Summary
Mice in which the genes for growth hormone (GH) or GH receptor (GHR −/−) are disrupted from conception are dwarfs, possess low levels of IGF‐1 and insulin, have low rates of cancer and diabetes, and are extremely long‐lived. Median longevity is also increased in mice with deletion of hypothalamic GH‐releasing hormone (GHRH), which leads to isolated GH deficiency. The remarkable extension of longevity in hypopituitary Ames dwarf mice can be reversed by a 6‐week course of GH injections started at the age of 2 weeks. Here, we demonstrate that mutations that interfere with GH production or response, in the Snell dwarf, Ames dwarf, or GHR −/− mice lead to reduced formation of both orexigenic agouti‐related peptide (AgRP) and anorexigenic proopiomelanocortin (POMC) projections to the main hypothalamic projection areas: the arcuate nucleus (ARH), paraventricular nucleus (PVH), and dorsomedial nucleus (DMH). These mutations also reduce hypothalamic inflammation in 18‐month‐old mice. GH injections, between 2 and 8 weeks of age, reversed both effects in Ames dwarf mice. Disruption of GHR specifically in liver (LiGHRKO), a mutation that reduces circulating IGF‐1 but does not lead to lifespan extension, had no effect on hypothalamic projections or inflammation, suggesting an effect of GH, rather than peripheral IGF‐1, on hypothalamic development. Hypothalamic leptin signaling, as monitored by induction of pStat3, is not impaired by GHR deficiency. Together, these results suggest that early‐life disruption of GH signaling produces long‐term hypothalamic changes that may contribute to the longevity of GH‐deficient and GH‐resistant mice.
Keywords: aging, dwarf mice, growth hormone, hypothalamus, inflammation, longevity
Abbreviations
- CR
calorie restriction
- GH
growth hormone
- GHR
growth hormone receptor
- CNS
central nervous system
- LepR‐b
leptin receptor
- Stat3
signal transducer and activator of transcription 3
- POMC
proopiomelanocortin
- ARH
arcuate nucleus of the hypothalamus
Among genetically altered long‐lived mice, animals with growth hormone (GH) deficiency, such as hypopituitary Ames (df/df) and Snell (dw/dw) dwarf mice, or GH insensitivity, such as the GH receptor (GHR) gene‐disrupted mouse (GHR−/−), stand out by virtue of the magnitude of life extension and consistency of the findings across sexes, genetic background, and diets (Bartke & Brown‐Borg, 2004; Bartke, 2008). These remarkably long‐lived animals are characterized by reduced postnatal growth rate, delayed development, and reduced adult body size (Junnila et al., 2013). Mice lacking Ghrh, or Ghrhr (Flurkey et al., 2001; Sun et al., 2013), are also long‐lived, as are mice lacking PAPP‐A, a protease specific for IGF‐1‐binding proteins (Conover & Bale, 2007), further implicating GH and/or IGF‐1 tonicity in the regulation of mammalian aging rate and lifespan.
Growth hormone is active in the central nervous system, influencing feeding behavior and a sense of well‐being in humans (Blissett et al., 2000). GH feeds back on the hypothalamus and regulates its own secretion (Kamegai et al., 1996). The arcuate nucleus of the hypothalamus (ARH) contains neurons that produce the anorexigenic peptide melanocyte‐stimulating hormone (MSH) and other neurons that coexpress the orexigenic peptides neuropeptide Y (NPY) and agouti‐related protein (AgRP), which regulate food intake and energy expenditure (Myers, 2004). The ARH neurons interact with pituitary cells that produce GH, through the opposing actions of somatostatin (SS) and GH‐releasing hormone (GHRH), derived from the periventricular nucleus and ARH of the hypothalamus (Muller et al., 1999). Systemic administration of GH induces expression of the c‐fos gene, a marker of neuronal activity, in the hypothalamic NPY and somatostatin neurons (Kamegai et al., 1994). The majority of NPY mRNA‐containing cells in the hypothalamic arcuate nucleus express the GHR gene, and NPY mRNA expression is downregulated in GHR−/− mice (Peng et al., 2001), suggesting that NPY neurons in the ARH mediate the feedback effect of GH on the hypothalamus.
There is growing evidence that the first few weeks of life may be a period in which GH action can influence the course of aging and that the time window for this developmental programming of aging may be relatively short (Bartke et al., 2013). Ames dwarf mice injected with GH between 2 and 8 weeks of age do not live longer than littermate control mice (Panici et al., 2010), suggesting that the extended lifespan of Ames dwarf mice depends upon lower availability of GH during the first few weeks of life, rather than on the level of GH action after the 8th week of life. Skin‐derived fibroblasts from GH‐injected dwarf mice also failed to show the enhanced resistance to lethal stresses observed in cells from uninjected dwarf mice (Panici et al., 2010). In contrast, injections of GH starting at 4 weeks of age did not reduce extended lifespan in Snell dwarf (Vergara et al., 2004) or in Ames dwarf mice (A. Bartke, unpublished data), suggesting that the susceptibility to GH effects on longevity may be diminished shortly after weaning. Conversely, diminution of nutrient availability and IGF‐1 levels in the first few weeks of life can lead to increased lifespan. Mice derived from cross‐fostered crowded litters (CL), in which 12 pups are present (‘CL’ mice), are longer lived than mice from cross‐fostered litters of 8 pups, showing that reduced nutrient availability in the first 3 weeks of life can itself lead to extended lifespan (Sun et al., 2009). Crowded litter mice have lower levels of IGF‐1 at weaning than controls (Sun et al., 2009), consistent with the idea that low levels of GH and/or IGF‐1 early in life may have long‐lasting benefits for health and longevity. These findings indicate that some important mechanisms of aging are programmed by endocrine signaling during the first few weeks of development.
