthermore aspirin on its own also, has free radical scavenging properties, which can inhibit DNA strand breakage and block lipid peroxidation of cell membranes.[43,44] Aspirin can go another step further by antagonising the effects produced by nuclear factor kappa-B and TNF-alfa, thus commemorating its role for unstable vitiligo and promoting a halt in disease progression.[45,46] Aspirin thus helps in changing an unstable active form of vitiligo to the stable form. Thus, aspirin may be beneficial in vitiligo only when the disease demonstrates signs of activity. Low doses of aspirin up to 300 mg per day is sufficient for this indication.[47] Duration for the same may need to be continued for a minimum period of 12 weeks, or till signs of disease activity are controlled. In childhood vitiligo too aspirin can be safely given during the active disease phase.[42]
Kawasaki's disease
Kawasaki's disease (KD) is an acute vasculitis involving the small and medium-sized blood vessels, associated with a constellation of cutaneous and systemic features.[48,49] The antithrombotic property of aspirin has been utilized while treating KD.[50,51] Based on the acute, subacute, and chronic phases of the disease, there is variation in aspirin dosing. According to the American Heart Association, 80–100 mg/kg/day of aspirin is given during the acute phase of the disease.[52] and 3–5 mg/kg/day during the subacute phase. In the chronic phase if coronary artery abnormalities are seen, then the same dose as the subacute phase is continued, till there is absence of coronary lesions on echocardiography.[53]
Postherpetic neuralgia
Postherpetic neuralgia (PHN) refers to a chronic resistant pain that persists at the site of viral rash even after rash resolution. Aspirin, used topically has proven efficacious for this indication. King[54] demonstrated the analgesic activity of aspirin by crushing aspirin tablets in chloroform and applying it over the affected site. De Benedittis et al.[55,56] further proved the same response after using aspirin/diethyl ether mixture while treating PHN. Aspirin brings about pain relief in PHN by neuronal membrane stabilization, denervation hypersensitivity, and inhibition of prostaglandin synthesis.[57,58] Topical aspirin used for treating PHN is obtained by crushing aspirin tablets of strength 375 mg to a fine powder and dissolving it in solvents such as diethyl ether or chloroform to get a final concentration of 75 mg of aspirin per mL of solution. However, dispensing aspirin in these inflammable solvents has its own disadvantage and safer solvents for the same would definitely be a better alternative. One such solvent used for the same is, Vaseline intensive moisturizing lotion, which has shown to be effective with aspirin in managing PHN as demonstrated by Kassirer et al.[59] and Balakrishnan et al.[60] Here aspirin tablet of strength 375 mg is powdered and dissolved in 5 mL of the above-mentioned moisturizing lotion to get a solution containing 75 mg/mL of aspirin. This paste is then uniformly applied over the hyperesthetic skin. Applications are done every 8 h for a period of at least 3 weeks in order to experience pain relief.
Mastocytosis
Mastocytosis is a rare disorder characterized by an abnormal population of mast cells in various organs of the body, namely, the skin, bone marrow, gastrointestinal tract, lymph nodes, spleen, and liver.[61] The role of aspirin in mastocytosis, is to alleviate the abnormal flushing seen during attacks. Aspirin acts by blocking the COX-2 enzyme, and reducing the elevated prostaglandin levels, associated with mastocytosis.[62,63] However caution is warranted while using aspirin because aspirin perse could even degranulate mast cells. Aspirin is therefore reserved for patients who have a vascular collapse that cannot be averted by the usage of H1 and H2 antagonists alone.[64] Treatment with aspirin should always be taken up in a hospital setting, starting with doses of aspirin ranging from 81 mg twice daily to 500 mg twice daily,[63] with prior administration of antihistaminics.[65] Aspirin is usually administered till the acute episode is brought under full control, with normalization of urinary 11-beta prostaglandin F2 alfa levels.
Niacin-induced cutaneous changes
Niacin has been found to be effective in the management of dyslipidemia. However facial flushing, associated with bothersome pruritus, may affect patient compliance. This complication of niacin is mediated by niacin G protein coupled receptor 109A expressed by Langerhans cells in the epidermis.[66,67] When these receptors are activated there is a release of arachidonic acid from the cellular stores of lipids via phospholipase A2.[68] Arachidonic acid is further sequentially metabolised by COX-1 and 2 to produce prostaglandins. Aspirin acts by blocking these cyclooxygenase enzymes, thus preventing prostaglandin release.[69,70,71] Studies done by Cefali et al.[72] and Whelam et al.[73] have demonstrated the benefit of aspirin in this condition. A double blinded placebo-controlled trial by Thakkar et al.[74] demonstrated similar efficacy of aspirin for niacinamide-induced flushing. Usually it is best to administer aspirin 30 min prior to niacin intake. The daily dose commonly used is 325 mg.[75] Further aspirin usage here does not interfere with the therapeutic benefit of niacin in dyslipidemia.[76]
Hughes’ syndrome
Hughes’ syndrome (HS) or antiphospholipid syndrome is a prothrombotic disorder manifesting with a constellation of cutaneous and systemic features. The most common complication occurring is recurrent abortion.[77] Low-dose aspirin employing 75–100 mg of the drug as a daily regimen has been found to be useful here.[78,79] Aspirin acts by decreasing platelet aggregation, thus preventing thrombosis in the uteroplacental circulation. Secondly, aspirin also reduces thromboxane to prostacyclin ratio and improves placental blood flow.[80] In this way, aspirin has shown to have a significant impact in reducing the rate of fetal resorption. Higher dosing has not shown any improvement in the thrombotic episodes in comparison to the low-dosing protocol.[81]
Sunburn reaction
An acute cutaneous response secondary to excessive exposure to ultraviolet (UV) rays is referred to as a sunburn reaction. Once UVB is absorbed by the DNA in the skin, a chain of events follow, wherein numerous inflammatory mediators such as prostaglandins, lipoxygenase products, adhesion molecules, reactive oxygen radicals, and cytokines such as TNF-alfa are released into the local mileu.[82] Along with these, histamine and substance P released from mast cells also enhance the inflammatory response.[83,84] Aspirin acts by reducing the production of these mediators and decreasing the effects produced by these chemokines in the sunburn reaction.[85] Post–UV light exposure it has been seen that a single oral dose of aspirin is effective enough to delay the production of erythema.[86] A double blinded crossover study, with 3.6 g of aspirin administered to sunburn patients over 9 h in three divided doses, 30 min prior to UVB exposure, along with a control placebo group revealed a significant reduction of erythema in the aspirin-treated group 4–6 h post–sun exposure.[87] Synder and Eaglstein[88] demonstrated the benefit of intradermal aspirin injections in patients prone to sunburn. Edwards et al.[89] have also highlighted the efficacy of oral aspirin in sunburn reactions.
