Abstract
Objective
The aim of the present study was to assess the effects of the changes in the management of paracetamol overdose recommended by the UK Commission for Human Medicines on rates of hospital admission.
Methods
An interrupted time series analysis was carried out on data for hospital admissions for paracetamol poisoning for England between January 2010 and June 2014, and for Scotland between January 2010 and Sept. 2014. The main outcome measure was admissions to hospital with paracetamol poisoning (T39.1), as defined by first position coding in children and adults.
Results
The time series analysis (Jan 2010 to June 2014) showed that admission rates for paracetamol poisoning were steady from 2010 to the date of change (September 2012), with an estimated 269 [95% confidence interval (CI) 252.5, 285.5] child (0–14 years) and 3541 (95% CI 3454, 3628) adult admissions per month. In September 2013, 12 months after the change, there were an estimated additional 116 [37.3% (95% CI 17.2–67.4)] child and 426 [12.5 % (95% CI 4.5–19.6)] adult admissions. Thus, in the year before the change (September 2011 to August 2012) there were 45,181 (3500 child and 41,681 adult) admissions, and in the year after (September 2012 to August 2013) there were 50,198 (4779 child and 45,419 adult) admissions. The overall proportion of child admissions was significantly greater after the change (Chi‐square 32.486, P < 0.001), emphasizing the disproportionate effect in children.
Conclusions
Changes to the management guidelines for paracetamol poisoning in September 2012 were rapidly implemented but have particularly increased paediatric hospital admissions for paracetamol poisoning. This impact in children, who are at low risk of mortality from paracetamol toxicity, appears excessive.
Keywords: children, epidemiology, hospital admissions, paracetamol, poisoning
What is Already Known About this Subject
In 2012, the Commission for Human Medicines (CHM) advised changes to the treatment nomogram for paracetamol poisoning in the UK.
We have previously shown that this produced a significant increase in presentations and admissions in adults in three UK hospitals.
What this Study Adds
Unexpectedly, we found a much larger increase in child admissions than in adults.
As this age group is at lowest risk of toxicity in overdose, this effect is both unexpected and disproportionate.
There is a need to review the CHM advice on paracetamol overdose, particularly in children.
Introduction
In September 2012, changes in the management of paracetamol poisoning were made in the UK following recommendations from the Commission for Human Medicines (CHM). This followed a review of indications for the use of acetylcysteine after the death of a young woman who had ingested 7 g of paracetamol not been treated at presentation, with a paracetamol concentration of 103 mg l–1 at 4 h, as she was not regarded as having a higher risk of toxicity 1. The changes included a change in the nomogram limits determining the need for the antidote acetylcysteine, with the adoption of a single nomogram line for the treatment of acute poisoning starting at 100 mg l–1 at 4 h after ingestion, which declined with a half‐life of 4 h, and is generally referred to as the ‘100 mg line’. The previous practice of assessing risk factors for patients above this line but below a line starting at 200 mg l–1 at 4 h (the ‘200 mg line’) was abandoned and CHM advised that risk factors should no longer be considered in the assessment of paracetamol poisoning. CHM also recommended that all patients with staggered overdose should be treated with acetylcysteine without specifying the ingested dose limits.
These changes placed the UK in a different position to most other major countries in the management of paracetamol overdose 2. We subsequently studied the effect of this policy on presentations, admissions and numbers of adult patients treated in three large UK hospitals, in Edinburgh, London and Newcastle upon Tyne. This research demonstrated significant increases in hospital presentations, admissions and the use of acetylcysteine and suggested that, if the changes were representative of those across the UK, they would generate substantial additional workload and costs to the National Health Service (NHS). Furthermore, these changes in management practice were expected to have only limited effects on mortality 1, calling them into question on cost–benefit grounds 3. Our previous hospital‐based study 3 was limited to the effect on adults; data on children had not been available to us as the hospital units involved in the study treated only adults. We now present data on national hospital activity for paracetamol overdose in England and Scotland, with a separate analysis for adults (≥15 years) and children (<15 years).
Methods
Data for all hospital admissions in England and Scotland for paracetamol poisoning (T39.1) were obtained from the Health and Social Care Information Centre (HSCIC) for England and from the Information Services Division of the National Services Scotland (ISD) for Scotland. Data were available from January 2010 to June 2014 for England, and January 2010 to September 2014 for Scotland. Trained clerks record the coding of diagnosis after patient discharge. Paracetamol poisoning is coded under T39.1. Coding structures allow for up to six diagnoses, and the principal diagnosis should appear in position 1 of the code for an admission. In the present work, we used the primary code in which paracetamol poisoning was mentioned for the main analysis, stratified by age and gender.
