Abstract
Aim
The aim of this review was to provide an updated overview of awareness, knowledge and views of off‐label prescribing in children.
Method
A literature search using electronic databases including PubMed, Medline, Scopus, Science Direct, Springer Link, Proquest, Ebsco Host and Google Scholar was conducted. Additional articles were identified by reviewing the bibliography of retrieved articles. The articles were searched with any of the following medical subject headings (MeSH) terms in the title: attitude, awareness, knowledge, experience, view, off‐label, pediatric, paediatric and children. The inclusion criteria were full text articles published in English between January 2004 and February 2015 and reported outcome related to awareness, knowledge and views regarding off‐label prescribing in children. Editorials, reviews, notes, conference proceedings, letters and studies reporting prevalence of off‐label prescribing were excluded. The articles were scrutinized using thematic analysis.
Results
Eleven studies conducted among doctors, community pharmacists, paediatric nurses, parents and children met the inclusion criteria. Nine themes were developed through document analysis which included main domains such as knowledge, awareness and views on off‐label drug use in children, choice of information sources, reasons and suggestions to reduce off‐label prescribing, concern regarding obtaining consent and participation in clinical trials.
Conclusion
The studies reviewed reported that the majority of doctors and community pharmacists were familiar with the term off‐label prescribing but knowledge among parents was low. Awareness on off‐label prescribing in children remains low among all study participants. There is a mismatch between views on off‐label prescribing in children of study participants and the finding of previous studies.
Keywords: attitude, awareness, children, off‐label, paediatric, views
Introduction
Prescribing in paediatric patients poses various challenges such as inadequate evidence due to limited studies done among the paediatric population, variation in drug disposition and off‐label prescribing 1. Off‐label prescribing is defined as ‘drugs prescribed and used outside their licensed indications with respect to dosage, age, indication or route’ 2. In general, off‐label prescription rates ranged from 10.5–80%, and higher rates were found in younger vs. older paediatric patients and in the hospital vs. community settings 2, 3, 4, 5, 6. The most common category of off‐label prescribing in children was dosage 2, 4, 5.
Legislations, protocols, procedures, government circulars and local guidelines are all strategies implemented to help healthcare providers play their role in making sure off‐label prescribing provides its intended benefit with minimum negative impact. But awareness, knowledge, views as well as attitude are crucial factors which will ensure actualization of these strategies. It is unclear to what extent these factors have been studied and no systematic review of awareness, knowledge and views of off‐label prescribing in children is currently available. Therefore we undertook this systematic review to provide an updated overview of the awareness, knowledge and views of off‐label prescribing in children.
The aim of this review is to provide an updated overview of the awareness, knowledge and views of off‐label prescribing in children.
Method
Literature search
PubMed, Medline, Scopus, Science Direct, Springer Link, Proquest, Ebsco Host and Google Scholar were searched using text words and medical subject headings (MeSH) including attitude, awareness, experience, view, knowledge, off‐label, paediatric and children. Additional articles were identified by reviewing the bibliography of the retrieved articles.
Inclusion and exclusion criteria
The inclusion criteria were full text articles published between January 2004 and February 2015, in English and reported outcome data related to awareness, knowledge and views of study participants regarding off‐label prescribing in children. Editorials, reviews, notes, conference proceedings and letters as well as studies reporting the prevalence of off‐label prescribing were excluded.
Article selection and review
The abstract of the articles was studied to determine inclusion of the article into this review if the title did not provide sufficient information to determine eligibility. The method used to select study articles for inclusion is summarized by adapting the PRISMA Group flow for study selection 7 (Figure 1). The following information was extracted from eligible studies: (i) identification of study, (ii) study details (study design, setting, study period, method), (iii) details of study participants, (iv) outcome measures, (v) results and (vi) limitations, strengths and recommendation discussed by the authors.
Figure 1.
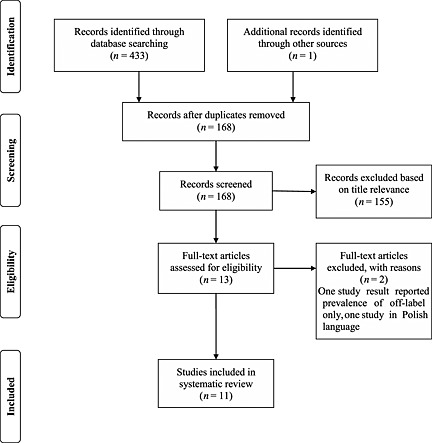
Quorum flowchart for study selection process
Quality assessment
Quality assessment of the eligible studies was evaluated independently by two review authors using a quality checklist for survey questionnaire 8 and a quality checklist for qualitative studies 9. Disagreements between the reviewers were resolved by discussion.
Data analysis
Statistical pooling of data was not conducted due to the variations of studies in designs, participants and outcome measures. Rather, results of the included studies were scrutinized using thematic analysis 10 by thorough reading and rereading in order to identify themes and groupings of similar themes (where appropriate) as they ‘emerged’ from the article 11. The final process of refinement and modification of the themes was conducted by discussion with two other reviewers.