Livedoid vasculopathy
This is a disorder predominantly involving the lower limbs, and characterized by painful ulcers, associated with ivory white plaques surrounded by areas of hyperpigmentation and telengiectasias. There are a multitude of causative factors involved, out of which altered coagulation and increased platelet activation contribute significantly.[90] Owing to the thrombogenic mechanisms involved, therapy with anticoagulants has been found to be beneficial.[91,92] Aspirin at doses not higher than 325 mg per day is given to patients, as higher doses inhibit prostacycline formation, which may increase the thrombotic tendency.[93] Combination of aspirin with dipyrimadole is often helpful.[94] Another useful synergism is the concomitant use of aspirin and pentoxifylline for atrophie blanche, with a better outcome than using either of the drugs alone.[95] Duration of treatment depends on how the patient is responding to the therapy given. However, on an average, treatment usually goes on for around 2 months to a year.[94] Remission begins first by the reduction in pain followed by re-epithelialization of the ulcers over a period of several months.
Lepra reactions
Aspirin given in doses of 600–1200 mg 4–6 times daily, has been found to be effective in mild type 1 lepra reactions. These reactions are clinically characterized by mild erythematous to oedematous plaques without any systemic disturbance or subjective or objective nerve involvement.[96] To start with, 600 mg of aspirin may be administered, up to 6 times per day and can be gradually tapered with reductions of 300–600 mg per week till the therapeutic outcome is achieved.[97]
Pruritus associated with polycythemia vera
In the above indication too, aspirin has been found beneficial. Given in a dosing of 300 mg twice daily or 1 h prior to bathing, aspirin showed promise in this scenario. Aspirin acts by directly suppressing the prostaglandin metabolism within the mast cells and preventing its degranulation.[98]
Pressure urticaria and Non-immunologic urticaria
Pressure urticaria has shown benefit with aspirin. With 3.9 g of aspirin administered to patients, in four divided doses for 3 days, a significant improvement in the painful pressure lesions have been noticed. However, no response is noted in the urticarial lesions following aspirin intake.[99] Nonimmunologic urticaria (NIU) has also shown improvement following administration of aspirin. Two doses of 1 g of aspirin administered a few hours prior to contactant exposure produces a favorable response in patients with NIU. However NIU associated with dimethyl sulfoxide exposure did not show any benefit with aspirin.[100]
Relapsing polychondritis
There have been a few case reports of relapsing polychondritis responding favorably to aspirin therapy.[101,102]
Systemic lupus erythematosus
In some cases of systemic lupus erythematosus (SLE) presenting with fever, cutaneous lesions, arthritis, and pyrexia, but without major organ involvement the beneficial role of aspirin at dosages of 3–6 g per day has been seen.[103] Aspirin's role in these patients is directed mainly toward alleviating the symptoms of pain associated with arthralgia and malaise and also in bringing the temperature down. Caution, however is mandated with the use of aspirin in these patients as they are at a greater risk for nonsteroidal anti-inflammatory drug (NSAID)-induced asceptic meningitis, hypertension, altered renal function, and deranged hepatic parameters after a long-term use of aspirin. Another subset of SLE patients requiring aspirin are those with positive antiphospholipid antibodies, who require long-term anticoagulation.[104,105]
Kasabach Merritt's syndrome
Kasabach Merritt's syndrome (KMS) is considered to be a consumption coagulopathy associated with a vascular tumor that has a tendency to enlarge rapidly. Within the enlarging tumor, there is platelet sequestration, which is responsible for the thrombocytopenia associated with KMS.[106] Aspirin has been used as an additional therapy here, usually in combination with dipyridamole.[107] However, the role of aspirin in KMS is limited.[108,109]
Malignant melanoma
The use of aspirin has been associated with marked reduction in melanoma risk. Studies have demonstrated a dose-dependent reduction in the proliferation of B16 murine melanoma cells and SK-28 human melanoma cells following aspirin intake.[110] Other mechanisms involved include oxidation of aspirin by tyrosinases present in the melanoma cells and depletion of intracellular glutathione with eventual formation of reactive oxygen species, all of which exert a toxic effect on the mitochondria of the melanoma cells, thus stopping its proliferation.[111,112] Aspirin also inhibits COX-2, which is increasingly expressed in the melanoma cells, and halts advancement of the tumor.[113,114] Further contributory effects include suppression of nuclear factor kappa-B and antiapoptotic transcription factor, which puts a stop to melanoma progression.[44,115,116,117] Proven studies[118,119,120] in this regard have been summarized in Table 1.