The data providers amalgamated data from age categories with very small numbers, in order to protect patient confidentiality, even though we had no details of individual patients other than the fact that an admission had occurred. Thus, for Scotland and small strategic health authorities (SHAs) in England, full analysis by all age groups was not always possible. We therefore examined general trends across all age groups and differences in adults (15 years and over) and children (14 years and younger) only at a national level. We also used all position coding to evaluate coding in different SHAs in England and examine the standardization of the data coding approach across England.
We considered it unlikely that the absolute rates of paracetamol overdose would change over such a short period in the absence of any other factors, and also that the effect of the Medicines and Healthcare Products Regulatory Agency (MHRA) advice would effectively produce a step change in admission rate at, or soon after, its introduction. Segmented regression analysis of interrupted time series was therefore used 4 to examine the effect of the change in MHRA guidelines for the management of paracetamol toxicity in September 2012. This analysis sets the time change at a prespecified time and controls for baseline level and trend when estimating expected changes in the number of admissions after the change in guidelines. Segmented regression was used to estimate the monthly number of admissions that might have occurred after September 2012 if the guidelines had not changed, and compared with the estimated number with the guideline change. Data were available for 33 months before the change and the 21 or 24 months after the change, for England and Scotland, respectively. Slope and change in level regression coefficients were used to estimate the absolute and relative difference in September 2013, 12 months after the change in guidelines.
Due to the presence of autocorrelation, model fitting was performed using restricted maximum likelihood (REML) with an autoregressive process of order 1 for the residuals, rather than least squares regression. The Durbin–Watson statistic of all final regression models was close to 2, indicating that no significant autocorrelation was present. Confidence intervals (CIs) for absolute effects were calculated using the method of Zhang 5, and confidence intervals for relative effects were estimated using the bias‐corrected and accelerated method from 5000 bootstraps of the original data. A more detailed analysis of admissions in children was performed on English data (not available in Scotland owing to small numbers) in young children (under 5 years) and those aged 5–14 years.
Finally, in order to assess the actual date that the step changes occurred, we used the ‘strucchange’ package (available from the Comprehensive R Archive Network (CRAN); https://cran.r‐project.org/) to identify the date of the most significant change (breakpoint) in the monthly admission rate over the whole period, with the calculation of 95% confidence interval of dates of change 6, 7, in order to compare with the actual date of the MHRA guidance.
Results
Figure 1 shows time series plots of monthly hospital admissions with paracetamol poisoning between January 2010 and June 2014 (full data in Table S1). A significant month‐to‐month change in the trend of admissions was not seen for either adults or children prior to the guideline change, and the baseline rates of admissions per month were 269 (95% CI 252.5, 285.5) in children and 3541 (95% CI 3454, 3628) in adults. Comparison of the pre‐ and post‐guideline trends estimates the excess number of monthly admissions resulting. Paracetamol poisoning admissions increased in children [+79.7 (95% CI 28.0, 131.5), P = 0.004] and in adults [+230 (95% CI −11.4 to 441), P = 0.098], although the change in the latter was not statistically significant using this analysis.
Figure 1.
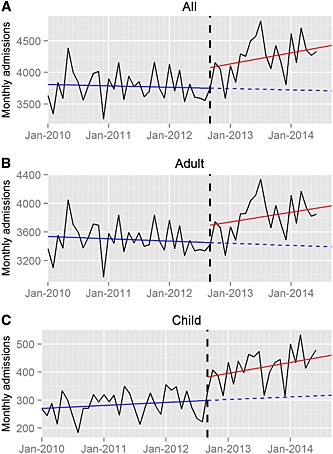
Time series plots for hospital admissions with paracetamol poisoning, between January 2010 and July 2014. Data are shown for all patients (A), adults (B) and children (C). The vertical dashed black line denotes the date of the Medicines and Healthcare Products Regulatory Agency guideline change, September 2012. The solid blue line shows the trend before the guideline change, the solid red line the trend after the change and the blue dashed line the predicted trend if the guideline change had not occurred
We present total admissions in the 2 years (September to August) prior to the change (2010–11 and 2011–12) and the first year afterwards (2012–13) in Table 1. There was month‐to‐month variability in admissions (Table S1), and in order to estimate the effect more precisely, we also calculated the predicted number of admissions in September 2013, 12 months after the guideline change, in two ways. Firstly, based on the pre‐guideline trends, we calculated an estimated baseline with no MHRA change and, secondly, using the post‐guideline change trends we estimated the absolute effect of the change (Table 2). Using this method, there were significant changes in both groups, with an estimated 116 (95% CI 63, 169) or 37.3% (95% CI 17.2%, 67.4%) additional child admissions, and 426 (95% CI 148, 704) or 12.5% (95% CI 4.5%, 19.9%) additional adult admissions. For comparison, the actual total numbers in August 2012 (immediately prechange) were 222 child and 3335 adult admissions, and in September 2013 (1 year post‐change) there were 400 child and 3660 adult admissions (Table S1). The disproportionate effect on child admissions was emphasized by the fact that the proportion of admissions in children was significantly greater after the change than it was beforehand (Chi‐square 32.486, P < 0.001), indicating a greater effect of the advice on childhood admissions.