Results
Overview of the included studies
Eleven studies met the inclusion criteria. Ten studies used quantitative methods while one study utilized a qualitative method. The individual studies included in this review are presented in Table 1. The majority of eligible studies were found to be of good quality (Tables 2 and 3). Through the process of data analysis, nine themes were developed and are outlined in the following section.
Table 1.
An overview of the studies
| Title (Author, year, reference) | Country | Study aim | Study design (duration) | Method | Target group | Survey instrument/tool characteristics | Outcome domains | Response rate (%) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Awareness about and views of parents on the off‐label drug use in children (Bang et al., 2014) 14 | India | To explore awareness among parents regarding both off‐label drug use in children | Cross‐sectional study (1 year) | Face‐to‐face interview using structured questionnaire | Parents (n = 400) | Validated, structured questionnaire | Parental views on safety and labelled use of drugs in children | 98.8 (400/405) | Participants felt that drugs used in children in hospital (89.5%) and community setting (80.3%) were either safe or extremely safe |
| To explore willingness to allow their child to participate in clinical research | |||||||||
| Comprised of 18 questions | Awareness regarding off‐label use in children | 30% parents were aware of off‐label drug use in children | |||||||
| Face validity done for translated version of questionnaire (English to Hindi and Marathi languages) | Communication from healthcare worker about off‐label drug use in children | 93% wanted to be informed whenever drug prescribed in off‐label manner | |||||||
| Parental views on off‐label drug use in children | 73% felt the off‐label drug use is illegal and 57% would ask for a change to labelled drug | ||||||||
| Willingness of allowing child participation in clinical trial | Participants not keen to allow their healthy children to participate in clinical trials participate | ||||||||
| Results from the 2012–2013 paediatric national survey on off‐label drug use in children in Spain (OL‐PED study) (Pérez et al., 2014) 15 | Spain | To estimate the current state of knowledge on off‐label use of drugs among Spanish paediatricians | Multicentre, descriptive, cross‐sectional study (8 months) | Online questionnaire | Paediatricians (n = 673) | Comprised of 10 questions | Knowledge and views on off‐label prescribing | 7.5 (673/9027) | 75.1% of participants knew the meaning of off‐label use |
| To assess the need to adopt measures to improve current practice | Distributed via email | Informing parents/guardians and documentation | 47% knew the importance of noting the off‐label use in medical records. However only 22% wrote it in the medical records | ||||||
| To estimate how well informed they are about legal liabilities arising from off‐label use of medications and to ascertain the sources paediatricians use to obtain information on drugs | View on factors of support from the point of view of medical liability | ||||||||
| Sources of information used to obtain information on drugs | |||||||||
| A questionnaire‐based study in Calabria on the knowledge of off‐label drugs in paediatrics (Saullo et al., 2013) 20 | Italy | To evaluate the knowledge of off‐label drugs in paediatricians of Calabria region in Italy | 2‐phase cross‐sectional study (8 months) | Online anonymous questionnaire | Paediatricians (n = 85) | Comprised of 10 questions; all question had tick box answers | Drugs and their off‐label use | 47.3 (85/180) | 40% used off‐label drugs ‘sometimes’ |
| General guidelines on risk and limitation of off‐label drugs in clinical practice | 74% did not have good knowledge about this practice | ||||||||
| Children's view on unlicensed/off‐label paediatric prescribing and paediatric clinical trials (Mukattash et al., 2012) 22 | Northern Ireland | To explore the views and perspectives of children on the unlicensed/off‐label use of medicines in children and on the participation of children in clinical trials | Cross‐sectional study (not reported) | Focus group discussions | School children (n = 123) | Sessions moderated by trained researcher | Views on the unlicensed use of medicines in children | 63.4 (123/194) | Pupils viewed unlicensed/off‐label use of medicine in children as unsafe and unethical |
| Facilitator was familiarized with focus group sessions by conducting 2 simulation sessions | Informing parents/guardians and children | Pupils felt it is necessary to test medicines in children to improve availability of licensed products | |||||||
| Study guide was used to ensure uniformity of focus group discussions | Clinical trials and willingness to participate | They felt that older children and parents should be informed when drug used in off‐label manner | |||||||
| Illness and participation in clinical trials | |||||||||
| Healthcare professional experiences and attitudes on unlicensed/off‐label paediatric prescribing and paediatric clinical trials (Mukattash et al., 2011) 16 | Northern Ireland | To investigate the knowledge and views of a range of healthcare professionals regarding the use of unlicensed/off‐label medicines in children and the participation of children in clinical trials | Cross‐sectional study (not reported) | Prospective questionnaire survey | Consultant paediatricians, general practitioners, community pharmacists, paediatric nurses (n = 563) | Survey instrument designed after an extensive review of the literature | Experiences and views of healthcare professionals regarding the use of unlicensed and off‐label medicines in children | 46.5 (563/1212) | More familiarity with the term unlicensed compared to off‐label prescribing |
| Comprised of 39 questions | Parental involvement in decision making regarding the medicine being prescribed for their child | 30.7% reported informing parents/guardians on the use of unlicensed/off‐label medicines in children | |||||||
| Questionnaire was piloted using a small number of healthcare professionals (n = 20; pharmacists and doctors) and respondents indicated that the questionnaire was clear and easy to understand | Information sources that healthcare professionals use when prescribing, dispensing and administering unlicensed/off‐label medicines to children | Willingness to be actively involved in clinical trials is highest among consultant paediatricians | |||||||
| Distributed via postal services and email | Paediatric clinical trials | ||||||||