Table 1.
Summary of studies proving the role of aspirin in malignant melanoma
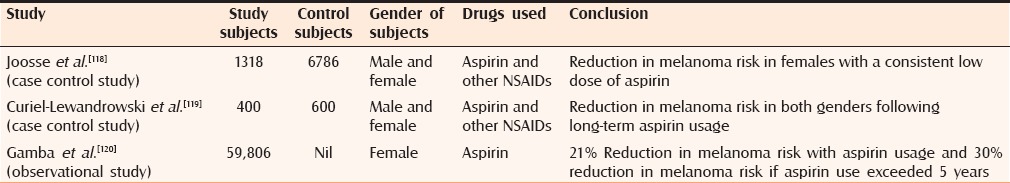
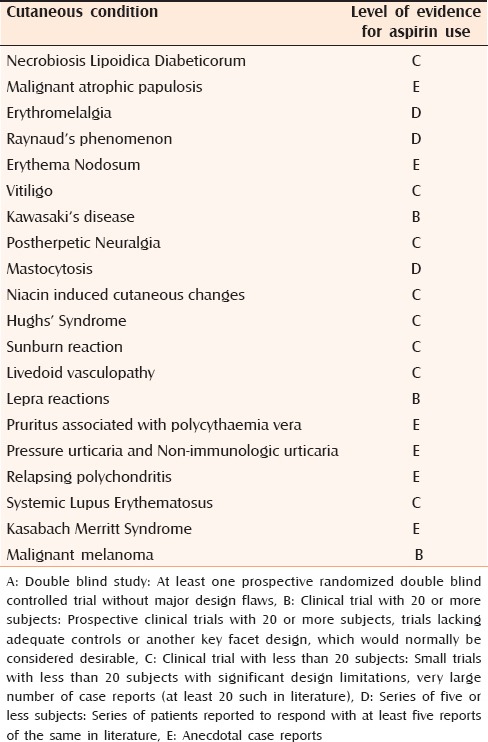
Table 2.
Level of evidence for the use of aspirin in various dermatoses mentioned in the review
CONTRAINDICATIONS
Aspirin is contraindicated in patients who are allergic to NSAIDs and also in patients with asthma, allergic rhinitis, nasal polyps, and peptic ulcer disease. Another contraindication for aspirin usage, are children and young teenagers with viral infections because of the risk of precipitating Reye's syndrome. It should be avoided during pregnancy especially in the third trimester because it could lead to closure of the ductus arteriosus in the fetus. Nursing mothers should also avoid aspirin as it is secreted in breast milk.[9]
ADVERSE EFFECTS
The commonest side effect, even with low doses of aspirin is peptic ulcer disease. Other toxicities include angioedema, urticaria, asthma exacerbation, and rhinitis.[32] Rarer adverse effects are prolongation of prothrombin time, thrombocytopenia, tinnitus, and interstitial nephritis. Chronic usage of aspirin for cutaneous disorders and malignant melanoma, has not been associated with major side effects apart from gastrointestinal bleeding, which too has been reported in 2% of subjects. Overall, aspirin has a well-tolerable safety profile.
CONCLUSION
Aspirin although an old and inexpensive drug, has shown promise for a variety of dermatologic disorders. With its role in reduction of melanoma risk aspirin has also found to have a place in oncology. With further advances in the field of molecular pharmacology, in the near future more newer indications could be discovered for aspirin, one of the oldest drugs in the field of medicine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Stone E. An account of the success of the bark of the willow tree in the cure of agues. Philos Trans. 1763;53:195–200. [Google Scholar]
- 2.Dreser H. Pharmakologisches uber aspirin (acetylsalicylsaure). Pfluger's. Arch. 1899;76:306–18. [Google Scholar]
- 3.Weiss HJ, Aledort LM. Impaired platelet-connective-tissue reaction in man after aspirin ingestion. Lancet. 1967;2:495–7. doi: 10.1016/s0140-6736(67)91658-3. [DOI] [PubMed] [Google Scholar]
- 4.Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I: Acetylation of a particulate fraction protein. J Clin Invest. 1975;56:624–32. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loll PJ, Picot D, Garavito RM. The structural basis of aspirin inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol. 1995;2:637–43. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- 6.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Ann Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 7.López-Farré A, Caramelo C, Esteban A, Alberola ML, Millás I, Montón M, et al. Effects of aspirin on platelet-neutrophil interactions: Role of nitric oxide and endothelin-1. Circulation. 1995;91:2080–8. doi: 10.1161/01.cir.91.7.2080. [DOI] [PubMed] [Google Scholar]
- 8.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101:1206–18. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 9.Grosser T, Smyth E, FitzGerald GA. Anti-inflammatory, anti-pyretic and analgesic agents; pharmacotherapy of gout. In: Brunton L, Chabner B, Knollman B, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw Hill; 2011. pp. 959–1004. [Google Scholar]
- 10.Levy G. Clinical pharmacokinetics of aspirin. Pediatrics. 1978;62(Suppl):867–72. [PubMed] [Google Scholar]
- 11.Reid SD, Ladizinki B, Lee K, Baibergenova A, Alavi A. Update on necrobiosis lipoidica: A review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783–91. doi: 10.1016/j.jaad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 12.Imtiaz KE, Khaleeli AA. Squamous cell carcinoma developing in necrobiosis lipoidica. Diabet Med. 2001;18:325–8. doi: 10.1046/j.1464-5491.2001.00452.x. [DOI] [PubMed] [Google Scholar]
- 13.Kota SK, Jammula S, Kota SK, Meher LK, Modi KD. Necrobiosis lipoidica diabeticorum: A case-based review of literature. Indian J Endocrinol Metab. 2012;16:614–20. doi: 10.4103/2230-8210.98023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karkavitsas K, Muller JA, Dowd PM, Kerby JD. Aspirin in the management of necrobiosis lipoidica. Acta Derm Venereol. 1982;62:183. [PubMed] [Google Scholar]
- 15.Heng MC, Song MK, Heng MK. Healing of necrobiotic ulcers with antiplatelet therapy. Correlation with plasma thromboxane levels. Int J Dermatol. 1989;28:195–7. doi: 10.1111/j.1365-4362.1989.tb02465.x. [DOI] [PubMed] [Google Scholar]
