Abstract
Aim:
The aim of the present study is to evaluate diffusion tensor tractography (DTT) as a tool for detecting diffuse axonal injury in patients of acute, mild, and moderate traumatic brain injury (TBI), using two diffusion variables: Fractional anisotropy (FA) and mean diffusivity (MD). The correlation of these indices with the severity of post-concussive symptoms was also assessed.
Materials and Methods:
Nineteen patients with acute, mild, or moderate TBI and twelve age- and sex-matched healthy controls were recruited. Following Magnetic Resonance Imaging (MRI) on a 3.0-T scanner, DTT was performed using the ‘fiber assignment by continuous tracking’ (FACT) algorithm for fiber reconstruction. Appropriate statistical tools were used to see the difference in FA and MD values between the control and patient groups. In the latter group, the severity of post-concussive symptoms was assessed six months following trauma, using the Rivermead Postconcussion Symptoms Questionnaire (RPSQ).
Results:
The patients displayed significant reduction in FA compared to the controls (P < 0.05) in several tracts, notably the corpus callosum, fornix, bilateral uncinate fasciculus, and bilateral superior thalamic radiations. Changes in MD were statistically significant in the left uncinate, inferior longitudinal fasciculus, and left posterior thalamic radiation. A strong correlation between these indices and the RPSQ scores was observed in several white matter tracts.
Conclusion:
Diffusion tensor imaging (DTI)-based quantitative analysis in acute, mild, and moderate TBI can identify axonal injury neuropathology, over and above that visualized on conventional MRI scans. Furthermore, the significant correlation observed between FA and MD indices and the severity of post-concussive symptoms could make it a useful predictor of the long-term outcome.
Keywords: Diffusion tensor imaging, diffusion tensor tractography, mild and moderate traumatic brain injury, post-concussive symptoms, tractography
Introduction
The post-concussion syndrome (PCS), a common sequel of traumatic brain injury (TBI) is a symptom complex comprising of headache, sleep disturbance, neuropsychiatric symptoms, and cognitive impairment.[1] Although it has been described most often in the setting of mild TBI, it occurs after moderate and severe TBI and whiplash injuries as well.[2] Traumatic brain injury has become a global health problem of major concern today, owing to the high incidence of road traffic accidents, sports injuries, terrorist operations, and war-related injuries. Brain trauma leads to diffuse axonal injury (DAI) secondary to abrupt acceleration/deceleration and/or rotational/vibrational forces, which cause axonal shearing.[3,4] It has been postulated that DAI plays a key role in persistent neurological and cognitive impairments observed after TBI, owing to disruption in connectivity of different regions in the brain. Several autopsy studies.[5,6] and animal studies[7] have documented ultrastructural axonal damage following TBI. Unfortunately, DAI is often missed or underestimated on CT scans and conventional MR protocols. Hence, there is inadequate correlation between the clinical outcome and the scan results.[8] Thus, a better method to assess the integrity of these white matter tracts is imperative for a better understanding of the extent of injury and for prognostication.
Diffusion tensor imaging (DTI) provides a window into the microstructural white matter integrity. This technique is sensitive to the diffusion of water molecules in the biological tissues. When the diffusion is uniform in all directions, it is termed as ‘isotropic’. In body tissues, such as the axons, diffusion is highly directional, owing to the natural barriers (cell membranes, myelin, and adjacent axons), and therefore, become ‘anisotropic’.[9] With axonal damage or disruption of the myelin integrity, there is loss of anisotropy. This can be detected by several indices, the most commonly used ones being - fractional anisotropy (FA), which depicts the degree of alignment of the white matter tracts, and mean diffusivity (MD), which represents the degree of overall restrictions to water diffusion.[10]
Maps depicting changes in anisotropy have been useful in detecting the sites of injury. However, they may not offer adequate insight into the specific pathways that have been disrupted. This can be effectively performed by DTI tractography (DTT), which provides a three-dimensional representation of the white matter tracts. The objective of the present study was to assess the usefulness of DTT, in detecting white matter changes at an early stage, which may be potentially reversible by therapeutic maneuvers. The association of these changes with the severity of post-concussive symptoms was also assessed.
Materials and Methods
Patients and control subjects
Twenty-seven patients with mild-to-moderate TBI were initially recruited in this prospective longitudinal (short-term) cohort study. A set of 12 normal, healthy controls with no prior history of TBI were also scanned using the same MR protocol. This cohort was matched for age and sex, and was subject to the same exclusion criteria as the patients [Table 1]. All patients were analyzed within the first seven days of injury. Similarity of the patient and control groups was confirmed with χ2 (sex) and Student t (age) tests. Inclusion criteria for TBI cases included patients aged between 16 and 65 years, who sustained mild or moderate traumatic brain injury within seven days of the scan. These patients were referred by a neurosurgeon, based on a detailed history and clinical examination. The exclusion criteria were pre-existing neurological disorders or a prior history of TBI, presence of focal lesions with the volume greater than 10 ml, visible on a cranial CT (including contusion, extra-axial hematoma, and/or intraparenchymal hemorrhages), neurological/psychiatric conditions that could result in abnormal MRI findings (i.e. prior brain tumor, Alzheimer's disease/mild cognitive impairment, human immunodeficiency virus (HIV) encephalopathy, schizophrenia, etc.), and severe TBI, with a Glasgow Coma Scale (GCS) <9. The criteria used for defining mild TBI, were in accordance with those postulated by the American Congress of Rehabilitation Medicine, wherein, at least one of the following conditions was met, namely: (1) Any period of loss of consciousness; (2) any loss of memory for events immediately before or after the accident; (3) any alteration in the mental state at the time of the accident (e.g. feeling dazed, disoriented or confused), and (4) focal neurological deficit(s) that may or may not be transient, but where the severity of the injury did not exceed the following: Loss of consciousness for less than 30 minutes, an initial Glasgow Coma Scale (GCS) of 13-15, and post-traumatic amnesia (PTA) of less than 24 hours.[11] The criteria for moderate brain injury were similar to those of mild TBI except for a GCS score of 9-12.[12]
Table 1.