| 4‐week window to complete the questionnaire | Dose‐related issues when prescribing unlicensed and off‐label medicines in children | ||||||||
| Perceptions and attitudes of Jordanian paediatricians towards off‐label paediatric prescribing(Mukattash et al., 2011) 17 | Jordan | To investigate the knowledge and views of Jordanian paediatricians regarding off‐label prescribing in children | Cross‐sectional study (not reported) | Prospective questionnaire survey | Hospital based paediatricians (n = 250) | Survey instrument designed after an extensive review of the literature | Experiences and views of paediatricians regarding off‐label prescribing to children | 83 (250/300) | 69% of participants were familiar with the term off‐label medicines but only 28% knowingly prescribed off‐label medicine to children |
| Comprised of 39 questions | Parental involvement in decision making regarding the medicine being prescribed for their child | Majority did not obtain informed consent or tell parents when they prescribe off‐label medicine | |||||||
| Face and content validity done by panel of experts | |||||||||
| Survey instrument was piloted prior to use | Dose – related issues with off‐label prescribing | ||||||||
| Survey instrument was personally delivered | |||||||||
| Collected after 4 weeks | |||||||||
| Off‐label, off‐limits? Parental awareness and attitudes towards off‐label use in paediatrics (Lenk et al., 2009) 13 | Germany | To explore knowledge and view regarding off‐label prescribing and participation in clinical trials | Cross‐sectional study (4 months) | Questionnaire survey | Parents (n = 94) | Comprised of three sections | Knowledge and view of off‐label use in paediatrics | Parents of children with renal disease: 54 (43/80) Parents of healthy children 64 (51/80) | Knowledge about off‐label drug use was poor in both groups |
| Participation in clinical trials | Refusal to off‐label drug use was low | ||||||||
| Parents with poor knowledge about off‐label drug use tend to refuse to volunteer their child for participation in clinical trials | |||||||||
| Public awareness and views on unlicensed use of medicines in children (Mukattash et al., 2008) 12 | Northern Ireland | To explore awareness and views of the general public on unlicensed use of medicines in children | Multicentre, cross‐sectional study (4 months) | Face‐to‐face interview using structured questionnaire | Parents (n = 1000) | Draft survey was examined for fitness for purpose and face validity in focus group | Views on safety and labelled use of drugs in children | Not reported | Majority had no previous knowledge about unlicensed/off‐label drug use |
| To explore participation of children in clinical trials | Final version was piloted in a sample of 20 members of public | Awareness regarding off‐label use in children | Most parents felt that they should be told when drug prescribed unlicensed/off‐label manner | ||||||
| Interview conducted by trained interviewer | Communication from healthcare worker about off‐label drug use in children | Views on clinical participation varied according health status of children | |||||||
| Views on off‐label drug use in children | |||||||||
| Willingness of allowing child participation in clinical trial | |||||||||
| Attitudes and experiences of community pharmacists towards paediatric off‐label prescribing: a prospective study (Stewart et al., 2007) 21 | United Kingdom | To identify community pharmacist experiences of, and attitudes towards paediatric off‐label prescribing | Cross‐sectional study (not reported) | Prospective questionnaire survey | Community pharmacists (n = 482) | Comprised of 21 questions with combination of tick‐box responses and written comments | Knowledge of and reasons for paediatric off‐label prescribing | 32.1 (482/1500) | Familiarity with concept of off‐label prescribing (73%), mainly gained through dispensing experience (64%) |
| Piloted prior to use | Classes of drugs dispensed off‐label | 40% knowingly dispensed off‐label prescription to children | |||||||
| Most common reasons for concerns when dispensing off‐label | Most common reason of off‐label prescription was age (84.6%) | ||||||||
| Sources of information for dispensing to children | Major concern regarding off‐label drug use is lack of dosage data (60%) | ||||||||
| Transfer of information to parents and prescribers | Majority feels that pharmacist should inform parents and prescribers if drug is used in off‐label manner (66% and 78%) | ||||||||
| A prospective questionnaire assessment of attitudes and experiences of off‐label prescribing among hospital based paediatricians (McLay et al., 2006) 18 | Scotland | To assess current attitudes of hospital based paediatricians to off‐label prescribing, and the performance of clinical trials in children | Cross‐sectional study (1 year) | Prospective questionnaire survey | Hospital based consultants and specialist registrars (n = 151) | Comprised of 24 questions with combination of tick‐box responses and written comments | Perceived reasons for off‐label prescribing | 59 (151/257) | Familiarity with concept of off‐label prescribing (92.8%) |
| Concerns about off‐label prescribing | 90% knowingly prescribed off‐label prescription to children | ||||||||
| Parental and GP involvement | Most common reason of off‐label prescription was age | ||||||||
| Paediatric clinical trials | Major concern regarding off‐label drug use is lack of efficacy data (63.8%) | ||||||||
| Off‐label prescribing to children: attitudes and experience of general practitioners (Ekins‐Daukes et al., 2005) 19 | Scotland | To identify experience with and attitudes towards paediatric off‐label prescribing in primary care | Cross‐sectional study (not reported) | Prospective questionnaire survey | General practitioners (n = 202) | Questionnaire was piloted prior to use | Prior knowledge of off‐label prescribing and licensing recommendations | 58 (202/346) | Majority familiar with off‐label concept (705) but 40% admitted knowingly prescribing off‐label dugs |
| Attempt to increase response rate was made | Acknowledgement of and perceived problems with off‐label prescribing Prescribing information sources used in practice | Ranked development of paediatric formulations and clearer dosage information highly as a means to reducing off‐label prescribing | |||||||
| Comprised of 13 questions | Importance of proposed methods or reducing off‐label prescribing | ||||||||
| Collected after 4 weeks |
Table 2.