- 16.Beck HI, Bjerring P, Rasmussen I, Zachariae H, Stenbjerg S. Treatment of necrobiosis lipoidica with low-dose acetylsalicylic acid. A randomized double-blind trial. Acta Derm Venereol. 1985;65:230–4. [PubMed] [Google Scholar]
- 17.Statham B, Finlay AY, Marks R. A randomized double blind comparison of aspirin and dipyridamole combination versus a placebo in the treatment of necrobiosis lipoidica. Acta Derm Venereol. 1981;61:270–1. [PubMed] [Google Scholar]
- 18.Vázquez-Doval FJ, Ruiz de Erenchun F, Paramo JA, Quintanilla E. Malignant atrophic papulosis. A report of two cases with altered fibrinolysis and platelet function. Clin Exp Dermatol. 1993;18:441–4. doi: 10.1111/j.1365-2230.1993.tb02246.x. [DOI] [PubMed] [Google Scholar]
- 19.Stahl D, Thomsen K, Hou-Jensen J. Degos’ disease treated with platelet-suppressive drugs. Lancet. 1977;2:46–7. doi: 10.1016/s0140-6736(77)90052-6. [DOI] [PubMed] [Google Scholar]
- 20.Drucker CR. Malignant atrophic papulosis: Response to antiplatelet therapy. Dermatologica. 1990;180:90–2. doi: 10.1159/000247999. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell SW. A rare vasomotor neurosis of the extremities and on maladies with which it may be confounded. Am J Med Sci. 1878;76:2–36. [Google Scholar]
- 22.Michiels JJ, Abele J, Steketee J, van Vliet HH, Vuzevski VD. Erythromelalgia caused by platelet-mediated arteriolar inflammation and thrombosis in thrombocythemia. Ann Intern Med. 1985;102:466–71. doi: 10.7326/0003-4819-102-4-466. [DOI] [PubMed] [Google Scholar]
- 23.Kurzrock R, Cohen PR. Erythromelalgia: Review of clinical characteristics and pathophysiology. Am J Med. 1991;91:416–22. doi: 10.1016/0002-9343(91)90160-y. [DOI] [PubMed] [Google Scholar]
- 24.van Genderen PJ, Michiels JJ. Erythromelalgia: A pathognomonic microvascular thrombotic complication in essential thrombocythemia and polycythemia vera. Semin Thromb Hemost. 1997;23:357–63. doi: 10.1055/s-2007-996109. [DOI] [PubMed] [Google Scholar]
- 25.Preston FE. Aspirin, prostaglandins, and peripheral gangrene. Am J Med. 1983;74:55–60. doi: 10.1016/0002-9343(83)90529-6. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan SG, Yesudian PD, Jayaraman M, Janaki VR, Raj BJ. Erythromelalgia responding to aspirin. Indian J Dermatol Venereol Leprol. 1996;62:204–5. [PubMed] [Google Scholar]
- 27.Bakst R, Merola JF, Franks AG, Jr, Sanchez M. Raynaud's phenomenon: Pathogenesis and management. J Am Acad Dermatol. 2008;59:633–53. doi: 10.1016/j.jaad.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Goyle KB, Dormandy JA. Abnormal blood viscosity in Raynaud's phenomenon. Lancet. 1976;1:1317–8. doi: 10.1016/s0140-6736(76)92651-9. [DOI] [PubMed] [Google Scholar]
- 29.Wigley FM. Clinical practice. Raynaud's phenomenon. N Engl J Med. 2002;347:1001–8. doi: 10.1056/NEJMcp013013. [DOI] [PubMed] [Google Scholar]
- 30.Akerkar SM, Bichile LS. Therapeutic options for systemic sclerosis. Indian J Dermatol Venereol Leprol. 2004;70:67–75. [PubMed] [Google Scholar]
- 31.Requena L, Requena C. Erythema nodosum. Dermatol Online J. 2002;8:4. [PubMed] [Google Scholar]
- 32.Forman SB, Garret AB. Vasoactive and antiplatelet agents. In: Wolvorton SE, editor. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia: Elsevier Saunders; 2007. pp. 405–15. [Google Scholar]
- 33.Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC, Tobin DJ, et al. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB activated pseudocatalase. J Investig Dermatol Symp Proc. 1999;4:91–6. doi: 10.1038/sj.jidsp.5640189. [DOI] [PubMed] [Google Scholar]
- 34.Maresca V, Roccella M, Roccella F, Camera E, Del Porto G, Passi S, et al. Increased sensitivity to peroxidative agents as a possible pathogenetic factor of melanocyte damage in vitiligo. J Invest Dermatol. 1997;109:310–3. doi: 10.1111/1523-1747.ep12335801. [DOI] [PubMed] [Google Scholar]
- 35.Jimbow K, Chen H, Park JS, Thomas PD. Increased sensitivity of melanocytes to oxidative stress and abnormal expression of tyrosinase-related protein in vitiligo. Br J Dermatol. 2001;144:55–65. doi: 10.1046/j.1365-2133.2001.03952.x. [DOI] [PubMed] [Google Scholar]
- 36.Dell’Anna ML, Urbanelli S, Mastrofrancesco A, Camera E, Iacovelli P, Leone G, et al. Alterations of mitochondria in peripheral blood mononuclear cells of vitiligo patients. Pigment Cell Res. 2003;16:553–9. doi: 10.1034/j.1600-0749.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 37.Beazley WD, Gaze D, Panske A, Panzig E, Schallreuter KU. Serum selenium levels and blood glutathione peroxidase activities in vitiligo. Br J Dermatol. 1999;141:301–3. doi: 10.1046/j.1365-2133.1999.02980.x. [DOI] [PubMed] [Google Scholar]