Demographic characteristics of participants

In the group of patients with TBI, the severity of post-concussive symptoms was assessed using the Rivermead Postconcussion Symptoms Questionnaire (RPSQ).[13] These patients were followed up for a period of six months, and interviewed at the end of six months for post-concussive symptoms using this questionnaire, which was a simple and reliable measure of outcome after head injury. This questionnaire was administered by a rater who was blinded to the DTI results. The patients were questioned about 16 symptoms, which were commonly encountered after head injury, on a five-point scale, from 0 to 4. The scoring was based on the severity of the symptoms as perceived by the patient, in comparison to his/her pre-injury (baseline) level. The scoring was done after six months, because any post-concussive symptoms in the acute phase could have been partly attributable to the direct effects of trauma (e.g. scalp injury producing headache) and also secondary to the mental and emotional strain induced by the accident. Of the 27 patients, two had a suboptimal DTI study and one had a large intraparenchymal hemorrhagic contusion, while two had large extradural/subdural hematomas. They were, therefore, excluded from the study. Three patients were excluded, as they could not be followed up at six months for assessment of the outcome. A total of 19 patients (13 with mild and six with moderate TBI) were finally included. The mean and standard deviation of the TBI and the control subjects’ ages were 27 ± 8.2 (range 18-50 years) and 28 ± 6.3 years (range 20-45 years), respectively. Written informed consent was obtained from each subject. The Institutional Review Board approved of the study.
Image acquisition
The MRI data was collected on a Siemens Skyra 3.0T scanner using a 20-channel phased array head coil. The MR protocol was as follows: (a)Three-plane localizer imaging (repetition time in milliseconds (msec) [TR]/echo time in msec [TE]: 8.6/4.0), (b)Fluid-attenuated inversion recovery (FLAIR) imaging (TR/TE/inversion time in msec [TI]: 9000/81/2500), (c) axial fast spin-echo T2-weighted (5600/100), (d) axial T1-weighted inversion recovery (2000/12/859), (e) sagittal T2-weighted spin echo imaging (5401/87), and (f) Susceptibility weighted imaging [SWI] (28/20). This was followed by the DTI sequence, which is detailed subsequently in Table 2.
Table 2.
Summary of MR protocol
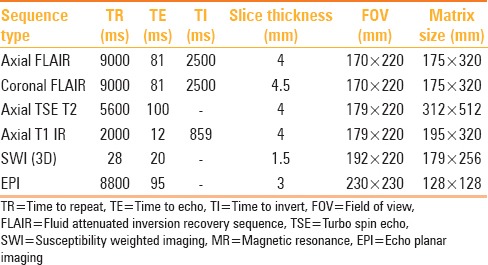
Diffusion tensor imaging protocol
For DTI, a single shot echo planar (EPI) DTI sequence was used, with a total scan time of approximately seven minutes. The image was acquired in the axial plane with an image matrix of 128 × 128 and slice thickness of 3 mm, with no inter-slice gap, and a field of view (FOV) of 230 × 230 mm. Thirty isotropic gradient directions with b = 1000 s/mm2 and one b = 0 acquisition were used. To enhance the signal-to-noise ratio and reduce the phase fluctuations, magnitude-constructed images were repeated (averages = 2) and temporally averaged.
Post-processing
Diffusion tensor imaging tractography (DTT) was performed to assess the diffusion tensor properties of the major white matter tracts. The DTI data was distortion-corrected for shear, scale, rotation, and translation using the Automated Image and Registration (AIR) package. The distortion-corrected data was then interpolated to attain isotropic voxels and decoded to obtain the tensor field for each voxel. The tensor field data was then diagonalized using the analytical diagonalization method to obtain the eigen values (λ1, λ2, and λ3) and three orthonormal eigen vectors (e1, e2, and e3). The tensor field data was then used to compute the DTI metrics (FA and MD) using the standard equations.
Subsequently the white matter (WM) segmentation and DT tractography were done using the in-house JAVA-based software, which used the principal eigenvector field segmentation (PEVFS) methodology.[14] The PEVFS helped determine the most promising seed point from where fiber tracking ought to be initiated. In this method, the DTI-derived FA and the principal eigenvector e1-field maps were utilized to construct a pre-segmented map - the stable fiber mass (SFM). The SFM is re-sliced into axial, coronal, and sagittal maps, which are color-coded into eight natural classes. These maps contain the signature segments of different fiber tracts. By a single mouse click into any pixel within this signature segment, the boundary of the region-of-interest (ROI) is automatically drawn and the fiber is tracked along successive slices using the FACT algorithm [Figure 1]. The ROI selection thus becomes user-independent, unlike in the classical DTT approaches, wherein, the ROI for fiber propagation is manually drawn. The DTI measures were calculated for the entire fiber. An FA threshold of 0.15 was used for fiber tracking. Major white-matter fiber tracts, including the corpus callosum (CC), superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF), corticospinal tracts (CST), cingulum (CNG), inferior fronto-occipital fasciculus (IFO), superior cerebellar peduncle (SCP), middle cerebellar peduncle (MCP), inferior cerebellar peduncle (ICP), anterior thalamic radiations (ATR), superior thalamic radiations (STR), posterior thalamic radiations (PTR), and uncinate fasciculus and fornix were generated and quantified using this JAVA-based software.