Recent evidence has suggested that hypothalamic inflammatory pathways may influence mouse lifespan in either direction (Zhang et al., 2013). Activation of NF‐kB dependent cytokine production and glial activity increases with age in the hypothalamus, and augmentation of this effect, either genetically or by viral‐mediated gene delivery, can shorten mouse lifespan (Zhang et al., 2013). More impressively, blunting hypothalamic inflammatory signals can increase lifespan in mice (Zhang et al., 2013). Glial activity and production of mRNA for the inflammatory cytokine TNF‐α are diminished in CL mice (Sadagurski et al., 2015), consistent with the idea that lower hypothalamic inflammation may modulate aging processes and lifespan in mice.
Our previous work has shown that hypothalami of CL mice, in addition to diminished steady‐state inflammation, also differ from controls in two other ways: increased density of both orexigenic and anorexigenic neural projections and increased sensitivity to leptin (Sadagurski et al., 2015). We therefore assessed these endpoints in three varieties of long‐lived mice with diminished GH and IGF‐1 activity. In addition, we evaluated the effects of early‐life GH injection into pituitary dwarf mice and examined hypothalami in mice in which liver‐specific disruption of GHR leads to lower IGF‐1 levels but not to lifespan extension.
Results
Development of ARH projections requires GH signaling
We evaluated three aspects of hypothalamic function in long‐lived mice with GH and/or IGF‐1 defects: (i) hypothalamic axonal projections; (ii) indices of inflammatory signals, each of which we had found to be altered in long‐lived CL mice; and (iii) responses to leptin injection.
To determine whether GH‐dependent pathway leads to alterations in hypothalamic axonal projections, we analyzed the immunoreactivity of AgRP‐ and α‐MSH‐containing fibers in three main hypothalamic projection areas: the ARH, PVH (paraventricular nucleus of the hypothalamus), and DMH (dorsomedial nucleus of the hypothalamus). Because in adult animals neurons that express AgRP are restricted to NPY‐containing neurons of the ARH, AgRP‐immunoreactive (IR) fibers serve as a marker for projections from ARH NPY neurons (Bouret et al., 2008). The density of AgRP‐IR fibers was severely reduced in the PVH, DMH, and the ARH of 5‐month‐old GHR−/− mice as compared to controls (Fig. 1A). Similar reduction in immunoreactive fibers was detected in young adult Snell dwarf mice (data not shown), as well as in Ames dwarf mice (presented below). The density of α‐MSH‐IR fibers in the PVH and DMH in GHR−/− mice was also lower than in control mice (P < 0.03) (Fig. 1B). The distribution pattern of AgRP‐IR fibers and α‐MSH‐IR fibers in the PVH was similar among Snell dwarf, GHR−/−, and their respective control mice, suggesting that GH alters the density but not the pattern of innervation. It is noteworthy that this decline in neuronal projections contrasts sharply with observations in CL mice, in which the same assays showed an increase in both AgRP and α‐MSH projections (Sadagurski et al., 2015).
Figure 1.
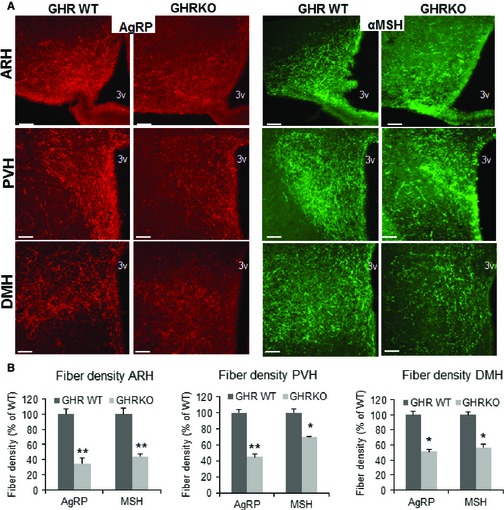
Effects of GH deficiency on hypothalamic neurocircuits. (A) Images and (B) quantification of agouti‐related peptide (AgRP) and α‐melanocyte‐stimulating hormone (α‐MSH)‐immunoreactive fibers innervating the ARH, PVH, and DMH at 6 months of age of GHR WT and GHR −/− male mice (n = 6/group). 3V, third ventricle. Scale bar: 200 μm. Error bars indicate SEM. *P < 0.05, **P < 0.001 versus GHR WT.
We also evaluated the effect of GH signaling on mRNA expression of hypothalamic neuropeptides critically involved in energy and glucose balance. The mRNA levels of orexigenic NPY, AgRP, and anorexigenic POMC were significantly reduced in 6‐month‐old female Snell dwarf (dw/dw) mice as compared to normal mice measured under fasting conditions (P < 0.0001) (Table 1). Similar results were seen in male mice (not shown). In addition, mRNA levels of these orexigenic and anorexigenic peptides were lower in 6‐month‐old female mice lacking GHR (GHR−/−) (P < 0.05) (Table 1), indicating that the change seen in the Snell dwarf mice reflected GH deficiency rather than the absence of TSH and prolactin which also characterize Snell and Ames dwarf mice.
Table 1.
Quantitative real‐time PCR analysis of hypothalamic agouti‐related peptide (AgRP), neuropeptide Y (NPY), and proopiomelanocortin (POMC) mRNA expression of 6‐month‐old Snell or GHR−/− female mice tested after 18 h of fasting (n = 8/group). Ratios are shown as mutant/control, with values < 0 indicating mRNAs expressed at lower levels in the mutants
P < 0.05.
P < 0.0001.
GH injections reverse the disruption in ARH projections in Ames dwarf mice
To determine whether transient exposure to GH, early in life, can reverse or prevent the disruption in ARH projections observed in GH‐deficient mice, we evaluated 18‐month‐old Ames dwarf mice that had been subjected to a series of GH injections between 2 and 8 weeks of age, using the method previously shown to reverse or prevent lifespan extension and to augment cellular stress resistance in Ames mice (Panici et al., 2010). In Ames dwarf mice that had received only control (i.e., saline) injections, we found diminished levels of both AgRP and α‐MSH neurons in the PVH, consistent with the deficit noted above for Snell dwarf and GHR−/− mice tested at younger ages (Fig. 2). Thus, hypothalamic re‐organization is produced on at least three background stocks (Ames, Snell, and GHR−/−) and is maintained at ages from 5 to 18 months. Ames dwarf mice that had been treated with GH in early life, however, did not differ from nonmutant control mice in AgRP and α‐MSH neurons in PVH (Fig. 2). Thus, as little as 6 weeks of exposure to systemic GH, early in postnatal life, leads to permanent changes in the density of hypothalamic neurons, paralleling the effects of GH injection on lifespan and cellular stress resistance (Panici et al., 2010) in Ames dwarf mice.