Quality assessment of quantitative studies included in systematic review
| Studies | Bang et al. 14 | Pérez et al. 15 | Saullo et al. 20 | Mukattash et al. 16 | Mukattash et al. 17 | Lenk et al. 13 | Mukattash et al. 12 | Stewart et al. 21 | McLay et al. 18 | Ekins‐Daukes et al. 19 |
|---|---|---|---|---|---|---|---|---|---|---|
| Quality assessment domains | ||||||||||
| Research question and design | ||||||||||
| a) Was there a clear research question, and was this important and sensible? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| b) Was a questionnaire the most appropriate research design for this question? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Sampling | ||||||||||
| c) Was the sampling frame sufficiently large and representative? | ✓ | ✓ | ? | ✓ | ✓ | ✗ | ✓ | ✓ | ? | ? |
| d) Did all participants in the sample understand what was required of them, and did they attribute the same meaning to the terms in the questionnaire? | ✓ | ✓ | ? | ? | ? | ? | ✓ | ? | ✓ | ? |
| Instrument | ||||||||||
| e) Were the claims for reliability and validity made, and are these justified? | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ |
| f) Did the questions cover all relevant aspects of the problem in a non‐threatening and non‐directive way? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| g) Were open‐ended (qualitative) and closed‐ended (quantitative) questions used appropriately? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| h) Was a pilot version administered to participants representative of those in the sampling frame, and the instrument modified accordingly? | ? | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Response | ||||||||||
| i) Have non‐responders been accounted for? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Coding and analysis | ||||||||||
| j) Was the analysis appropriate (e.g. statistical analysis for quantitative answers, qualitative analysis for open‐ended questions) and were the correct techniques used? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| k) Were adequate measures in place to maintain accuracy of data? | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Presentation of results | ||||||||||
| l) Have all relevant results (‘significant’ and ‘non‐significant’) been reported? | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| m) Is there any evidence of ‘data dredging’ (i.e. analyses that were not ‘hypothesis driven’)? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Bias | ||||||||||
| n) Is there evidence of any other bias (e.g. funding bias)? | ? | ✓ | ? | ✓ | ✓ | ✓ | ? | ✓ | ? | ? |
✓ = yes;
× = no;
NR = not reported;
? = unclear
Table 3.
Quality assessment of qualitative study included in systematic review
| Mukattash et al. 22 | |||
|---|---|---|---|
| Screening questions | Yes | No | Cannot tell |
| 1. Research question | / | ||
| Did the paper address a clear research question? | |||
| 2. Design | / | ||
| Was the study design appropriate to the research question? In particular, was a qualitative approach suitable and was the right design used? | |||
| 3. Context | / | ||
| Was the context of the study sufficiently well described that the findings can be related to other settings? | |||
| 4. Sampling | / | ||
| Did the researchers include sufficient cases/settings/observations so that conceptual rather than statistical generalizations could be made? | |||
| Were the authors' preconceptions and ideology adequately set aside? | |||
| 5. Data collection | / | ||
| Was the data collection process systematic, thorough and auditable? Were attempts made to identify and explore disconfirming examples? | |||
| 6. Data analysis | / | ||
| Were data analyzed systematically and rigorously? Did the analysis take account of all observations? Were sufficient data presented? Were disconfirming observations dealt with? | |||
| 7. Results | / | ||
| Were there any unintended consequences? | |||
| 8. Conclusions | / | ||
| Did the authors draw a clear link between data and explanation (theory)? | |||
| 9. Reflexivity | / | ||
| Were the authors' positions and roles clearly explained and the resulting biases considered? | |||
| 10. Ethics | / | ||
| Are there any ethical reservations about the study? | |||
| 11. Worth/relevance | / | ||
| Was this piece of work worth doing at all, and has it contributed usefully to knowledge? | |||
Parents' knowledge and views on safe and labelled drug use in children
Three studies explored knowledge and views of parents on safe and labelled drug use in children 12, 13, 14. In all three studies, parents without children were asked to assume that they had a child of their own. Results were also analyzed according to parents who had healthy children and those with ill children at the time the studies were conducted. Prior to knowing about off‐label drug use, most parents thought that all medicines prescribed to children in both the hospital and primary care setting had undergone a similar testing and licensing process as in the case of medicines for adults 12, 14. In a study conducted in India, 89.5% of parents felt that drugs prescribed to their children were either safe or extremely safe and parents with healthy children felt that drugs prescribed in the hospital were safe as compared with those prescribed by a family physician (89.4% vs. 81.3%, P < 0.05) 14. The views of parents regarding safety of drug use in children dropped drastically after they knew about the concept of off‐label prescribing 12, 14.