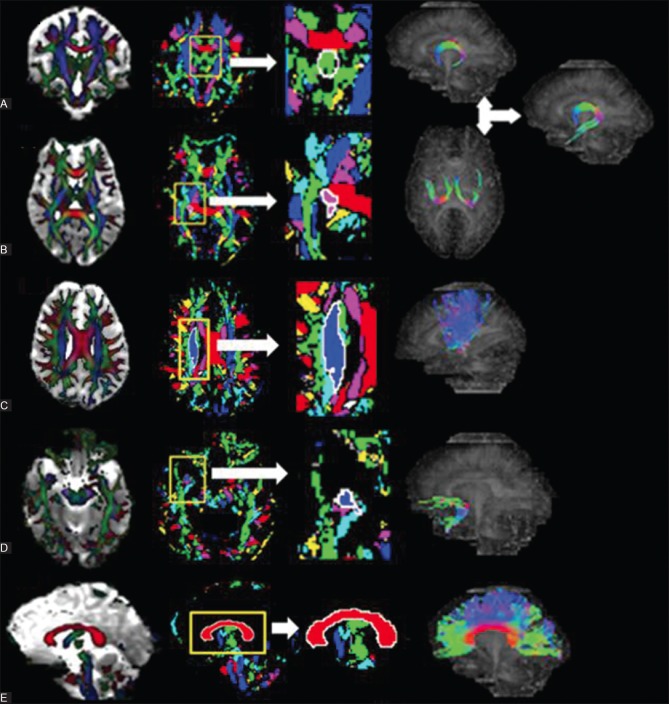
Figure 1 (A and B).
A schematic presentation of the fiber tract reconstruction for the fornix (C) superior thalamic radiation (D) uncinate fasciculus and (E) corpus callosum, in the study participants. Column (i) shows the FA map overlaid on the MD map. For each tract, the section perpendicular to the tract was taken. Column (ii) shows the stable fiber mass map, which contains the signature segments of each tract. Column (iii) is an enlarged view of the signature segment. By clicking into it, the boundary of the ROI used for fiber generation is automatically drawn (seen as white line). Column (iv) shows the respective tract generated by the FACT algorithm (Fornix is constructed by placing three ROIs, one on the coronal section (A-i) and two bilateral ROIs on the axial (B-i) sections, and finally adding the fibers using the OR function)
Statistical analysis
The differences in participant demographics between patients and controls were tested for statistical significance using the Student's t test for age and chi-square test for gender. An independent sample t test was performed to see the difference in FA and MD values between the controls and patient group. A P ≤ 0.05 was considered to be significant. Bivariate correlation was used to analyze the correlation of these indices with the severity of symptoms, as determined by the RPSQ. The analysis of variance (ANOVA), with post-hoc Bonferroni correction, was used to assess the differences in the FA and MD values between the three groups of mild TBI, moderate TBI, and the control group. The independent sample t test was performed to see the difference in the RPSQ scores between the mild and moderate TBI groups. This test was also used to assess if there was any significant difference in the RPSQ scores of mild TBI patients, with and without conventional MRI findings. All statistical analyses were performed using the SPSS (version 16.0, SPSS Inc, Chicago, IL, USA) statistical software.
Results
Of the 19 patients, 11 had no abnormality on the conventional MR sequences. Of the eight patients with conventional MR findings, four patients had sustained mild TBI, while the other four had moderate TBI. The conventional MRI revealed small intraparenchymal hemorrhagic contusions in two patients with mild TBI. The other two patients in this subgroup had multiple hemorrhagic foci (best seen on SWI sequences) consistent with DAI. Of the four patients with moderate TBI, one patient had a small intraparenchymal hemorrhagic contusion, while the remaining three patients had changes of DAI along with hemorrhagic contusions, of which two cases had additional small extra-axial collections. There was no statistically significant difference between the patients and control with regard to age and sex. Details of patient and control demographics are summarized in Table 1.
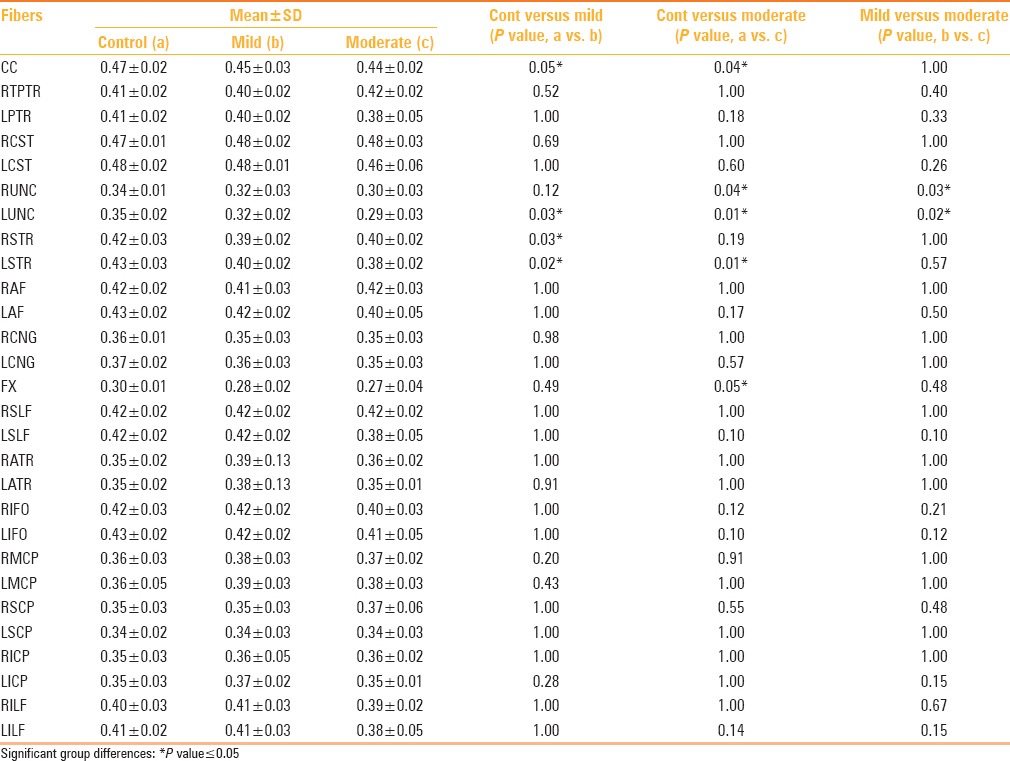
The patients displayed a significant reduction in FA compared to controls (P < 0.05) in several tracts notably the corpus callosum, fornix, bilateral uncinate fascicule, and bilateral superior thalamic radiations [Figure 2 and Table 3]. When the mild and moderate TBI groups were considered separately, it was observed that in the former group, significant differences were observed in the corpus callosum, left uncinate fasciculus, and bilateral superior thalamic radiations, compared to the controls [Table 4]. Likewise, the moderate TBI group showed significant differences from the controls in the corpus callosum, fornix, bilateral uncinate fascicule, and left superior thalamic radiations. Between the mild and moderate TBI groups, statistically significant differences in FA values were noted in the bilateral uncinate fasciculus [Table 4].