- 38.Dell’Anna ML, Maresca V, Briganti S, Camera E, Falchi M, Picardo M. Mitochondrial impairment in peripheral blood mononuclear cells during the active phase of vitiligo. J Invest Dermatol. 2001;117:908–13. doi: 10.1046/j.0022-202x.2001.01459.x. [DOI] [PubMed] [Google Scholar]
- 39.Baek BS, Kim JW, Lee JH, Kwon HJ, Kim ND, Kang HS, et al. Age related increase of brain cyclooxygenase activity and dietary modulation of oxidative status. J Gerontol A Biol Sci Med Sci. 2001;56:B426–31. doi: 10.1093/gerona/56.10.b426. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Sakamoto K. Reactive oxygen species up-regulates cyclooxygenase-2, p53, and Bax mRNA expression in bovine luteal cells. Biochem Biophys Res Commun. 2001;284:203–10. doi: 10.1006/bbrc.2001.4927. [DOI] [PubMed] [Google Scholar]
- 41.Zailaie MZ. The effect of acetylsalicylic acid on the release rates of leukotrienes B4 and C4 from cultured skin melanocytes of active vitiligo. Saudi Med J. 2004;25:1439–44. [PubMed] [Google Scholar]
- 42.Zailaie MZ. Aspirin reduces serum anti-melanocyte antibodies and soluble interleukin-2 receptors in vitiligo patients. Saudi Med J. 2005;26:1085–91. [PubMed] [Google Scholar]
- 43.Shi X, Ding M, Dond Z, Chan F, Ye J, Wang S, et al. Antioxidant properties of aspirin: Characterization of the ability of aspirin to inhibit silica-induced lipid peroxidation, DNA damage, NF-kappaB activation, and TNF-alpha production. Mol Cell Biochem. 1999;199:93–102. doi: 10.1023/a:1006934612368. [DOI] [PubMed] [Google Scholar]
- 44.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I (kappa) B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 45.Sharquie KE, Mehenna SH, Naji AA, AI-Azzawi H. Inflammatory changes in vitiligo: Stage I and II depigmentation. Am J Dermatopathol. 2004;26:108–12. doi: 10.1097/00000372-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Abel-Naser MB, Krüger-Krasagakes S, Krasagakis K, Gollnick H, Abdel-Fattah A, Orfanos LE. Further evidence for involvement of both cell mediated and humoral immunity in generalized vitiligo. Pigment Cell Res. 1994;7:1–8. doi: 10.1111/j.1600-0749.1994.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 47.Zailaie MZ. Effect of prolonged low-dose oral aspirin on the oxidative status of peripheral blood mononuclear cells of active vitiligo. Indian J Dermatol. 2005;50:9–16. [Google Scholar]
- 48.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arevugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 49.Burns JC. Commentary: Translation of Dr. Tomisaku Kawasaki's original report of fifty patients in 1967. Pediatr Infect Dis J. 2002;21:993–5. doi: 10.1097/00006454-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Burns JC, Glode MP, Clarke SH, Wiggins J, Jr, Hathaway WE. Coagulopathy and platelet activation in Kawasaki syndrome: Identification of patients at high risk for development of coronary artery aneurysms. J Pediatr. 1984;105:206–11. doi: 10.1016/s0022-3476(84)80114-6. [DOI] [PubMed] [Google Scholar]
- 51.Hamasaki Y, Ichimaru T, Tasaki H, Mujazaki S. Studies on the effect of long-term use of low dose aspirin in Kawasaki disease. Acta Paediatr Jpn. 1988;30:63–7. doi: 10.1111/j.1442-200x.1988.tb02498.x. [DOI] [PubMed] [Google Scholar]
- 52.Scuccimarri R. Kawasaki disease. Pediatr Clin North Am. 2012;59:425–45. doi: 10.1016/j.pcl.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Luca NJ, Yeung RS. Epidemiology and management of Kawasaki's disease. Drugs. 2012;72:1029–38. doi: 10.2165/11631440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54.King RB. Topical aspirin in chloroform and the relief of pain due to herpes zoster and postherpetic neuralgia. Arch Neurol. 1993;50:1046–53. doi: 10.1001/archneur.1993.00540100041012. [DOI] [PubMed] [Google Scholar]
- 55.De Benedittis G, Besana F, Lorenzetti A. A new topical treatment for acute herpetic neuralgia and post-herpetic neuralgia: The aspirin/diethyl ether mixture. An open-label study plus a double blind controlled clinical trial. Pain. 1992;48:383–90. doi: 10.1016/0304-3959(92)90088-S. [DOI] [PubMed] [Google Scholar]