Figure 2.
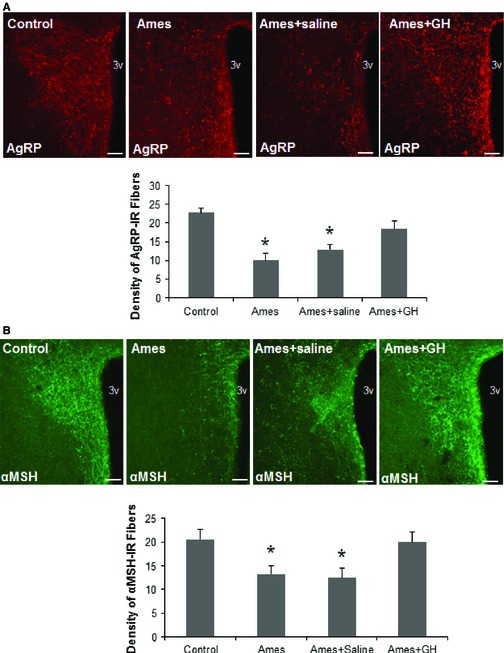
Effect of early‐life GH treatment on hypothalamic projections in Ames mice. Images and quantification of (A) AgRP and (B) α‐MSH‐immunoreactive fibers innervating the PVH at 18 months of age in control mice, Ames mice, Ames mice treated with saline injections at 2 weeks of age, and Ames mice treated with GH (n = 4 to 6 mice/group; all males.). 3V, third ventricle. Scale bar: 200 μm. Error bars indicate SEM. *P < 0.05 versus control.
Effect of GH on hypothalamic inflammation during aging
We evaluated two indices of hypothalamic inflammation: GFAP (glial fibrillary acidic protein, a marker of astrocyte activation) and production of TNF‐α by microglia. Immunofluorescent staining for GFAP was less intense in the ARH of 18‐month‐old Ames male mice (Fig. 3) compared to littermate control mice (P < 0.05), consistent with other work associating long lifespan with lower hypothalamic inflammation (Zhang et al., 2013). Thus, Ames dwarf mice resemble CL mice in that both mice have lower levels of hypothalamic inflammation. Early‐life treatment of Ames dwarf mice with GH, however, restored the number of GFAP+ cells to the level seen in age‐matched control mice. Mice given injections of saline, rather than GH, showed no such effect (Fig. 3).
Figure 3.
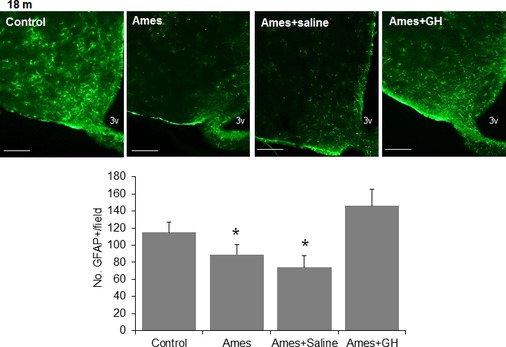
Hypothalamic astrogliosis in GH‐treated Ames mice. Representative images of astrocytes identified by immunofluorescent detection of GFAP protein in coronal sections of hypothalamus obtained from 18‐month‐old male control, Ames, and Ames treated either with saline or GH at early ages. Scale bar: 100 μm. Quantification of GFAP staining represents number of GFAP‐positive cells per field (error bars indicate SEM) in the region of the ARC (n = 4 to 6 mice/group), *P < 0.05 versus control.
To evaluate cytokine production by microglial cells, we stained for TNF‐α in the mediobasal hypothalamus (MBH) of 18‐month‐old mice and co‐stained for Iba‐1 as a marker for microglia. The number of immunoreactive TNF‐α‐positive cells was lower in Ames dwarf mice than in littermate controls, consistent with the GFAP data. Early‐life GH injections prevented this effect; such mice did not differ from control mice not bearing the Ames dwarf mutation (Fig. 4). Mice that had received saline instead of GH injections closely resembled uninjected Ames dwarf mice when evaluated at 18 months of age (Fig. 4B).
Figure 4.
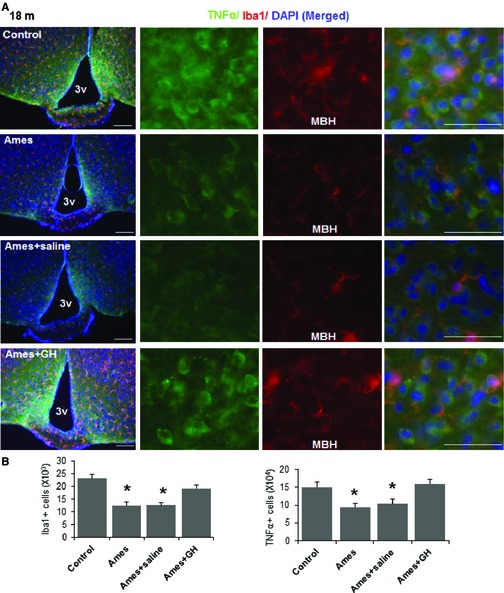
Hypothalamic inflammation in GH‐treated Ames mice. Brain sections of 18‐month‐old mice were analyzed for hypothalamic microglia and TNF‐α. (A) Representative images showing immunostaining in the MBH subregion of control and Ames dwarf mice, and in Ames mice that had been treated either with saline or GH. Scale bars: 100 μm (far left); 20 μm (right side panels).(B) Numbers of cells immunoreactive for Iba‐1, or TNF‐α in the hypothalamic mediobasal region (across the confocal microscopic field of serial sections) from indicated male mice (n = 4 to 6/group); error bars show SEM *P < 0.05 versus control.