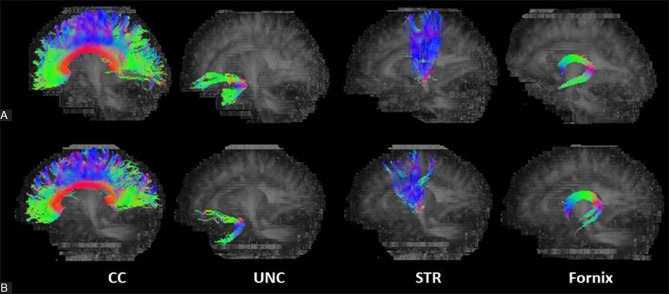
Figure 2 (A and B).
Representative fiber tractography of the corpus callosum (CC), uncinate fasciculus (UNC), superior thalamic radiation (STR), and fornix, in the controls and patients
Table 3.
Tractography differences in fractional anisotropy between TBI cases and controls
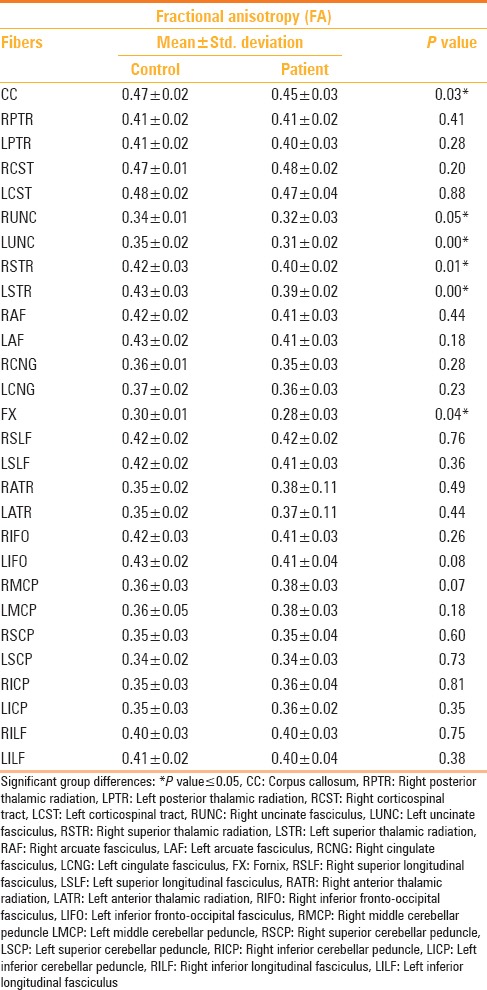
Table 4.
Tractography differences in fractional anisotropy between the three groups of control (a), mild TBI (b) and moderate TBI (c)
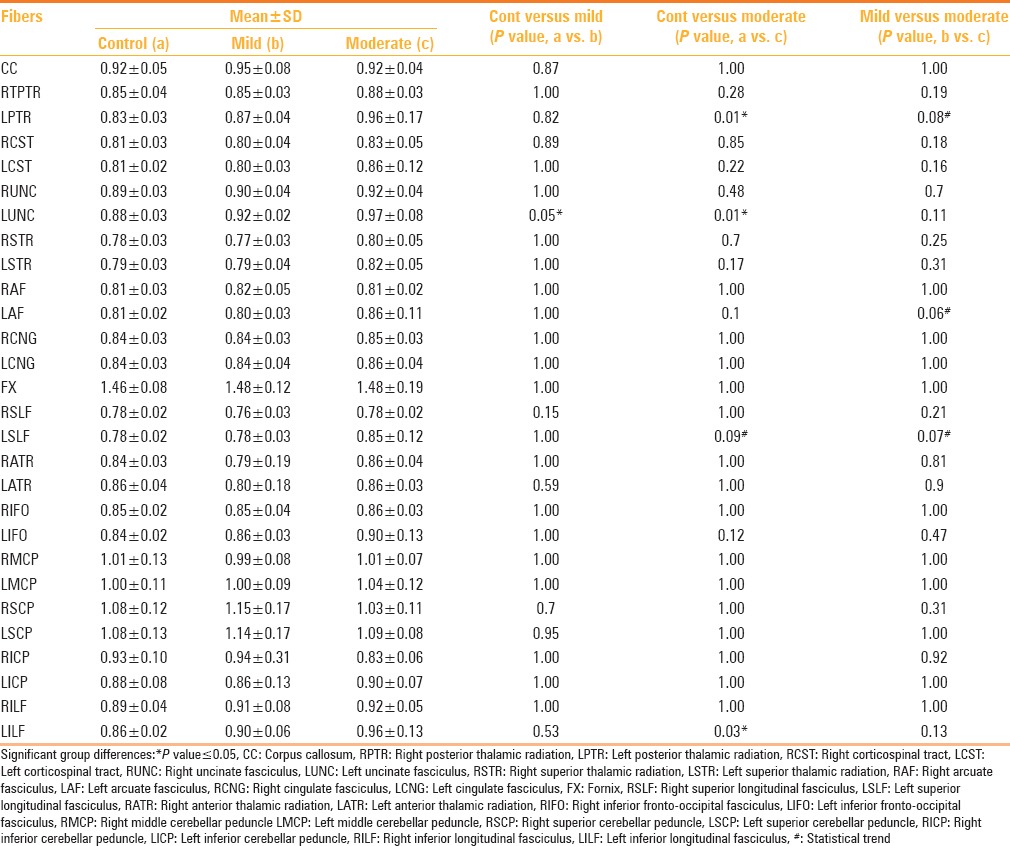
Changes in MD were statistically significant in the left uncinate fasciculus, left inferior longitudinal fasciculus, and left posterior thalamic radiation [Table 5]. When the mild TBI group was considered separately, a statistically significant difference in MD value was observed compared to the controls in the left uncinate fasciculus. A significant difference in the MD values between the moderate TBI and control groups was noted in the left uncinate fasciculus, left posterior thalamic radiation, and left inferior longitudinal fasciculus, with a statistical trend noted in the left superior longitudinal fasciculus. Between the mild and moderate TBI groups, there were no statistically significant (P < 0.05) differences in the MD values in any of the tracts, however, the MD values in the left posterior thalamic radiation, left superior longitudinal fasciculus, and left arcuate fasciculus showed a statistical trend [Table 6].