Table 1.
Annual admissions (September to August) overall, and for adults and children, for paracetamol poisoning in England and Scotland, September 2010 to August 2013. The Medicines and Healthcare Products Regulatory Agency issued new guidance in early September 2012
| YEAR | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2010–11 | 2011–12 | 2012–13 | |||||||
| England | Scotland | Total | England | Scotland | Total | England | Scotland | Total | |
| All | 41 259 | 4356 | 45 606 | 40 790 | 4391 | 45 181 | 44 845 | 5353 | 50 198 |
| Adult | 38 027 | 4091 | 42 118 | 37 586 | 4095 | 41 681 | 40 506 | 4913 | 45 419 |
| Child | 3223 | 261 | 3488 | 3204 | 296 | 3500 | 4339 | 440 | 4779 |
Table 2.
Estimated effects of the change in Medicines and Healthcare Products Regulatory Agency guidance in the management of paracetamol poisoning. Data shown are model‐calculated numbers for admissions without the change (first column) as opposed to the predicted effect of the change (second column) with 95% confidence intervals (CIs) in September 2013, 12 months after the guideline change. The proportion of child admissions is significantly greater post‐change (Chi‐square 32.486, P < 0.001)
| Estimated admissions for September 2013 | ||||
|---|---|---|---|---|
| Without guideline change | With guideline change | Absolute effect (95% CI) | Relative effect (95% CI) | |
| All | 3727 | 4270 | 543 (243, 843) | 14.6 % (6.0, 23.0) |
| Adult | 3418 | 3844 | 426 (148, 704) | 12.5 % (4.5, 19.9) |
| Child | 310 | 425 | 116 (63, 169) | 37.3 % (17.2, 67.4) |
To further understand the age groups of children affected by the MHRA change, additional time‐series plots of child hospital admissions by age in England are shown in Figure 2, and the resultant statistical analysis is shown in Table S2. There was a significant increase in monthly admissions both in children aged 0–4 [+26.3 (95% CI 6.2, 46.4), P = 0.014] and those aged 5–14 [+48.7 (95% CI 8.1, 89.3), P = 0.023] immediately after the guidance change. However, while the monthly admission rate post‐change continued to increase in those aged 5–14 [β3 = 4.9 (95% CI 2.0, 7.8), P = 0.002], it subsequently decreased significantly in those aged 0–4 [β3 = −2.9 (95% CI −4.4, −1.5), P < 0.001] (Figure 2 and Table S2).
Figure 2.
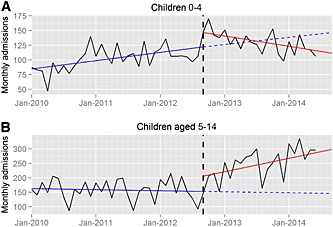
Time series plots for hospital admissions with paracetamol poisoning in England for children between January 2010 and July 2014. Data are shown for (A) children aged 0–4 and (B) children aged 5–14 years old. The vertical dashed black line denotes the date of the Medicines and Healthcare Products Regulatory Agency guideline change, September 2012. The solid blue line shows the trend before the guideline change, the solid red line the trend after the change and the blue dashed line the predicted trend if the guideline change had not occurred
Using breakpoint analysis, the most significant step changes in admission rate over the whole period were identified to have occurred in September 2012 (95% CI dates June 2012 to November 2012) for children and February 2013 (95% CI dates November 2012 to May 2013) for adults (Figure 1). This suggests that the issuing of the new MHRA guidance change took approximately 2 months to have an impact on adult hospital admissions but affected the monthly child admission rate immediately.
Discussion
The present study demonstrated a disproportionate increase in the admissions of children under the age of 15 years (Figure 1; Table 2; Table S1) following MHRA guidance in September 2012. One year after the change, the net effect was an estimated 37.3% (95% CI 17.2%, 67.4%) increase in monthly child admissions but a smaller increase of 12.5% (95% CI 4.5%, 19.9%) in adults (Tables 1 and 2) and a statistically significant increase in the proportion of paracetamol poisoning admissions in children (Chi‐square 6.414, P = 0.0113).