Liver‐specific deletion of GHR does not modulate hypothalamic α‐MSH and AgRP fiber density
Mice in which GHR is disrupted only in liver have 90% lower levels of serum IGF‐1 compared to controls, but do not show lifespan extension in either of two separate colonies (Dominick et al., 2015). GH action in other organs is, however, intact, and the mice have elevated serum GH as a result of disruption of IGF‐1‐dependent feedback circuits (List et al., 2014). We found that the density of AgRP and α‐MSH fibers in adult 6‐month‐old LiGHRKO male mice was comparable to that of control littermates (Fig. 5), suggesting that the changes in hypothalamic neuron projection density in GHR−/− mice were not caused by lower plasma IGF‐1 levels. Similarly, hypothalamic GFAP and TNF‐α were comparable between 6‐month‐old LiGHRKO mice and control littermates (Fig. S1).
Figure 5.
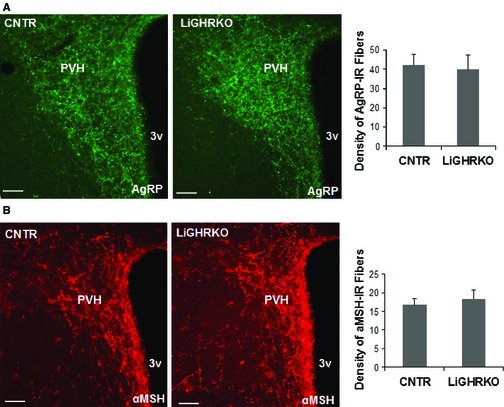
Images and quantification of agouti‐related peptide (AgRP) (A) and α‐melanocyte‐stimulating hormone (α‐MSH)‐immunoreactive fibers (B) innervating the paraventricular nucleus of the hypothalamus (PVH) at 6‐month‐old control and LiGHRKO male mice; (n = 4/group). 3V, third ventricle (Bregma: ‐0.82). Scale bar: 100 μm. Error bars reflect SEM.
Effect of GH deficiency on hypothalamic leptin signaling
Crowded litter mice are also hypersensitive to leptin signals, as measured by leptin‐induced increase in phosphorylation of Stat3 (Sadagurski et al., 2015). GHR−/− mice are obese, with elevated leptin levels, and leptin is known to be critical for formation of ARH projections (Baquedano et al., 2014). We therefore assessed whether alteration of projection pathways from the ARH in GHR−/− mice was due to reduced ability of leptin to promote ARH neurite extension. The leptin‐stimulated accumulation of pStat3 in the brain reflects cell‐autonomous LepR‐b‐induced intracellular signaling, which is impaired in states associated with diminished leptin action (Leinninger & Myers, 2008). The LepR‐b/Stat3 pathway plays a role in early circuit formation and controls the architecture of POMC, but not AgRP, projections (Bouret et al., 2012). Peripheral injection of leptin in 6‐month‐old GHR−/− mice robustly induced pStat3 immunoreactivity in the ARH after 1 h, similar to levels seen in the control mice (Fig. 6A). In addition, we detected a similar expression pattern of the LepR‐b mRNA in the GHR−/− and control mice at this age (Fig. 6B). Furthermore, peripheral injection of leptin similarly induced pStat3 in GHR−/− and control mice at 21 days of age (Fig. 6C). These results suggest that leptin‐induced intracellular signaling is not disturbed in GHR‐deficient mice and that the effects of GH on the architecture of ARH circuitry are not due to changes in leptin responses.
Figure 6.
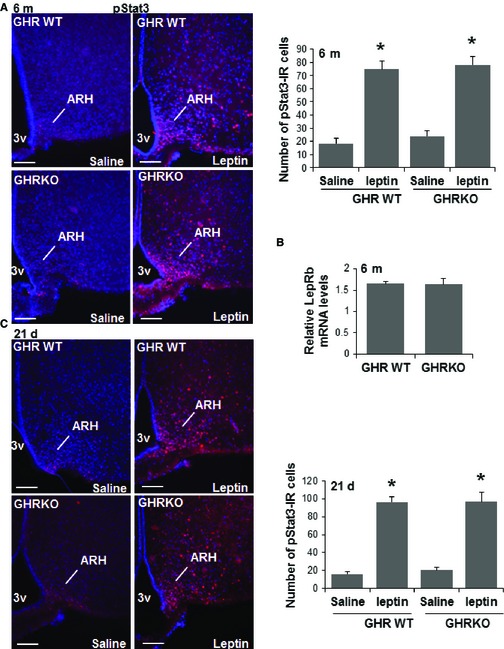
Hypothalamic leptin signaling in GHR −/− mice. Immunostaining for pStat3 1 h after intraperitoneal injection of vehicle or leptin (5 mg kg−1), with quantification of pStat3 immunoreactivity in (A) 6‐month‐old male mice and (C) 21‐day‐old male mice of the indicated groups (B) LepRb mRNA levels in 6‐month‐old male mice, 3V = third ventricle. Representative images from the hypothalamus are shown. Scale bar: 100 μm. (n = 4/each group), error bars reflect mean ± SEM. *P < 0.05 versus WT.
Discussion
We demonstrate here that three strains of dwarf mice (Ames, Snell, and GHR−/−) in which low GH action lead to lifespan extension have diminished density of both AgRP and α‐MSH fibers in the hypothalamus and lower levels of hypothalamic inflammation. Strikingly, these effects are prevented by transient early‐life GH treatment in at least Ames dwarf mice that also blocks the lifespan effect. Neither effect is seen in mice with liver‐specific disruption of GHR, which do not show the longevity increase found in mice with global disruption of GHR. These data suggest that the hypothalamus may make a critical contribution to the lifespan effect in these mice. Because long‐lived CL mice show changes in hypothalamic inflammation that parallel those reported here for GH mutant mice, but opposite changes in hypothalamic neuronal projection (Sadagurski et al., 2015) and because inhibition of hypothalamic inflammation has been reported to increase mouse lifespan (Zhang et al., 2013), we suspect that the decline in inflammation may be the more important factor for lifespan extension.