Table 5.
Tractography differences in mean diffusivity between TBI cases and controls
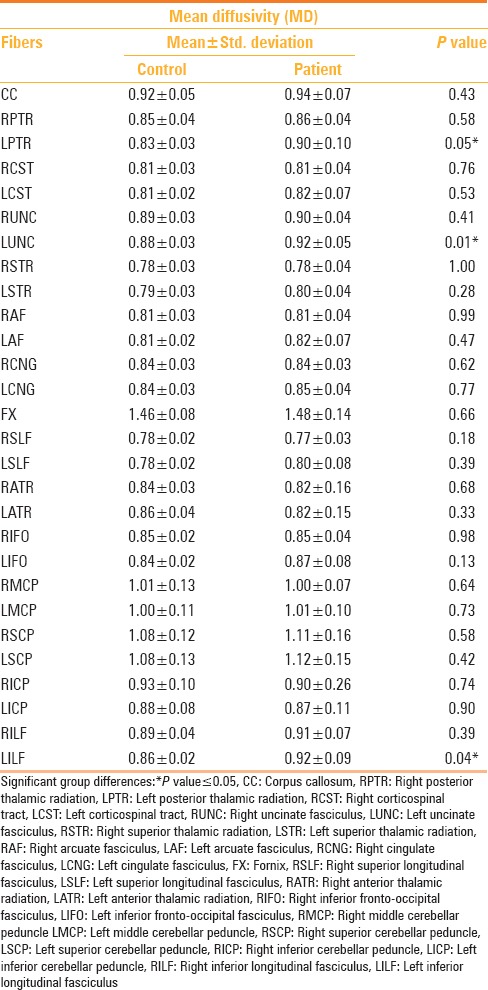
Table 6.
Tractography differences in mean diffusivity between the three groups of control (a), mild TBI (b) and moderate TBI (c)
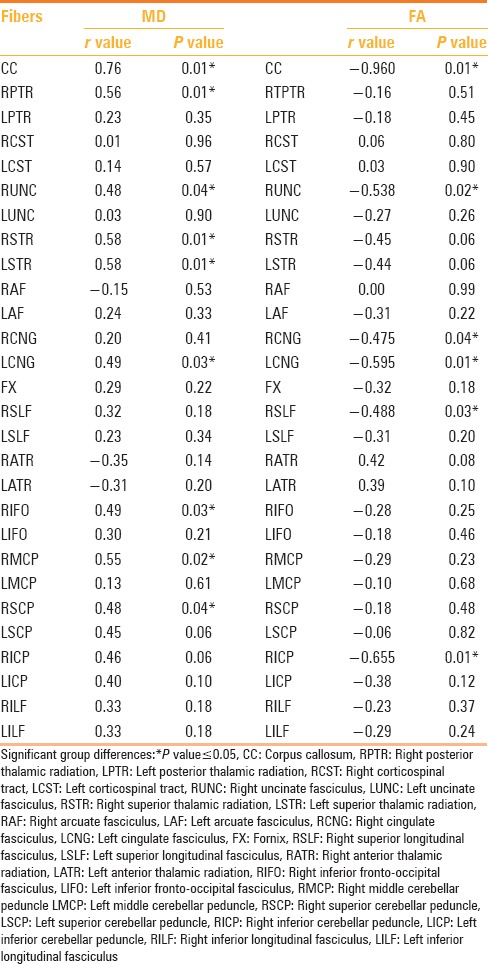
Bivariate correlation analysis in the 19 patients showed a strong positive correlation (P ≤ 0.05) between the FA values in the corpus callosum, right uncinate fasciculus, bilateral cingulum, right superior longitudinal fasciculus, and right inferior cerebellar peduncle, and the RPSQ scores. Likewise, a strong correlation between MD scores and the RPSQ scores (P ≤ 0.05) was observed in several white matter tracts, namely corpus callosum, right uncinate fasciculus, left cingulum, bilateral superior thalamic radiation, right posterior thalamic radiation, right inferior fronto-occipital fasciculus, right middle, and the superior cerebellar peduncle [Figures 3 and 4, Table 7]. There was no statistically significant difference in the RPSQ scores between the mild and moderate TBI group (P-value 0.92) and neither was there any statistically significant difference in the RPSQ scores between the TBI patients with mild abnormalities on the conventional MRI scans, and those without (P-value 0.75).
Figure 3.
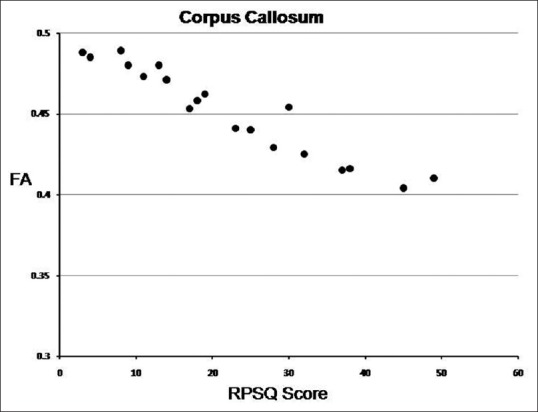
Scatter plot showing Rivermead post-concussion scores versus FA values of CC in 19 TBI subjects
Figure 4.