- 56.De Benedittis G, Lorenzetti A. Topical aspirin/diethyl ether mixture versus indomethacin and diclofenac/diethyl ether mixtures for acute herpetic neuralgia and post herpetic neuralgia: A double-blind crossover placebo-controlled study. Pain. 1996;65:45–51. doi: 10.1016/0304-3959(95)00155-7. [DOI] [PubMed] [Google Scholar]
- 57.Rowbotham MC. Topical analgesic agents. In: Field HL, Liebeskind JC, editors. Progress in Pain Research and Management. Seattle, WA: IASP Press; 1993. pp. 211–27. [Google Scholar]
- 58.Riccioppo Neto F. Further studies on the actions of salicylates on nerve membranes. Eur J Pharmacol. 1980;68:155–62. doi: 10.1016/0014-2999(80)90316-7. [DOI] [PubMed] [Google Scholar]
- 59.Kassirer MR, King RB, Robert A. Concerning the management of pain associated with herpes zoster and postherpetic neuralgia. Pain. 1988;35:368–9. doi: 10.1016/0304-3959(88)90150-9. [DOI] [PubMed] [Google Scholar]
- 60.Balakrishnan S, Bhushan K, Bhargava VK, Pandhi P. A randomized parallel trial of topical aspirin-moisturizer solution vs. oral aspirin for acute herpetic neuralgia. Int J Dermatol. 2001;40:535–8. doi: 10.1046/j.1365-4362.2001.01265.x. [DOI] [PubMed] [Google Scholar]
- 61.Hartmann K, Burns SB, Henz BM. Mastocytosis: Review of clinical and experimental aspects. J Invest Dermatol Symp Proc. 2001;6:143–7. doi: 10.1046/j.0022-202x.2001.00029.x. [DOI] [PubMed] [Google Scholar]
- 62.Alto WA, Clarcq L. Cutaneous and systemic manifestations of mastocytosis. Am Fam Physician. 1999;59:3047–54. [PubMed] [Google Scholar]
- 63.Butterfield JH. Survey of aspirin administration in systemic mastocytosis. Prostaglandins Other Lipid Mediat. 2009;88:122–4. doi: 10.1016/j.prostaglandins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Metcalfe DD. The treatment of mastocytosis: An overview. J Invest Dermatol. 1991;96:55–9. [PubMed] [Google Scholar]
- 65.Austen KF. Systemic mastocytosis. N Engl J Med. 1992;326:639–40. doi: 10.1056/NEJM199202273260912. [DOI] [PubMed] [Google Scholar]
- 66.Benyó Z, Gille A, Kero J, Csiky M, Suchánková MC, Nüsing RM, et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115:3634–40. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benyó Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal Langerhans cells. Mol Pharmacol. 2006;70:1844–9. doi: 10.1124/mol.106.030833. [DOI] [PubMed] [Google Scholar]
- 68.Lin LL, Lin AY, Knopf JL. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci U S A. 1992;89:6147–51. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andersson RG, Alberg G, Brattsand R, Ericsson E, Lundholm L. Studies on the mechanism of flush induced by nicotinic acid. Acta Pharmacol Toxicol (Copenh) 1977;41:1–10. doi: 10.1111/j.1600-0773.1977.tb02116.x. [DOI] [PubMed] [Google Scholar]
- 70.Eklund B, Kaijser L, Nowak J, Wennmalm A. Prostaglandins contribute to the vasodilation induced by nicotinic acid. Prostaglandins. 1979;17:821–30. doi: 10.1016/0090-6980(79)90055-8. [DOI] [PubMed] [Google Scholar]
- 71.Svedmyr N, Heggelund A, Aberg G. Influence of indomethacin on flush induced by nicotinic acid in man. Acta Pharmacol Toxicol (Copenh) 1977;41:397–400. doi: 10.1111/j.1600-0773.1977.tb02678.x. [DOI] [PubMed] [Google Scholar]
- 72.Cefali EA, Simmons PD, Stanek EJ, McGovern ME, Kissling CJ. Aspirin reduces cutaneous flushing after administration of an optimized extended-release niacin formulation. Int J Clin Pharmacol Ther. 2007;45:78–88. doi: 10.5414/cpp45078. [DOI] [PubMed] [Google Scholar]
- 73.Wheelam AM, Price SO, Fowler SF, Hainer BL. The effect of aspirin on niacin-induced cutaneous reactions. J Fam Pract. 1992;34:165–8. [PubMed] [Google Scholar]
- 74.Thakkar RB, Kashyap ML, Lewin AJ, Krause SL, Jiang P, Padley RJ. Acetylsalicylic acid reduces niacin extended-release induced flushing in patients with dyslipidemia. Am J Cardiovasc Drugs. 2009;9:69–79. doi: 10.1007/BF03256578. [DOI] [PubMed] [Google Scholar]
- 75.Jungnickel PW, Maloley PA, Vander Tuin EL, Peddicord TE, Campbell JR. Effect of two aspirin pretreatment regimens on niacin-induced cutaneous reactions. J Gen Intern Med. 1997;12:591–6. doi: 10.1046/j.1525-1497.1997.07118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaijser L, Eklund B, Olsson AG, Carlson LA. Dissociation of the effects of nicotinic acid on vasodilation and lipolysis by a prostaglandin synthesis inhibitor, indomethacin, in man. Med Biol. 1979;57:114–7. [PubMed] [Google Scholar]
- 77.McNeil HP, Chesterman CN, Krilis SA. Immunology and clinical importance of antiphospholipid antibodies. Adv Immunol. 1991;49:193–280. doi: 10.1016/s0065-2776(08)60777-4. [DOI] [PubMed] [Google Scholar]