We demonstrate a significant decrease in the density of both orexigenic and anorexigenic neurite projections in Snell, Ames, and GHR−/− dwarf mice, compared with normal mice, at ages ranging from 5–6 months to 18 months. Our data are consistent with previous reports that hypothalamic mRNA levels of NPY, AgRP, and POMC are decreased in Ames and Snell mice, compared to controls (Peng et al., 2001; Hurley et al., 2003). Similarly, hypothalamic mRNA levels of AgRP, POMC, and NPY are reduced in GHR−/− mice, with reduced inflammation in response to high‐fat diet (HFD) (Baquedano et al., 2014). The absence of GH action could plausibly underlie the decline in NPY and AgRP levels, because these neurons express GHR and require GH for growth (Chan et al., 1996; Bohlooly et al., 2005). Leptin and insulin are important anorexigenic hormones that signal to the hypothalamus, and both NPY/AgRP and POMC/CART neurons express receptors for these two hormones (Bouret & Simerly, 2007). We see no changes in hypothalamic responses to leptin, as measured by Stat3 phosphorylation, or in expression of LepR‐b in GHR−/− mice (Fig. 6). These data indicate that the GHR, which is expressed and competent during the time of the postnatal leptin surge, mediates the developmental actions of GH on ARH projections. Notably, both AgRP‐ and POMC‐containing projections appear affected by GH and GHR deficiency.
Changes in hypothalamic architecture that occur in the immediate postnatal period appear to be malleable, and are particularly susceptible to changes in hormonal signals (Bouret & Simerly, 2006). Consistent with this idea, Ames dwarf mice subjected to daily GH injections starting at the age of 2 weeks for 6 weeks showed a remarkable reduction in longevity, so that their lifespans no longer differed from that of genetically normal littermates (Panici et al., 2010). In contrast, GH treatment of Snell dwarf mice, when started at 4 weeks, did not influence lifespan (Vergara et al., 2004). GH injections started at 4 weeks of age also failed to reduce longevity in Ames dwarf mice (Bartke, unpublished). Although these protocols differed in important ways, including the intensity of the GH injection schedule, the disparities suggest that sensitivity of lifespan to GH signals may change between 2 weeks and 4 weeks of age in mice. Our new results show that Ames dwarf mice that had been subjected to a series of GH injections between 2 and 8 weeks of age did not show the decline in ARH neuronal projections in PVH seen in uninjected or saline‐injected Ames dwarf mice. Furthermore, hypothalamic inflammatory changes observed in Ames dwarf mice were reversed. Thus, a few weeks of exposure to systemic GH, early in postnatal life, leads to permanent changes in the hypothalamic circuitry and inflammatory responses.
Our findings suggest that the changes in hypothalamic branching patterns and midlife inflammatory tone seen in Ames and Snell dwarf mice are mediated principally by early‐life exposure to systemic GH, rather than to defects in prolactin or thyroid hormones (Masternak & Bartke, 2012). Food consumption, O2 utilization, when adjusted for body mass, is higher in dwarf and GHR−/− mice, and these mice also have higher adiposity (Meyer et al., 2004; Bonkowski et al., 2006). It is possible that their elevations in adiponectin and leptin, or reduced body temperature, contribute to their extended longevity (Bartke et al., 2013) and may themselves be influenced by alterations in hypothalamic status.
The absence of GH action, either globally or in mice with liver‐specific GH knockout, impairs liver IGF‐1 synthesis, allowing discrimination between direct effects of GH and those mediated by endocrine IGF‐1. Our data show that changes in hypothalamic neurite density seen in mice with global GHR deletion are not replicated in mice with liver‐specific disruption of GHR, suggesting that the hypothalamic changes are not brought about by lower plasma IGF‐1 levels. Disruption of IGF‐1 gene expression during early life causes growth retardation and defects in the development of metabolic organs that can alter energy homeostasis throughout adult life (Woods et al., 1996). GHR−/− mice have increased expression of hypothalamic IGF‐1 and IGF1R (Nyberg, 2000; Bartke, 2011), despite lower levels of plasma IGF‐1. Enhanced hypothalamic IGF‐1 expression in the mice with global disruption of GHR raises the possibility that compensatory increases in the local production and paracrine/autocrine actions of IGF‐1 could account for some characteristics of GH‐deficient mouse strains, because both GH and IGF‐1 exert neuroprotective effects (Sonntag et al., 2005). Liver‐specific GHR knockout mice (LiGHRKO) have a 90% decrease in circulating IGF‐1 levels and a 300% increase in circulating GH (List et al., 2014), although no data are available on the levels of IGF‐1 and IGF1R in the hypothalamus of LiGHRKO mice. Unaltered ARH circuitry in the LiGHRKO mice demonstrates a specific role of GH action in hypothalamic development.
Hypothalamic inflammation influences the set points of multiple homeostatic loops. A recent publication (Zhang et al., 2013) has shown that modulation of inflammatory circuits in the hypothalamus, involving NF‐kB/IKKb signals under the control of GnRH, can extend lifespan and delay multiple aspects of aging in mice. Long‐lived GH‐deficient and GH‐resistant mice have reduced levels of IL‐6 and TNF‐α, mediators of inflammation (Bartke, unpublished). Further, hypothalamic IL‐1β expression in Ames dwarf mice is significantly reduced in comparison with normal control animals (Bartke, unpublished). It was previously reported that GHR−/− mice have higher serum levels of TNF‐α and IL‐1 as compared to control mice independently of diet. The increase in serum inflammatory markers in GHR−/− mice was coincident with an inflammatory pattern in the hypothalamus. However, their mRNA levels were decreased in the hypothalamus suggesting that there may be increased uptake of these cytokines from the circulation (Baquedano et al., 2014). We have now found that hypothalamic inflammation is reduced in middle‐aged mice by the Ames dwarf mutation and that transient early‐life exposure to GH was able to reverse this phenotype.