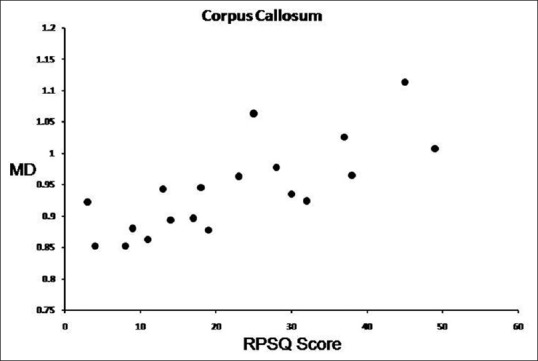
Scatter plot showing Rivermead post-concussion scores versus MD values of CC in 19 TBI subjects
Table 7.
Bivariate correlation analysis between tractography indices (FA and MD) and RPSQ scores in patients
Discussion
Diffuse axonal injury is an important mechanism in the pathophysiological changes underlying TBI. This occurs due to rapid acceleration/deceleration and rotation of brain structures of varying density.[3] Unfortunately, it is often undetected or underestimated on conventional CT and MR scans. This article presents an elegant means of localizing and quantifying the changes in white matter tracts, over and above that depicted on conventional MRI scans. The two most commonly used parameters for identification and quantification of the microstructural white matter integrity are FA and MD. FA is a measure of the directionality of water diffusion along the axon, relative to its perpendicular axis, with scores ranging from 0 to 1.[10] It is a sensitive parameter to detect injury, as the diffusion is more anisotropic in intact, compared to injured or compromised white matter.[15] MD is a measure of the average diffusion in all directions.[16]
There are several approaches for assessment of white-matter integrity on DTI. Conventional region of interest (ROI) analysis involves selecting an ROI within a specific anatomic region, and comparing the DTI parameters obtained between cases and controls.[17] However, it is time-consuming, calls for significant operator expertise in ROI placement and is restricted to those areas which are easily identifiable or thought to be the most significant. Hence, comparison of multiple brain regions across large groups is cumbersome.[18] Whole brain histogram analysis is a simple, automated and sensitive approach, which gives a quantitative global assessment of the overall extent of injury.[17] However, it does not provide information on individual tracts.
Voxel-based analysis (VBA) is another objective, automated method, which provides a voxel-by-voxel comparison of the FA values between two groups of brains. It helps identify sites of injury to specific the white matter regions in the entire brain, without any prior assumptions of the areas likely to be affected.[19] Although it provides a good overview of the regional changes in white matter integrity, it lacks information on the specific tracts involved. Tract-based spatial statistics (TBSS) is also an automated process that enables a voxel-wise comparison of FA values without the need for a pre-specified ROI. The FA image of all the subjects is aligned to a common target. A skeletonized mean-FA image is then generated. Each subject's aligned FA image is then projected onto the skeleton, followed by a voxel-wise statistical evaluation across subjects.[20]
Diffusion tensor tractography is a novel method, which provides an unprecedented three-dimensional visualization of white matter tracts. Tracking is initiated from a start location (or seed point) in a direction that is determined by the principle eigenvector at that point. White matter tracts are generated by identifying the neighboring voxels, which attain a pre-determined FA threshold, and lie within the projected path of the area of interest.[21] This procedure enables localization of damage to the specific tracts, thus enabling the correlation of morphological alterations with functional derangements. A major challenge is the correct placement of seed ROIs within the trajectory of a particular tract, which requires considerable operator expertise. The present study utilizes a novel, automated tractography method that circumvents the potential error that could arise subsequent to incorrect placement of seed ROIs.[14]
Prior studies have been undertaken to assess white matter changes in the DAI using the above methods. ROI analysis performed in the acute phase, revealed reduced FA in the corpus callosum, centrum semiovale, and internal and external capsules, with increased MD in the internal capsule and corpus callosum.[15,22] Another study incorporating the entire brain voxel-wise analysis in the acute phase of mild traumatic brain injury demonstrated clusters of low FA in the dorsolateral prefrontal cortex.[23] Several clusters also demonstrated high MD values. Another voxel-based comparison study by Rutgers et al.,[24] observed significantly raised ADC and decreased FA within the genu of the corpus callosum in the acute phase. DTI has been found to be useful in demonstrating axonopathy in the corpus callosum and peri-ventricular white matter, not just in the acute stage, but on follow-up as well, in regions of normal-appearing white matter on conventional MRI.[25]
Most previous studies have reported a decrease in FA and an increase in MD in the affected areas in the acute phase.[15,22,23,26] Our findings also corroborate with these studies, with a significant reduction in FA in several tracts, notably the corpus callosum, fornix, bilateral uncinate fascicule, and bilateral superior thalamic radiations, and increase in MD in the left uncinate fasciculus, left inferior longitudinal fasciculus and left posterior thalamic radiation. However, there have been reports with findings contradictory to the above studies, which show increased FA, with or without a decrease in MD in the acute phase, both in mild as well as severe TBI.[27,28,29] This has been explained by the authors as a transient phenomenon, likely owing to the axonal swelling, with subsequent restriction of diffusion in the intercellular space. This explanation needs to be validated on a larger number of subjects. The underlying cellular events, which can lead to changes in DTI indices are difficult to elucidate, and can possibly be clarified only in those cases where histology can be performed.[30]
Some of the differences in the observations of various studies may be due to differences in the nature of injury, age of the patient, timing of the scan, and methodology of the study. Errors in normalization, co-registration, and spatial smoothing can lead to discrepant findings. The signal-to-noise ratio of the raw data and limited spatial resolution of the EPI images may also influence the accuracy of the DTI indices. Accurate placement of ROIs is a labor-intensive task that requires considerable expertise, thus limiting the comparison of multiple brain regions across large groups.[18]
In comparison to the methodology in the studies mentioned earlier, DTT has the advantage of delineating the entire length of the white matter pathways through fiber propagation algorithms. Using DTT-based analysis in the acute phase, Wang et al.[21] has demonstrated a significant reduction in FA in the fornix, corpus callosum, and peduncular projections, which correlates well with the functional outcome as determined by the Glasgow outcome Scale-Extended (GOSE) scores. One of the prerequisites for tractography is the need for the accurate placement of seed ROIs to generate the trajectory of the white matter tract, which is time-consuming and requires considerable expertise. The present study utilizes a novel, carefully designed method for fiber tractography using the FACT algorithm, namely, the principal eigenvector field segmentation (PEVFS) methodology, which has proven to be a user-independent, robust, efficient, and reproducible method.[14] It obviates the potential error that could occur owing to incorrect placement of seed ROIs along the trajectory of the white matter tract. The results of our study are in concordance with those in several previous studies, with significantly reduced FA in the corpus callosum, fornix, bilateral uncinate fascicule, and bilateral superior thalamic radiations. Changes in MD have been statistically significant in the left uncinate fasciculus, left inferior longitudinal fasciculus, and left posterior thalamic radiation in our study.