To study the effect in children further, we examined admissions in those under 5 years of age and those aged 5–14 years in England (Figure 2). In children under 5 years there was initially a large surge in admissions, which gradually settled, but in those over 5–14 years the admissions continued to increase throughout the period of study. This suggests that paediatricians may have adapted their management approach in younger children in a different way to those aged over 4 years 8. Young children have a larger mass of liver in proportion to their body mass, and are thus normally considered to be less at risk from paracetamol overdose. The Office for National Statistical data have documented no reported fatalities due to accidental paracetamol poisoning alone in children aged less than 15 years between 2000 and 2011 (Flanagan R and Handley S, personal communication), and the MHRA was also unaware of any deaths in this age group at the time of their review 1. Although the adolescent risk of liver injury is thought to mirror that in adults, it would seem that fatal overdoses in this group are extremely rare. The effect of the MHRA guideline therefore seems to have affected the treatment of paracetamol poisoning in children in a way that was unexpected and unlikely to benefit the vast majority of those admitted. We believe that this is because CHM advice that risk factors should not be used in assessing paracetamol poisoning affects not only the management guidelines for acute poisoning, but also on those for chronic therapeutic excess, which is a common problem in children. Previous guidance for this indication stated that treatment was only needed if the ingestion exceeded 150 mg kg–1 day–1, unless risk factors were present; for that minority of patents, a dose limit of 75 mg kg–1 was recommended. After the CHM had recommended that risk factors should no longer be used, it became unclear which patients should be treated at this lower dose threshold. The CHM recommended that ‘clinical judgement’ should be used but did not specify the factors that should be taken into account in making such a judgement. Referral might also have been encouraged by the advice to treat all staggered overdoses, which might have captured repeated non‐intentional overdose. A dose of 75 mg kg–1 day–1 is lower than the therapeutic paracetamol dose for smaller adults and some children, and is a lower threshold than used anywhere else in the world.
The MHRA communicated the recommended changes via several mechanisms, including the British National Formulary and the National Poisons Information Service, including its online poisons information database TOXBASE. Senior NHS managers and hospital medical directors also received direct notification of the advice from the MHRA, thus contributing to uptake. Our findings showed that the net effect of this advice on NHS clinical activity appeared quickly, especially for children, and was fully effective by February 2013 (Figure 1). The predicted breakpoint (strucchange analysis) suggested a change in child admissions in September 2012 (95% CI June to November 2012). We can find no evidence that paediatricians had advance notice of the impending change in advice from the MHRA, and believe, therefore, that the effect of the advice on child admissions was almost immediate. Increases in adult admissions seem to have taken longer, with a model‐predicted breakpoint in February 2013 (95% CI November 2012 to May 2013). We are uncertain why this change was more rapid in children than adults, but these data suggest that paediatricians reacted more rapidly and consistently to the MHRA advice than those treating adults.
Our previous study, from three adult units, showed that more patients presented to hospital after the guidance change, and suggested that this was, in part, due to larger numbers of patients requiring assessment for staggered or chronic overdose. Overall, in that study, of all patients presenting with paracetamol overdose, an additional 7.1% were admitted in absolute terms (95% CI 4.0, 10.2, P < 0.001) 3. The findings for the adult admissions we now report from England and Scotland are consistent with these findings, in spite of the previously reported difficulties with hospital coding of poisoned patients 9. These effects also have implications for the use of long‐term admission data to study the epidemiology of paracetamol poisoning in the UK.
An acknowledged problem with NHS coding sets that we used is that they currently reflect only patients admitted to hospitals, and exclude patients discharged from emergency departments, and so we cannot measure the overall effect on all hospital activity nationally from national data sets, although these data are available from our earlier three‐hospital study. We could not identify any major difference in coding approaches in different health authority areas in England, based on the data available to us. There were differences in rates of admission in different parts of the UK, and the magnitudes of increase post‐change were similar but not uniform. We wished to present an overall view of the effect of the MHRA change across the UK, and a potential weakness of the present study is that we might have ignored small regional differences in response.
Finally, it is interesting to note that the Office for National Statistics data demonstrate that registered deaths from paracetamol poisonings were at a higher level in 2013 than in the previous 5 years, the annual rates being: 255 in 2009; 199 in 2010; 207 in 2011; 182 in 2012; and 226 in 2013 10. Thus, the MHRA change has not had any discernable effect on deaths. This is not surprising as deaths in patients assessed as below previous UK treatment thresholds are uncommon and most patients who die present to hospital too late for effective therapy. In their briefing document, the MHRA estimated that the change in guidance would save less than one death every 2 years, although there was some uncertainty about this estimate 1.