End‐of‐life necropsy data have been published for Ames, Snell, and GHR−/− mice (Ikeno et al., 2003, 2009; Vergara et al., 2004), showing that the extension of lifespan is not accompanied by a major shift in the relevant proportions of specific lethal illnesses. Most of the mutant mice, such as mice in the control groups, die of some form of neoplasia, rather than of metabolic diseases such as diabetes or atherosclerosis. Connections between hypothalamic inflammatory set points and lifelong cancer risk are obscure, but clearly deserve further investigation.
Nutritional excess is a key activator of metabolic inflammation in the hypothalamus, suggesting that interventions that can counteract hypothalamic neuroinflammation may protect against metabolic disorders and diabetes. The discovery that inhibition of hypothalamic inflammation can delay aging (Zhang et al., 2013) suggests that drugs that extend lifespan might be effective in inhibiting centrally regulated inflammatory processes. It will be important to determine the effects of drugs that extend mouse longevity, such as aspirin (Strong et al., 2008), rapamycin (Miller et al., 2011), and nordihydroguaiaretic acid, acarbose, and 17‐α‐estradiol (Harrison et al., 2014) on hypothalamic inflammatory processes elevated with age.
Methods
Animals
Procedures involved in this study were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA). GHRKO mice on a C57BL/6J background and homozygous WT littermates were used (Chandrashekar et al., 1999). Genotype was determined by PCR analysis of genomic DNA obtained from a tail sample collected at weaning. Ames dwarfs (Prop1df) and homozygous mice (df/df) were produced on a genetically heterogeneous background by mating heterozygous females and homozygous mutant males at Southern Illinois University, and sent to the University of Michigan. In this colony, the Prop1df mutation is maintained on a heterogeneous genetic background. Liver tissue‐specific GHR−/− (LiGHRKO) mice were generated as described previously (List et al., 2014). Animals were maintained under temperature‐ and light‐controlled conditions (20–23 °C, 12‐h light–dark cycle).
Growth hormone treatment
Groups of 15–17 Ames dwarf males were subjected to treatment with porcine GH (pGH) via subcutaneous (s.c.) injection [4 μg g−1 body weight (bw) per day], given 2× per day starting at the age of 2 weeks and continuing for 6 weeks. Twice‐daily saline‐treated dwarfs (df/df) of the same age were used as controls. After 6 weeks of treatment, animals were maintained normally and euthanized at 18 months of age. These mice were not exposed to any other manipulations.
Leptin treatment
For peripheral leptin treatment, mice were injected i.p. with either 5 mg kg−1 recombinant mouse leptin (provided by Dr. A Parlow, National Hormone and Pituitary Program, Torrance, CA, USA) or vehicle as previously described (Sadagurski et al., 2012). Mice were sacrificed 1 h after an i.p. injection of leptin or vehicle performed in overnight‐fasted animals.
RNA extraction and qPCR
Hypothalami were carefully dissected using Brain Matrices (Braintree Scientific, Braintree, MA, USA). Isolated mRNA from this tissue was analyzed using quantitative real‐time PCR. RNA was isolated using the Qiagen RNeasy Kit (Qiagen, Valencia, CA, USA), which was combined with the RNase‐Free DNase Set (Qiagen). RNA was reverse‐transcribed with the High Capacity cDNA RT Kit and amplified using TaqMan® Universal PCR‐Master Mix, NO AmpErase UNG with TaqMan® Assay‐on‐demand kits (Applied Biosystems, Foster City, CA, USA). Relative expression of target mRNAs was adjusted for total RNA content by beta‐actin RNA quantitative PCR. Quantitative PCR was performed on an ABI‐PRISM 7900 HT Sequence Detection system (Applied Biosystems). Each reaction was carried out in triplicates as previously described (Sadagurski et al., 2010). Primers used including the following: AgRP F: AGGGCA TCAGAAGGCCTGACCA, AgRP R: CTTGAAGAAGCGGCAGTAGCAC, POMC F: AAGAGCAGTGACTAAGAGAGGCCA, POMC R: ACATCTATGGAGGTCTGAAGCAGG, NPY F: TAGGTAACAAACGAATGG GG, NPY R: AGG ATGAGATGAGATGTGGG, LepR‐b R: GGAGACAGAGGCCCAGACATT, LepR‐b F: AAACTTCCCTCCAGTTCCAAAAG‐3.
Perfusion and immunolabeling
Mice were anesthetized (IP) pentobarbital and transcardially perfused with phosphate‐buffered saline (PBS) (pH 7.5) followed by 4% paraformaldehyde (PFA). Brains were postfixed, dehydrated, and then sectioned coronally (30 μm) using a sliding microtome, followed by immunohistochemical or immunofluorescent analysis as previously described (Patterson et al., 2010). For immunohistochemistry, free‐floating brain sections were pretreated by sequential incubations in 0.3% H2O2/1% NaOH, 0.3% glycine, and 0.03% SDS, followed by blocking in normal donkey serum (NDS). Sections were incubated with goat anti‐AgRP (1:1000; Phoenix Pharmaceuticals, Belmont, CA, USA), sheep anti‐α‐MSH (1:1000; Millipore, Temecula, CA, USA), rabbit anti‐GFAP (1:1000; Millipore), mouse anti‐TNF‐α (1:500; Abcam, Cambridge, MA, USA), or rabbit anti‐Iba‐1 (1:1000; Wako, Richmond, VA, USA) as primary antibodies, followed by Alexa Fluor‐conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA) as previously published (Munzberg et al., 2004; Bouret et al., 2008; Sadagurski et al., 2012; Zhang et al., 2013; Vogt et al., 2014). For pStat3 immunostaining, sections were pretreated for 20 min in 0.5% NaOH and 0.5% H2O2 in potassium PBS, followed by immersion in 0.3% glycine for 10 min. Sections were then placed in 0.03% SDS for 10 min and placed in 4% normal serum plus 0.4% Triton X‐100 plus 1% BSA for 20 min before incubation for 48 h with a rabbit anti‐pStat3 antibody (1:1000; Cell Signaling Technology, Danvers, MA, USA). Sections were mounted onto Superfrost Plus slides (Fisher Scientific, Hudson, NH, USA) and cover slips added with ProLong Antifade mounting medium (Invitrogen). Microscopic images were obtained using an Olympus FluoView 500 Laser Scanning Confocal Microscope (Olympus, Center Valley, PA, USA) equipped with a 20× objective.