Knowledge on off‐label drug use in children
Doctors
Six studies 15, 16, 17, 18, 19, 20 reported data on familiarity with the term ‘off‐label prescribing’ among doctors, with the majority being familiar (69.2 to 92.8%) 15, 16, 17, 18, 19. However, one study conducted in Italy 20 reported that 74% of paediatricians declared not to have a good knowledge about this practice. Specialist care doctors were more familiar with the term ‘off‐label prescribing’ compared with their primary care counterparts 15, 17, 18. Consultant paediatricians were found to be most familiar with the term ‘off‐label prescribing’ (83.3%, P < 0.05) when compared with other healthcare professionals 16 and neonatologists were the most familiar among hospital‐based paediatricians 17. Most general practitioners (GPs) were unaware that off‐label prescribing was commonplace in general practice 19. A study conducted in Jordan 17 reported more familiarity among hospital‐based paediatricians trained in the United Kingdom compared with those trained locally or in other countries (USA and other European countries). Over half of the respondents in two studies 16, 17 reported that doctors gained their familiarity and knowledge regarding off‐label prescribing through professional experience and post‐graduate studies.
Pharmacists
Two studies explored familiarity of community pharmacists with the term off‐label prescribing among children 16, 21. One study reported that 73% of community pharmacists admitted to being familiar with the concept of off‐label prescribing 21. However, a separate analysis of the latter study indicated that community pharmacists were more familiar with the term unlicensed medicines (93%) 16. Familiarity with off‐label prescribing was reported to be gained through dispensing experience (73%) 21 and undergraduate studies (50%) 16.
Parents
Three studies conducted among parents identified that knowledge of parents regarding off‐label drug use in children was relatively low (14 to 35%) 12, 13, 14. There was no statistical significant difference observed amongst the different socioeconomic classes, parents and non‐parents or between parents of sick or healthy children with regards to knowledge about off‐label drug use 12, 13, 14. A large proportion of parents also thought that off‐label drug use was illegal 14 and associated with increased occurrence of side effects 12.
Children
One study explored children's knowledge of off‐label drug use 22. This study combined the term ‘off‐label’ and ‘unlicensed’ as ‘unlicensed medicine use’ since the distinction between these two terms were not discussed in detail with children. The children were able to link licensing to medicine safety and to permission for the medicine to be prescribed 22.
Awareness and views on off‐label drug use in children
In this review, awareness is regarded as consciousness towards off‐label prescribing i.e. knowingly or unknowingly prescribing or dispensing off‐label medicines and views included inputs by study participants regarding categories of off‐label prescribing, common age group involved and disease state where medicine was commonly prescribed off‐label.
Doctors
Six studies conducted among doctors reported that the percentage of prescriptions knowingly written in an off‐label manner ranged between 25 to 90% 15, 16, 17, 18, 19, 20. All studies reported that most doctors prescribed off‐label medicines unknowingly 15, 16, 17, 18, 19, 20. A lower percentage of awareness was reported among hospital‐based paediatricians even though they were reported to be more familiar with the concept of off‐label prescribing 17. Awareness among hospital‐based consultant paediatricians and specialist registrars was reported to be higher 18.
Five studies highlighted the most common category of off‐label prescribing 16, 17, 18, 19, 20. Four of the studies found that most off‐label prescribing happened for patients of younger age groups than for which the products were licensed 16, 18, 19, 20 while one study reported that use of the product for a different indication than licensed as the most common category of off‐label prescribing 17. The majority of respondents in two studies reported neonates to be the most likely to receive off‐label prescriptions 16, 17. Respiratory and neurological diseases were found to be the two common disease states where drugs were prescribed off‐label for children 18, 20.
Pharmacists
The majority of community pharmacists were dispensing off‐label medicine unknowingly 16, 21. The most common category for a dispensed prescription being off‐label was for a younger patient than the recommended age 16, 21. The majority of community pharmacists indicated dispensing off‐label medicines for infants (age 1 to 23 months) 16.