In conclusion, the CHM advice to doctors in the UK on the management of paracetamol poisoning produced an increase in hospital activity that was rapid and larger in children than in adults. The reasons are likely to have been due to the nature of overdoses taken by children, and the fact that CHM advice was largely intended to reduce the number of death in adults. The implications of these findings are that the CHM advice was implemented rapidly but appears to have had unintended consequences for the management of paracetamol overdose in children. Action is needed to modify the guidance, to clarify the need for hospital admission and acetylcysteine in groups at very low risk of toxicity, especially children, in whom the risk of severe liver injury is particularly low in the UK.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work.
SHLT is a member of the Commission for Human Medicines and was a member of the MHRA paracetamol expert working group.
We are grateful to the Health and Social Care Information Centre (HSCIC) for England and from the Information Services Division of the National Services Scotland (ISD) for Scotland for helpful discussion and data provision.
Supporting information
Table S1. Monthly admissions in children (aged less than 15 years) and adults by month in England from 2010 to June 2014, Scotland from 2010 to September 2014, and overall from 2010 to June 2014.
Table S2. Segmented regression analysis for hospital admissions in England and Scotland with paracetamol poisoning between January 2010 and July 2014. Analyses include those for all admissions, adults (over 14 years) and children (<15 years) for England and Scotland. In addition, for England we show the changes in children 0–4 years and 5–14 years separately.
Narayan, H. , Thomas, S. HL. , Eddleston, M. , Dear, J. W. , Sandilands, E. , and Nicholas Bateman, D. (2015) Disproportionate effect on child admissions of the change in Medicines and Healthcare Products Regulatory Agency guidance for management of paracetamol poisoning: an analysis of hospital admissions for paracetamol overdose in England and Scotland . Br J Clin Pharmacol, 80: 1458–1463. doi: 10.1111/bcp.12779.
References
- 1. MHRA . Benefit risk profile of acetylcysteine in the management of paracetamol overdose [online]. Direct link to archived document available at http://www.mhra.gov.uk/home/groups/pl‐p/documents/drugsafetymessage/con184709.pdf (last accessed 25 September 2015).
- 2. Gosselin S, Hoffman RS, Juurlink DN, Whyte IM, Yarema M, Caro J. Treating acetaminophen overdose: thresholds, costs and uncertainties. Clin Toxicol 2013; 51: 130–3. [DOI] [PubMed] [Google Scholar]
- 3. Bateman DN, Carroll R, Pettie J, Yamamoto T, Elamin MEMO, Peart L, et al. Effect of the UK’s revised paracetamol poisoning management guidelines on admissions, adverse reactions, and costs of treatment. Br J Clin Pharmacol 2014; 78: 610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27: 299–309. [DOI] [PubMed] [Google Scholar]
- 5. Zhang F, Wagner AK, Soumerai SB, Ross‐Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol 2013; 62: 143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeileis A, Leisch F, Hornik K, Kleiber C. strucchange: an R package for testing for structural change in linear regression models. J Stat Software 2002; 7: 1–38. [Google Scholar]
- 7. Zeileis A, Shah A, Patnaik I. Testing, monitoring, and dating structural changes in exchange rate regimes. Comput Stat Data Anal 2010; 54: 1696–706. [Google Scholar]
- 8. Freeman R. MHRA recommendations on the use of intravenous acetylcysteine in paracetamol overdose. Arch Dis Child 2014; 99: 37–40. [DOI] [PubMed] [Google Scholar]
- 9. Wood DM, Conran P, Dargan PI. ICD‐10 coding: poor identification of recreational drug presentations to a large emergency department. Emerg Med J 2011; 28: 387–9. [DOI] [PubMed] [Google Scholar]
- 10. ONS . Deaths related to drug poisoning in England and Wales, 2013. In Statistical Bulletin [online]. Available at http://www.ons.gov.uk/ons/rel/subnational-health3/deaths-related-to-drug-poisoning/england-and-wales---2013/stb---deaths-related-to-drug-poisoning-in-england-and-wales--2013.html (last accessed 25 September 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Monthly admissions in children (aged less than 15 years) and adults by month in England from 2010 to June 2014, Scotland from 2010 to September 2014, and overall from 2010 to June 2014.
Table S2. Segmented regression analysis for hospital admissions in England and Scotland with paracetamol poisoning between January 2010 and July 2014. Analyses include those for all admissions, adults (over 14 years) and children (<15 years) for England and Scotland. In addition, for England we show the changes in children 0–4 years and 5–14 years separately.