Quantification
The density of AgRP and α‐MSH innervation of the PVH (bregma:‐0.82), DMH (bregma:‐1.58), and ARH (bregma:‐1.8) was determined by quantitative confocal microscopy using previously published methods (Patterson et al., 2010). For each animal, two sections through the PVH were acquired. Image analysis was performed using Image J analysis software (version 1.39t; National Institutes of Health, Bethesda, MD, USA). Each image plane was binarized to isolate the labeled fibers from the background as well as to compensate for differences in fluorescence intensity and was then skeletonized so that each fiber segment was 1 pixel thick. The integrated intensity was then calculated for each image, which reflects the total number of pixels in the skeletonized image and was proportional to the total length of labeled fibers in the image. This procedure was carried out on each image plane in the stack, and the values for all image planes in a stack were summed. The resulting value is an accurate index of fiber density in the volume sampled (Patterson et al., 2010).
For quantification of immunoreactive neurons, images of matched brain areas were taken from at least three sections containing the ARH of the hypothalamus for each brain between bregma −1.3 mm and −2.4 mm (according to the Franklin mouse brain atlas). Serial brain sections across the hypothalamus were made at 20 μm thickness, and every five sections were represented by one section with staining and cell counting. All sections were arranged from rostral to caudal to examine the distribution of labeled neurons. The images were quantified with Imaris (versions 6.4 and 7.0; Bitplane). The number of positive neurons was presented as means ± SEM.
Statistical analysis
Data sets with more than two groups were analyzed using one‐way analysis of variance (anova) followed by Tukey's post hoc test. For GH injections experiments, the statistical comparison of the untreated mutant mice was with untreated genetic controls, and the comparison of the GH‐treated mutant mice was with the saline‐injected mutant controls. Two‐tailed Student's t‐tests were used for comparisons involving only two groups. All data were presented as mean ± SEM. P < 0.05 was considered significant.
Funding
This project was supported by NIH grant R01‐AG019899 (to AB), by a Senior Scholar Award from the Ellison Medical Foundation (to RAM) and by the UM Nathan Shock Center, P30‐AG013283 (to RAM). MS was supported by a pilot grant from Shock Center AG‐13283, the Pepper Center AG‐024824, and a Feasibility Grant from the Michigan Diabetes Research Center (P30DK020572).
Conflict of interest
No conflict of interests, financial or otherwise, are declared by the authors.
Supporting information
Fig. S1 Hypothalamic inflammation in LiGHRKO mice.
Acknowledgments
The authors thank Dr. A. Parlow, National Hormone and Pituitary Program (NHPP) for recombinant mouse leptin, and Amanda Keedle and Sabrina Van Roekel for husbandry assistance. JJK is supported by the state of Ohio's Eminent Scholar Program that includes a gift by Milton and Lawrence Goll.
References
- Baquedano E, Ruiz‐Lopez AM, Sustarsic EG, Herpy J, List EO, Chowen JA, Frago LM, Kopchick JJ, Argente J (2014) The absence of GH signaling affects the susceptibility to high‐fat diet‐induced hypothalamic inflammation in male mice. Endocrinology 155, 4856–4867. [DOI] [PubMed] [Google Scholar]
- Bartke A (2008) New findings in gene knockout, mutant and transgenic mice. Exp. Gerontol. 43, 11–14. [DOI] [PubMed] [Google Scholar]
- Bartke A (2011) Single‐gene mutations and healthy ageing in mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Brown‐Borg H (2004) Life extension in the dwarf mouse. Curr. Top. Dev. Biol. 63, 189–225. [DOI] [PubMed] [Google Scholar]
- Bartke A, Sun LY, Longo V (2013) Somatotropic signaling: trade‐offs between growth, reproductive development, and longevity. Physiol. Rev. 93, 571–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blissett J, Harris G, Kirk J (2000) Effect of growth hormone therapy on feeding problems and food intake in children with growth disorders. Acta Paediatr. 89, 644–649. [DOI] [PubMed] [Google Scholar]
- Bohlooly YM, Olsson B, Bruder CE, Linden D, Sjogren K, Bjursell M, Egecioglu E, Svensson L, Brodin P, Waterton JC, Isaksson OG, Sundler F, Ahren B, Ohlsson C, Oscarsson J, Tornell J (2005) Growth hormone overexpression in the central nervous system results in hyperphagia‐induced obesity associated with insulin resistance and dyslipidemia. Diabetes 54, 51–62. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A (2006) Long‐lived growth hormone receptor knockout mice show a delay in age‐related changes of body composition and bone characteristics. J. Gerontol. A Biol. Sci. Med. Sci. 61, 562–567. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Simerly RB (2006) Developmental programming of hypothalamic feeding circuits. Clin. Genet. 70, 295–301. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Simerly RB (2007) Development of leptin‐sensitive circuits. J. Neuroendocrinol. 19, 575–582. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB (2008) Hypothalamic neural projections are permanently disrupted in diet‐induced obese rats. Cell Metab. 7, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Bates SH, Chen S, Myers MG Jr, Simerly RB (2012) Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J. Neurosci. 32, 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YY, Steiner RA, Clifton DK (1996) Regulation of hypothalamic neuropeptide‐Y neurons by growth hormone in the rat. Endocrinology 137, 1319–1325. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Bartke A, Coschigano KT, Kopchick JJ (1999) Pituitary and testicular function in growth hormone receptor gene knockout mice. Endocrinology 140, 1082–1088. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK (2007) Loss of pregnancy‐associated plasma protein A extends lifespan in mice. Aging Cell 6, 727–729. [DOI] [PubMed] [Google Scholar]