- 78.Elder MG, De Swiet M, Robertson A, Elder MA, Flloyd E, Hawkins DF. Low dose aspirin in pregnancy. Lancet. 1988;i:410. doi: 10.1016/s0140-6736(88)91198-1. [DOI] [PubMed] [Google Scholar]
- 79.Balasch J, Carmona F, López-Soto A, Font J, Creus M, Fábregues F, et al. Low-dose aspirin for prevention of pregnancy loses in women with primary antiphospholipid syndrome. Hum Reprod. 1993;8:2234–9. doi: 10.1093/oxfordjournals.humrep.a138009. [DOI] [PubMed] [Google Scholar]
- 80.Canesi B, Brucato A. Anti-phospholipid antibodies and pregnancy. Haematologica. 2005;1:6–12. [Google Scholar]
- 81.Ruiz-Irastorza G, Khamashta MA, Hughes-GR Antiaggregant and anticoagulant therapy in systemic lupus erythematosus and Hughes’ syndrome. Lupus. 2001;10:241–5. doi: 10.1191/096120301667789546. [DOI] [PubMed] [Google Scholar]
- 82.Hruza LL, Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993;100:35–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- 83.Walsh LJ. Ultraviolet B irradiation of skin induces mast cell degranulation and release of tumor necrosis factor-alpha. Immunol Cell Biol. 1993;73:226–33. doi: 10.1038/icb.1995.37. [DOI] [PubMed] [Google Scholar]
- 84.Farr PM, Shuster S. Sunburn and neuropeptide depletion. Br J Dermatol. 1995;133:1012. doi: 10.1111/j.1365-2133.1995.tb06949.x. [DOI] [PubMed] [Google Scholar]
- 85.Driscoll MS, Wagner RF., Jr Clinical management of the acute sunburn reaction. Cutis. 2000;66:53–8. [PubMed] [Google Scholar]
- 86.Gruber CM, Jr, Ridolfo AS, Nickander R, Mikulaschek WM. Delay of erythema of human skin by anti-inflammatory drugs after ultraviolet irradiation. Clin Pharmacol Ther. 1972;13:109–13. doi: 10.1002/cpt1972131109. [DOI] [PubMed] [Google Scholar]
- 87.Miller WS, Ruderman FR, Smith JG., Jr Aspirin and ultraviolet light-induced erythema in man. Arch Dermatol. 1967;95:375–8. [PubMed] [Google Scholar]
- 88.Synder DS, Eaglstein WH. Intradermal anti-prostaglandin agents and sunburn. J Invest Dermatol. 1974;62:47–50. [Google Scholar]
- 89.Edwards EK, Jr, Horwitz SN, Frost P. Reduction of erythema response to ultraviolet light by nonsteroidal antiinflammatory agents. Arch Dermatol Res. 1982;272:263–7. doi: 10.1007/BF00509055. [DOI] [PubMed] [Google Scholar]
- 90.Papi M, Didona B, De Pità O, Frezzolini A, Di Giulio S, De Matteis W, et al. Livedo vasculopathy vs small vessel cutaneous vasculitis: Cytokine and platelet P-selectin studies. Arch Dermatol. 1998;134:447–52. doi: 10.1001/archderm.134.4.447. [DOI] [PubMed] [Google Scholar]
- 91.Jorizzo JL. Livedoid vasculopathy: What is it? Arch Dermatol. 1998;134:491–3. doi: 10.1001/archderm.134.4.491. [DOI] [PubMed] [Google Scholar]
- 92.McCalmont CS, McCalmont TH, Jorizzo JL, White WL, Leshin B, Rithberger H. Livedo vasculitis: Vasculitis or thrombotic vasculopathy? Clin Exp Dermatol. 1992;17:4–8. doi: 10.1111/j.1365-2230.1992.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 93.Kern AB. Atrophie blanche. Report of two patients treated with aspirin and dipyridamole. J Am Acad Dermatol. 1982;6:1048–53. [PubMed] [Google Scholar]
- 94.Drucker CR, Duncan WC. Antiplatelet therapy in atrophie blanche and livedo vasculitis. J Am Acad Dermatol. 1982;7:359–63. doi: 10.1016/s0190-9622(82)70123-9. [DOI] [PubMed] [Google Scholar]
- 95.Lee SS, Ang P, Tan SH. Clinical profile and treatment outcome of livedoid vasculitis: A case series. Ann Acad Med Singapore. 2003;32:835–9. [PubMed] [Google Scholar]
- 96.Pfaltzgraff RE. The management of reaction in leprosy. Int J Lepr Other Mycobact Dis. 1989;57:103–9. [PubMed] [Google Scholar]
- 97.Shahiduzzaman GK, Kamal SM, Ahad MA, Islam R. Leprosy: An overview. Med Today. 2011;23:44–50. [Google Scholar]
- 98.Jackson N, Burt D, Crocker J, Boughton B. Skin mast cells in polycythaemia vera: Relationship to the pathogenesis and treatment in pruritus. Br J Dermatol. 1987;116:21–9. doi: 10.1111/j.1365-2133.1987.tb05787.x. [DOI] [PubMed] [Google Scholar]
- 99.Sussman GL, Harvey RP, Schocket AL. Delayed pressure urticaria. J Allergy Clin Immunol. 1982;70:337–42. doi: 10.1016/0091-6749(82)90022-7. [DOI] [PubMed] [Google Scholar]
- 100.Lahti A, Väänänen A, Kokkonen EL, Hannuksela M. Acetylsalicylic acid inhibits non-immunologic contact urticarial. Contact Dermatitis. 1987;16:133–5. doi: 10.1111/j.1600-0536.1987.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 101.Kremer J, Gates SA, Parhami N. Relapsing polychondritis excellent response to naproxen and aspirin. J Rheumatol. 1979;6:719–20. [PubMed] [Google Scholar]