Parents
The majority of parents (59%) involved in the study done in India thought that doctors would not knowingly prescribe drugs which were not fully tested for use in children 14. Contrarily, 84.2% of parents from a study conducted in Northern Ireland thought that their doctors would knowingly prescribe a drug which was not fully tested for use in children 12.
Children
Children trusted that doctors/pharmacists had adequate knowledge to decide the dose of medicines untested in children 22.
Choice of information sources
In the community setting, the British National Formulary (BNF) was the most commonly used by GPs and community pharmacists 19, 21. In a study conducted among healthcare workers in Northern Ireland, the British National Formulary for Children (BNFc) was the most commonly used information source 16. Contrarily, Spanish paediatricians tended to use protocols or clinical guidelines more frequently 15. Less frequently used information sources included the manufacturer's summary of product characteristics, Monthly Index of Medical Specialties, conference and meeting proceedings, colleagues' opinion, national guidelines and the local formularies 15, 16, 19, 21. A study among Scottish primary care practitioners reported no respondent used any of the available paediatric formularies 19.
Reasons for off‐label prescribing
Four studies evaluated the reasons for prescribing off‐label medicines 18, 19, 20, 21. The reasons established were lack of paediatric dosage information 19, 21, lack of appropriate paediatric formulations 19, 20, hospital consultants' advice 19, lack of licensed alternative 18, 19 and lack of clinical trials data 21.
Suggestions to reduce off‐label prescribing
Two studies highlighted measures to reduce off‐label prescribing, which included increasing the number of clinical trials in children, making more appropriate formulations available for young children and having clearer and consistent dosage information 16, 19. The GPs placed clear and consistent labelling as the most important approach 19 but healthcare professionals, as a whole, ranked making more appropriate formulations available for younger children as the most important approach to reduce off‐label prescribing 16.
Communication with parents and guardians
The majority of healthcare workers 16 and parents 12, 14 agreed that when a drug was prescribed in an off‐label manner, the information should be disclosed to the parents. However, the rate reported for such practice remained low (4.8–32.4%) 15, 16, 17, 18. Hospital‐based paediatricians felt that parents should not be told when a medicine had been prescribed in an off‐label manner for their children 17, 18. Only one third of hospital‐based paediatricians admitted to informing a child's GP that they were prescribing an off‐label medicine 18. The community pharmacists felt that the pharmacist had a responsibility to inform the prescriber and parents when medicines were prescribed off‐label for children 21. However, the doctors were deemed to be the most appropriate personnel to inform the parents in both primary and specialist care settings 12, 14. Once they knew that their children were prescribed off‐label drugs, most parents would ask for a change of drugs to one that had been fully tested and licensed for use in children 14 or they would use the medicine for their child with caution 12. The percentage of refusal of off‐label use was higher among parents of healthy children compared with parents of ill children 13. Besides parents, children also thought that the child should be told when off‐label medicines were used to create alertness to potential side effects 22.
Concern regarding informed consent
Verbal consent was more common in both the community and hospital setting when informing parents about off‐label prescribing 16, 17. The majority of hospital‐based consultant paediatricians and specialist registrars did not seek informed consent from parents when prescribing off‐label medicine 18. Spanish paediatricians felt that supporting actions from medical liability should include informing the parents, making a note of it in the medical records and to have protocols or clinical practice guidelines to endorse the action. However, most of them admitted not making a note in the medical records if the parents were verbally informed 15.
Participation in clinical trials
Doctors
Two studies conducted among doctors evaluated their responses regarding clinical trials in children 16, 18. Over half of the respondents in these two studies believed that all new medicines and medicines used in off‐label manner should undergo clinical trials in children 16, 18. A third of the respondents in a study conducted in Scotland believed that generic medicines too should be tested in children 18. Hospital‐based paediatricians were more willing to be actively involved in clinical trials compared with GPs (53% vs. 20.1%) 16, 18. Among all, consultant paediatricians reported the most willingness to help with clinical trials (94.4%) 16. The majority stated that they would allow their own children to take part in clinical trials 16, 18 depending on the child's health status and the benefit from participating in such trials 16.
Community pharmacists
The majority of community pharmacists (64.9%) believed that medicines used in off‐label manner should undergo clinical trials in children but not all are willing to be actively involved in paediatric clinical trials (41.4%) 16. Community pharmacists' willingness to consent for their children to take part in clinical trials was associated with the worsening of the child's health status and benefit from the clinical research 16.
Paediatric nurses
The response from paediatric nurses regarding participation in clinical trials was limited and extracted from a study done among healthcare professionals 16. The majority of paediatric nurses believed that off‐label medicines should not undergo clinical trials in children and they were not willing to be actively involved in clinical trials (i.e. help in recruiting patients) 16.