TBI can lead to impairment of the neurocognitive function, particularly in motor skills, attention, memory, processing speed, and executive function.[31] Identification and quantification of the extent of disruption to the white matter tracts, on DTI, can enable the correlation of these changes with the neurocognitive scores, owing to the fact that individual pathways are correlated to specific brain functions. For instance, changes in the hippocampal/fornix pathway (HC/FX), within a month of mild-to-moderate traumatic head injury, have been found to be associated with memory impairment.[32] Likewise, low FA values in the dorsolateral prefrontal cortex, in the acute phase, correlated well with a poorer executive function, as seen in the study by Lipton et al.[23] Kraus and Niogi et al.[33,34] have found an association between FA and cognitive performance, which is most pronounced in higher grades of injury. Using diffusion tensor tractography, Pal et al. have found a good correlation between the DTI indices in several white matter tracts, and neuropsychological test scores in patients with moderate frontal lobe injury.[35] Another study by Kumar et al. on patients with mild and moderate traumatic brain injury has shown that although DTI abnormalities in the regions of the corpus callosum are higher in patients with moderate compared to mild TBI, in moderate TBI, significantly decreased FA is found only in the genu, compared to that in mild TBI. Furthermore, this is associated with a poor neuropsychological outcome six months post injury. Moderate TBI shows poorer NPT scores compared to mild TBI, but this does not reach statistical significance.[36] Although several studies have established a correlation between DTI indices and cognitive measures, others have not.[37,38] The variability in results may be due to the variability in patient characteristics, such as, the severity of injury, time since injury, nature of damage, for example, edema, hematoma, increased intracranial pressure, and so on.[30] Also variation in the methodology for establishing indices of the brain injury may also play a significant role.
In addition to the sometimes subtle cognitive deficits that get unearthed on detailed neuropsychological testing, patients who have sustained TBI, often complain of clinically overt and persistent post-concussion symptoms, such as, headache, fatigue, sleep disturbances, dizziness, and the like. This may significantly impact their performance and overall quality of life. Two clinical criteria – the International Classification of Diseases (ICD-10) and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) - are often used to define the syndrome.[39] The severity of post-concussion symptoms is commonly assessed by the Rivermead Post-concussion Symptoms Questionnaire (RPSQ).[40,41] Very few studies have attempted to assess the correlation between the microstructural white matter changes detected on DTI and the extent of post-concussive symptoms. A previous study by Smits et al.,[42] has found a correlation between the severity of the post-concussive syndrome and the reduction in white matter integrity, as observed in patients with mild TBI, using the tract-based spatial statistics (TBSS) approach. To the best of our knowledge, this is the first study that utilizes the diffusion tensor tractography approach for assessing the correlation between the microstructural white matter changes underlying mild-moderate TBI in the acute phase, with severity of post-concussive symptoms, making it potentially useful in predicting the outcome.
Our study had certain limitations. The sample size was relatively small and the patients were evaluated only on DTI during the acute phase after injury. Longitudinal studies would be helpful, to delineate the changes in DTI indices with time and correlate them with the long-term outcome. Although our methodology for DTT has proven to be robust and reliable, the possibility of multiple crossing fibers in a voxel, with possible damage to a single fiber, can lead to potential errors.
Conclusion
The current study shows that DTI using tractography is a useful objective measure to document injury in the acute phase of mild-moderate TBI. Furthermore, the significant correlation observed between FA and MD indices in several white matter tracts and the severity of post-concussive symptoms can make it a useful predictor of the functional outcome.