- 102.McAdam LP, O’Hanlon MA, Bluestone R, Pearson CM. Relapsing polychondritis: Prospective study of 23 patients and review of the literature. Medicine (Baltimore) 1976;55:193–215. [PubMed] [Google Scholar]
- 103.Schur PH. The clinical management of systemic lupus erythematosus. In: Decker JL, editor. Systemic Lupus Erythematosus Management. New York: Grune and Stratton; 1983. pp. 259–81. [Google Scholar]
- 104.Giles IP, Isenberg DA. Autoimmune disorders and vasculitides. In: Warell DA, Cox TM, Firth JD, editors. Oxford Textbook of Medicine. 5th ed. Oxford New York: Oxford University Press; 2010. pp. 3649–704. [Google Scholar]
- 105.Longo DL, Kasper DL, Jameson JL, Fauci AS, Hausen SL, Loscalzo J. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw Hill; 2012. Disorsers of joints and adjacent tissues; pp. 2649–863. [Google Scholar]
- 106.Hall GW. Kasabach-Merritt syndrome: Pathogenesis and management. Br J Haematol. 2001;112:851–62. doi: 10.1046/j.1365-2141.2001.02453.x. [DOI] [PubMed] [Google Scholar]
- 107.Koerper MA, Addiego JE, Jr, deLorimier AA, Lipow H, Price D, Lubin BH. Use of aspirin and dipyridamole in children with platelet trapping syndromes. J Pediatr. 1983;102:311–4. doi: 10.1016/s0022-3476(83)80550-2. [DOI] [PubMed] [Google Scholar]
- 108.Hagerman LJ, Czapek EE, Donnellan WL, Schwartz AD. Giant hemangioma with consumption coagulopathy. J Pediatr. 1975;87:766–8. doi: 10.1016/s0022-3476(75)80305-2. [DOI] [PubMed] [Google Scholar]
- 109.Lang PG, Dubin HV. Hemangioma-thrombocytopenia syndrome; A disseminated intravascular coagulopathy. Arch Dermatol. 1975;111:105–7. [PubMed] [Google Scholar]
- 110.Ordan O, Rotem R, Jaspers I, Flescher E. Stress responsive JNK mitogen-activated protein kinase mediates aspirin-induced expression of B16 melanoma cellular proliferation. Br J Pharmacol. 2003;138:1156–62. doi: 10.1038/sj.bjp.0705163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vad NM, Yount G, Moridani MY. Biochemical mechanism of acetylsalicylic acid (Aspirin) selective toxicity toward melanoma cell lines. Melanoma Res. 2008;18:386–99. doi: 10.1097/CMR.0b013e3283107df7. [DOI] [PubMed] [Google Scholar]
- 112.Chiu LC, Tong KF, Ooi VE. Cytostatic and cytotoxic effects of cyclooxygenase inhibitors and their synergy with docosahexaenoic acid on the growth of human skin melanoma A-375 cells. Biomed Pharmacother. 2005;59(Suppl 2):S293–7. doi: 10.1016/s0753-3322(05)80049-6. [DOI] [PubMed] [Google Scholar]
- 113.Kuźbicki Ł, Lange D, Strączyńska-Niemiec A, Chwirot BW. The value of cyclooxygenase-2 expression in differentiating between early melanomas and histopathologically difficult types of benign human skin lesions. Melanoma Res. 2012;22:70–6. doi: 10.1097/CMR.0b013e32834defec. [DOI] [PubMed] [Google Scholar]
- 114.Chwirot BW, Kuźbicki Ł. Cyclooxygenase-2 (COX-2):First immunohistochemical marker distinguishing early cutaneous melanomas from benign melanocytic skin tumors. Melanoma Res. 2007;17:139–45. doi: 10.1097/CMR.0b013e3280dec6ac. [DOI] [PubMed] [Google Scholar]
- 115.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 116.Franco AV, Zhang XD, Van Berkel E, Sanders JE, Zhang XY, Thomas WD, et al. The role of NF-kappa B in TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of melanoma cells. J Immunol. 2001;166:5337–45. doi: 10.4049/jimmunol.166.9.5337. [DOI] [PubMed] [Google Scholar]
- 117.Ivanov VN, Fodstand O, Ronai Z. Expression of ring finger-deleted TRAF2 sensitizes metastatic melanoma cells to apoptosis via up-regulation of p38, TNFalpha and suppression of NF-kappaB activities. Oncogene. 2001;20:2243–53. doi: 10.1038/sj.onc.1204314. [DOI] [PubMed] [Google Scholar]
- 118.Joosse A, Koomen ER, Casparie MK, Herings RM, Guchelaar HJ, Nijsten T. Non-steroidal anti-inflammatory drugs and melanoma risk: Large Dutch population-based case-control study. J Invest Dermatol. 2009;129:2620–7. doi: 10.1038/jid.2009.201. [DOI] [PubMed] [Google Scholar]
- 119.Curiel-Lewandrowski C, Nijsten T, Gomez ML, Hollestein LM, Atkins MB, Stern RS. Long-term use of nonsteroidal anti-inflammatory drugs decreases the risk of cutaneous melanoma. Results of a United States case-control study. J Invest Dermatol. 2011;131:1460–8. doi: 10.1038/jid.2011.58. [DOI] [PubMed] [Google Scholar]
- 120.Gamba CA, Swetter SM, Stefanick ML, Kubo J, Desai M, Spaunhurst KM, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: The Women's Health Initiative. Cancer. 2013;119:1562–9. doi: 10.1002/cncr.27817. [DOI] [PMC free article] [PubMed] [Google Scholar]