Parents
Three studies involving parents evaluated their response regarding participation of their children in clinical trials 12, 13, 14. The majority of parents would allow their children to participate in clinical trials if their children were suffering from life‐threatening conditions but their willingness reduced as the health status of the child improved 12, 14. Well‐informed parents were more willing to volunteer their children for participation in clinical trials 13. Monetary incentives and the child's age were not factors influencing the decision of the parents to allow their child's participation in a clinical trial 12, 14.
Children
School children were willing to take part in clinical trials only if they were seriously ill and if the medicine might help them but felt that those medicines should not be tested in babies 22. Children also felt that they must give informed consent prior to their participation in clinical trials 22.
Discussion
This review was limited by the small number of studies (11 studies) which included responses from doctors, pharmacists, nurses, parents and children. Different methods were used in the studies to explore awareness, knowledge and views of off‐label prescribing among children, making a direct comparison impossible but some common points are worth highlighting.
Familiarity with off‐label prescribing was assessed in doctors 16, 17, community pharmacists 16, 21 and parents 12, 13, 14 but the reason for familiarity was reported only for healthcare professionals, pointing out professional experience, post‐graduate and undergraduate training 16, 17, 21 as the main reasons. The variation in reasons of familiarity was due to different rates of off‐label drug use reported in different settings 2, 6 and difference in undergraduate and post‐graduate curricula 23, 24, 25, 26, 27. This review showed that most doctors did not reveal information regarding off‐label drug use to parents 15, 16, 17, 18, which could have resulted in a low level of familiarity to off‐label prescribing among parents.
Five studies 16, 18, 19, 20, 21 reporting views of study participants on off‐label prescribing in children highlighted age as the common category of off‐label drug use. Previous literature and systematic reviews 2, 3, 5, comprising a total of 78 studies, have concluded dose as the common category, accentuating a mismatch between views of study participants of this review and the finding of previous studies.
Even though various references were available specifically for paediatric patients 28, 29, 30, this review showed that most doctors and pharmacists tended to rely on other sources to obtain information regarding drug use in children, while expressing lack of paediatric dosage information as a major concern, an issue highlighted since the term ‘therapeutic orphan’ was coined by Shirkey in 1968 31.
Doctors, pharmacists, parents and children were supportive of paediatric clinical trials to address the issue of off‐label medicine use. Even though a clinical trial was the most valid means of obtaining data 32, 33, this review found that conducting clinical trials in children was not the most favoured means to reduce off‐label prescribing.
Most studies included in this review used a self‐reported, questionnaire‐based method of data collection, resulting in low response rates which could have been overcome by face to face or focus group interviews. Further research should address possible gaps identified from the reviewed studies, such as lack of communication among healthcare providers and parents. Additionally, a structured intervention programme derived from learning needs assessment will foster awareness, knowledge and view of healthcare professionals.
Conclusion
This systematic review described major behavioural aspects of doctors, community pharmacists, paediatric nurses, parents and children to off‐label prescribing. In general, the studies reported that the majority of doctors and community pharmacists were familiar with the term off‐label prescribing but knowledge among parents was low. Awareness regarding off‐label prescribing in children remained low among all study participants. There seemed to be a mismatch between views of the study participants on off‐label prescribing in children and the finding of previous studies.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Balan, S. , Hassali, M. A. , and Mak, V. S. L. (2015) Awareness, knowledge and views of off‐label prescribing in children: a systematic review. Br J Clin Pharmacol, 80: 1269–1280. doi: 10.1111/bcp.12750.
References
- 1. Sutcliffe AG. Prescribing medicines for children: major problems exist, but there are some promising developments. BMJ 1999; 319: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pandolfini C, Bonati M. A literature review on off‐label drug use in children. Eur J Pediatr 2005; 164: 552–8. [DOI] [PubMed] [Google Scholar]
- 3. Kimland E, Odlind V. Off‐label drug use in pediatric patients. Clin Pharmacol Ther 2012; 91: 796–801. [DOI] [PubMed] [Google Scholar]
- 4. Magalhães J, Rodrigues A, Roque F, Figueiras A, Falcão A, Herdeiro M. Use of off‐label and unlicenced drugs in hospitalised paediatric patients: a systematic review. Eur J Clin Pharmacol 2015; 71: 1–13. [DOI] [PubMed] [Google Scholar]