- Dominick G, Berryman DE, List EO, Kopchick JJ, Li X, Miller RA, Garcia GG (2015) Regulation of mTOR activity in snell dwarf and GH receptor gene‐disrupted mice. Endocrinology 156, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE (2001) Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl Acad. Sci. USA 98, 6736–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA (2014) Acarbose, 17‐alpha‐estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 2, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley DL, Birch DV, Almond MC, Estrada IJ, Phelps CJ (2003) Reduced hypothalamic neuropeptide Y expression in growth hormone‐ and prolactin‐deficient Ames and Snell dwarf mice. Endocrinology 144, 4783–4789. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A (2003) Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 58, 291–296. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A (2009) Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J. Gerontol. A Biol. Sci. Med. Sci. 64, 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ (2013) The GH/IGF‐1 axis in ageing and longevity. Nat. Rev. Endocrinol. 9, 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamegai J, Minami S, Sugihara H, Higuchi H, Wakabayashi I (1994) Growth hormone induces expression of the c‐fos gene on hypothalamic neuropeptide‐Y and somatostatin neurons in hypophysectomized rats. Endocrinology 135, 2765–2771. [DOI] [PubMed] [Google Scholar]
- Kamegai J, Hasegawa O, Minami S, Sugihara H, Wakabayashi I (1996) The growth hormone‐releasing peptide KP‐102 induces c‐fos expression in the arcuate nucleus. Brain Res. Mol. Brain Res. 39, 153–159. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Myers MG Jr (2008) LRb signals act within a distributed network of leptin‐responsive neurones to mediate leptin action. Acta Physiol. (Oxf). 192, 49–59. [DOI] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ (2014) Liver‐specific GH receptor gene‐disrupted (LiGHRKO) mice have decreased endocrine IGF‐I, increased local IGF‐I, and altered body size, body composition, and adipokine profiles. Endocrinology 155, 1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Bartke A (2012) Growth hormone, inflammation and aging. Pathobiol. Aging Age Relat. Dis. 2, 17293‐DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer CW, Klingenspor M, Rozman J, Heldmaier G (2004) Gene or size: metabolic rate and body temperature in obese growth hormone‐deficient dwarf mice. Obes. Res. 12, 1509–1518. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de CR, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R (2011) Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 66, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller EE, Locatelli V, Cocchi D (1999) Neuroendocrine control of growth hormone secretion. Physiol. Rev. 79, 511–607. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Flier JS, Bjorbaek C (2004) Region‐specific leptin resistance within the hypothalamus of diet‐induced obese mice. Endocrinology 145, 4880–4889. [DOI] [PubMed] [Google Scholar]
- Myers MG Jr (2004) Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog. Horm. Res. 59, 287–304. [DOI] [PubMed] [Google Scholar]
- Nyberg F (2000) Growth hormone in the brain: characteristics of specific brain targets for the hormone and their functional significance. Front. Neuroendocrinol. 21, 330–348. [DOI] [PubMed] [Google Scholar]
- Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM (2010) Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long‐lived mutant mice. FASEB J. 24, 5073–5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CM, Bouret SG, Park S, Irani BG, Dunn‐Meynell AA, Levin BE (2010) Large litter rearing enhances leptin sensitivity and protects selectively bred diet‐induced obese rats from becoming obese. Endocrinology 151, 4270–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XD, Park S, Gadelha MR, Coschigano KT, Kopchick JJ, Frohman LA, Kineman RD (2001) The growth hormone (GH)‐axis of GH receptor/binding protein gene‐disrupted and metallothionein‐human GH‐releasing hormone transgenic mice: hypothalamic neuropeptide and pituitary receptor expression in the absence and presence of GH feedback. Endocrinology 142, 1117–1123. [DOI] [PubMed] [Google Scholar]
- Sadagurski M, Norquay L, Farhang J, D'Aquino K, Copps K, White MF (2010) Human IL6 enhances leptin action in mice. Diabetologia 53, 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Leshan RL, Patterson C, Rozzo A, Kuznetsova A, Skorupski J, Jones JC, DePinho RA, Myers MG Jr, White MF (2012) IRS2 signaling in LepR‐b neurons suppresses FoxO1 to control energy balance independently of leptin action. Cell Metab. 15, 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Landeryou T, Cady G, Bartke A, Bernal‐Mizrachi E, Miller RA (2015) Transient early food restriction leads to hypothalamic changes in the long‐lived crowded litter female mice. Physiol. Rep. 3, pii: e12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS (2005) Growth hormone and insulin‐like growth factor‐1 (IGF‐1) and their influence on cognitive aging. Ageing Res. Rev. 4, 195–212. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE (2008) Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 7, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Sadighi Akha AA, Miller RA, Harper JM (2009) Life‐span extension in mice by preweaning food restriction and by methionine restriction in middle age. J. Gerontol. A Biol. Sci. Med. Sci. 64, 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R, Bartke A (2013) Growth hormone‐releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. eLife 2, e01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara M, Smith‐Wheelock M, Harper JM, Sigler R, Miller RA (2004) Hormone‐treated snell dwarf mice regain fertility but remain long lived and disease resistant. J. Gerontol. A Biol. Sci. Med. Sci. 59, 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt MC, Paeger L, Hess S, Steculorum SM, Awazawa M, Hampel B, Neupert S, Nicholls HT, Mauer J, Hausen AC, Predel R, Kloppenburg P, Horvath TL, Bruning JC (2014) Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high‐fat feeding. Cell 156, 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods KA, Camacho‐Hubner C, Savage MO, Clark AJ (1996) Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin‐like growth factor I gene. N. Engl. J. Med. 335, 1363–1367. [DOI] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D (2013) Hypothalamic programming of systemic ageing involving IKK‐beta, NF‐kappaB and GnRH. Nature 497, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Hypothalamic inflammation in LiGHRKO mice.