Acknowledgement
We gratefully acknowledge the invaluable contributions of Dr. R K Gupta and Dr. R K Rathore who have devised the principal eigenvector field segmentation methodology for diffusion tensor tractography, and for their constant support and guidance.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Bazarian JJ, Wong T, Harris M, Leahey N, Mookerjee S, Dombovy M. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj. 1999;13:173–89. doi: 10.1080/026990599121692. [DOI] [PubMed] [Google Scholar]
- 2.Haas DC. Chronic post-traumatic headaches classified and compared with natural headaches. Cephalalgia. 1996;16:486–93. doi: 10.1046/j.1468-2982.1996.1607486.x. [DOI] [PubMed] [Google Scholar]
- 3.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: An analysis of 45 cases. Ann Neurol. 1982;12:557–63. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 4.Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25:370–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Bigler ED. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J Int Neuropsychol Soc. 2004;10:794–806. doi: 10.1017/S1355617704105146. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheimer DR. Microscopic lesions in the brain following head injury. J Neurol Neurosurg Psychiatry. 1968;31:299–306. doi: 10.1136/jnnp.31.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Povlishock JT. Traumatically induced axonal injury: Pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- 8.Kelly AB, Zimmerman RD, Snow RB, Gandy SE, Heier LA, Deck MD. Head trauma: Comparison of MR and CT--experience in 100 patients. Am J Neuroradiol. 1988;9:699–708. [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bihan D. Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed. 1995;8:375–86. doi: 10.1002/nbm.1940080711. [DOI] [PubMed] [Google Scholar]
- 10.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 11.Kay T, Harrington DE, Adams R, Andersen T, Berrol S, Cicerone K, et al. Definitions of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8:86–7. [Google Scholar]
- 12.MacKenzie JD, Siddiqi F, Babb JS, Bagley LJ, Mannon LJ, Sinson GP, et al. Grossman RI. Brain atrophy in mild or moderate traumatic brain injury: A longitudinal quantitative analysis. AJNR Am J Neuroradiol. 2002;23:1509–15. [PMC free article] [PubMed] [Google Scholar]
- 13.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The rivermead post concussion symptoms questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242:587–92. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 14.Rathore RK, Gupta RK, Agarwal S, Trivedi R, Tripathi RP, Awasthi R. Principal eigenvector field segmentation for reproducible diffusion tensor tractography of white matter structures. Magn Reson Imaging. 2011;29:1088–100. doi: 10.1016/j.mri.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Inglese M, Markani S, Johnson G, Cohen BA, Silver JA, Gonen O, et al. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. J Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 16.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson RR, Meda SA, Vasudevan S, Kou Z, Govindarajan KA, Hanks RA, et al. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J Neurotrauma. 2007;24:446–59. doi: 10.1089/neu.2006.0153. [DOI] [PubMed] [Google Scholar]
- 18.Porter EJ, Counsell SJ, Edwards AD, Allsop J, Azzopardi D. Tract-based spatial statistics of magnetic resonance images to assess disease and treatment effects in perinatal asphyxial encephalopathy. Pediatr Res. 2010;68:205–9. doi: 10.1203/PDR.0b013e3181e9f1ba. [DOI] [PubMed] [Google Scholar]
- 19.Marquez de la Plata CD, Yang FG, Wang JY, Krishnan K, Bakhadirov K, Paliotta C, et al. Diffusion tensor imaging biomarkers for traumatic axonal injury: Analysis of three analytic methods. J Int Neuropsychol Soc. 2011;17:24–35. doi: 10.1017/S1355617710001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Wang JY, Bakhadirov K, Devous MD, Sr, Abdi H, McColl R, Moore C, et al. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol. 2008;65:619–26. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- 22.Arfanakis K, Haughton V, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- 23.Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252:816–24. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- 24.Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: A diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008;29:514–9. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, et al. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma. 2009;26:481–95. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- 26.Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22:115–22. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- 27.Field AS, Hasan K, Jellison BJ, Arfanakis K, Alexander AL. Diffusion tensor imaging in an infant with traumatic brain swelling. AJNR Am J Neuroradiol. 2003;24:1461–4. [PMC free article] [PubMed] [Google Scholar]
- 28.Bazarian JJ, Zhong J, Blythe B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: A pilot study. J Neurotrauma. 2007;24:1447–59. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 29.Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–55. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 30.Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, et al. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42:503–14. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy MR, Wozniak JR, Muetzel RL, Mueller BA, Chiou HH, Pantekoek K, et al. White matter and neurocognitive changes in adults with chronic traumatic brain injury. J Int Neuropsychol Soc. 2009;15:130–6. doi: 10.1017/S1355617708090024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh M, Jeong J, Hwang D, Sungkarat W, Gruen P. Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn Reson Imaging. 2010;28:22–40. doi: 10.1016/j.mri.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain. 2007;130:2508–19. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 34.Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29:967–73. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pal D, Gupta RK, Agarwal S, Yadav A, Ojha BK, Awasthi A, et al. Diffusion tensor tractography indices in patients with frontal lobe injury and its correlation with neuropsychological tests. Clin Neurol Neurosurg. 2012;114:564–71. doi: 10.1016/j.clineuro.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Kumar R, Gupta RK, Husain M, Chaudhry C, Srivastava A, Saksena S, et al. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: Its correlation with neuropsychometric tests. Brain Inj. 2009;23:675–85. doi: 10.1080/02699050903014915. [DOI] [PubMed] [Google Scholar]
- 37.Sherer M, Stouter J, Hart T, Nakase-Richardson R, Olivier J, Manning E, et al. Computed tomography findings and early cognitive outcome after traumatic brain injury. Brain Inj. 2006;20:997–1005. doi: 10.1080/02699050600677055. [DOI] [PubMed] [Google Scholar]
- 38.Yount R, Raschke KA, Biru M, Tate DF, Miller MJ, Abildskov T, et al. Traumatic brain injury and atrophy of the cingulate gyrus. J Neuropsychiatry Clin Neurosci. 2002;14:416–23. doi: 10.1176/jnp.14.4.416. [DOI] [PubMed] [Google Scholar]
- 39.McCauley SR, Boake C, Pedroza C, Brown SA, Levin HS, Goodman HS, et al. Postconcussional disorder: Are the DSM-IV criteria an improvement over the ICD-10? J Nerv Ment Dis. 2005;193:540–50. doi: 10.1097/01.nmd.0000172592.05801.71. [DOI] [PubMed] [Google Scholar]
- 40.Messé A, Caplain S, Pélégrini-Issac M, Blancho S, Lévy R, Aghakhani N, et al. Specific and evolving resting-state network alterations in post-concussion syndrome following mild traumatic brain injury. PLoS One. 2013;8:e65470. doi: 10.1371/journal.pone.0065470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall EC, Lund E, Brown D, Murdock KR, Gettings L, Scalea TM, et al. How are you really feeling? A prospective evaluation of cognitive function following trauma. J Trauma Acute Care Surg. 2014;76:859–65. doi: 10.1097/TA.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 42.Smits M, Houston GC, Dippel DW, Wielopolski PA, Vernooij MW, Koudstaal PJ, et al. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology. 2011;53:553–63. doi: 10.1007/s00234-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]