- 5. Lindell‐Osuagwu L, Korhonen M, Saano S, Helin‐Tanninen M, Naaranlahti T, Kokki H. Off‐label and unlicensed drug prescribing in three paediatric wards in Finland and review of the international literature. J Clin Pharm Ther 2009; 34: 277–87. [DOI] [PubMed] [Google Scholar]
- 6. Cuzzolin L, Atzei A, Fanos V. Off‐label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin Drug Saf 2006; 5: 703–18. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA StatementThe PRISMA statement. Ann Intern Med 2009; 151: 264–9. [DOI] [PubMed] [Google Scholar]
- 8. Boynton PM, Greenhalgh T. Selecting, designing, and developing your questionnaire. BMJ 2004; 328: 1312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mays N, Pope C. Quality in qualitative health research In: Qualitative Research in Health Care, Third edn. Oxford, UK: Blackwell Publishing Ltd, 2007; 82–101. [Google Scholar]
- 10. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3: 77–101. [Google Scholar]
- 11. Pope C, Mays N. Qualitative research in health care. Oxford, UK: John Wiley & Sons, 2013. [Google Scholar]
- 12. Mukattash T, Millership J, Collier P, McElnay J. Public awareness and views on unlicensed use of medicines in children. Br J Clin Pharmacol 2008; 66: 838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lenk C, Koch P, Zappel H, Wiesemann C. Off‐label, off‐limits? Parental awareness and attitudes towards off‐label use in paediatrics. Eur J Pediatr 2009; 168: 1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bang V, Mallad A, Kannan S, Bavdekar SB, Gogtay NJ, Thatte UM. Awareness about and views of parents on the off‐label drug use in children. Int J Risk Saf Med 2014; 26: 61–70. [DOI] [PubMed] [Google Scholar]
- 15. Piñeiro Pérez R, Ruiz Antorán MB, Avendaño Solá C, Román Riechmann E, Cabrera Garcia L, Cilleruelo Ortega MJ, Mellado Peña MJ. Results from the 2012–2013 paediatric national survey on off‐label drug use in children in Spain (OL‐PED study). Anal Pediatr (Barcelona, Spain : 2003)2014; 81: 16–21. [DOI] [PubMed] [Google Scholar]
- 16. Mukattash T, Hawwa AF, Trew K, McElnay JC. Healthcare professional experiences and attitudes on unlicensed/off‐label paediatric prescribing and paediatric clinical trials. Eur J Clin Pharmacol 2011; 67: 449–61. [DOI] [PubMed] [Google Scholar]
- 17. Mukattash T, Wazaify M, Khuri‐Boulos N, Jarab A, Hawwa A, McElnay J. Perceptions and attitudes of Jordanian paediatricians towards off‐label paediatric prescribing. Int J Clin Pharm 2011; 33: 964–73. [DOI] [PubMed] [Google Scholar]
- 18. McLay JS, Tanaka M, Ekins‐Daukes S, Helms PJ. A prospective questionnaire assessment of attitudes and experiences of off label prescribing among hospital based paediatricians. Arch Dis Child 2006; 91: 584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ekins‐Daukes S, Helms PJ, Taylor MW, McLay JS. Off‐label prescribing to children: attitudes and experience of general practitioners. Br J Clin Pharmacol 2005; 60: 145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saullo F, Saullo E, Caloiero M, Menniti M, Carbone C, Chimirri S, Paletta L, Gallelli L. A questionnaire‐based study in Calabria on the knowledge of off‐label drugs in pediatrics. J Pharmacol Pharmacother 2013; 4: S51–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stewart D, Rouf A, Snaith A, Elliott K, Helms PJ, McLay JS. Attitudes and experiences of community pharmacists towards paediatric off‐label prescribing: a prospective survey. Br J Clin Pharmacol 2007; 64: 90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mukattash T, Trew K, Hawwa A, McElnay J. Children's views on unlicensed/off‐label paediatric prescribing and paediatric clinical trials. Eur J Clin Pharmacol 2012; 68: 141–8. [DOI] [PubMed] [Google Scholar]
- 23. Jamshed S, Babar ZUD, Masood I. The PharmD degree in developing countries. Am J Pharm Educ 2007; 71 (Article 125). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hubball H, Burt H. Learning outcomes and program‐level evaluation in a four‐year undergraduate pharmacy curriculum. Am J Pharm Educ 2007; 71 (Article 90). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al‐Wazaify M, Matowe L, Albsoul‐Younes A, Al‐Omran OA. Pharmacy education in Jordan, Saudi Arabia, and Kuwait . Am J Pharm Educ 2006; 70 (Article 18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al‐Wazaify M, Albsoul‐Younes A. Pharmacy in Jordan . Am J Health Syst Pharm 2005; 62: 2548. [DOI] [PubMed] [Google Scholar]
- 27. Sosabowski MH, Gard PR. Pharmacy education in the United Kingdom . Am J Pharm Educ 2008; 72 (Article 130). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shann F. Drug doses: Collective Pty Limited. Victoria, Australia, 2010. [Google Scholar]
- 29. Taketomo CK, Hodding J, Kraus D. Pediatric and neonatal dosage handbook. Hudson, OH: Lexi‐Comp, 2012. [Google Scholar]
- 30. Committee PF. British National Formulary for Children (2009). London: BMJ Group, 2009. [Google Scholar]
- 31. Shirkey H. Editorial comment: therapeutic orphans. J Pediatr 1968; 72: 119–20. [DOI] [PubMed] [Google Scholar]
- 32. Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet 2004; 364: 803–11. [DOI] [PubMed] [Google Scholar]
- 33. Steinbrook R. Testing medications in children. N Engl J Med 2002; 347: 1462. [DOI] [PubMed] [Google Scholar]


