Abstract
Hormonal contraceptives are highly prevalent. Currently, little is known about Irish hormonal contraceptive trends to date since the 1995 British media contraceptive controversy. The aim of this study was to examine recent trends in contraceptive use in Ireland and to determine the frequency of co‐prescriptions with important interacting medications. Approximately 40% of the Irish population are prescribed 70% of total medicines under the Irish GMS scheme. Medicines were identified using the WHO Anatomical Therapeutic Chemical (ATC) classification system. Regression analysis was used to examine trends over time. Of all contraceptives dispensed in 2013, oral contraceptives were used the most (74%) and long acting reversible contraceptives (LARCs) the least (7.5%). Fourth generation combined oral contraceptives (COCs) predominated, although a slight significant decline was shown (P < 0.0001). Second and third generation COCs were significantly increasing and decreasing, respectively (P < 0.0001). Progestin‐only pills were significantly increasing (P < 0.0001 across age groups). Low rates of contraceptive co‐prescribing with important interacting drugs are shown. However, 93.6% of those on enzyme‐inducing anti‐epileptic medications were co‐prescribed ineffective contraception containing <50 μg oestrogen.Irish prescribing trends of second and third generation COCs have remained consistent since 1995. The slow decline in fourth generation COC uptake follows new evidence of an increased risk of venous thromboembolism (VTE) reported in 2011. The low, but increasing, uptake of LARCs is consistent with other countries. Co‐prescribing practices involving hormonal contraceptives requires continued vigilance. This study emphasizes the need to optimize co‐prescribing practices involving hormonal contraceptives and anti‐epileptic medications and highlights the need to address the barriers to the currently low uptake of LARC methods in Ireland.
Keywords: antiepileptic medications, combined oral contraceptives (COCs), co‐prescribing trends, drug interactions, long acting reversible contraceptives (LARCs)
Introduction
Oral contraceptive pills (OCPs) have been available since the 1960s, although it was not until 1978 that the Irish Family Planning Act legalized the provision of contraceptives under prescription. Despite the recognized benefits, OCPs have long remained under intense media scrutiny from the ongoing fears of venous thromboembolism (VTE), albeit low absolute risks, associated with these hormonal contraceptives.
In 1995 the British Committee of Safety of Medicines issued a warning outlining a two‐fold increased risk of VTE associated with third generation OCPs compared with second generation OCPs 1. This led to widespread media attention, which influenced prescribing practices across Europe in favour of second generation hormonal contraceptives. Recently, in 2012, further media attention in France led to a formal request to the European Medicines Agency (EMA) in 2013 to re‐assess the risk profile of third and fourth generation combined oral contraceptives 2. Overall the risk of VTE for second and third generation COCs is 5–12 per 10 000 women years, which remains lower than that associated with pregnancy and the post‐partum period, 29 per 10 000 and 300–400 per 10 000 women years, respectively. 3.
Since the 1995 British contraception warning, in Ireland, third generation COC uptake fell from 50% to 30%, whilst second generation COCs' rose from 31% to 43% from 1995 to 1996 4. Little is currently known about the Irish hormonal contraceptive trends to date, including the appropriateness of co‐prescribing with other important medications. This is particularly important when co‐prescribed medications have teratogenic potential capable of altering contraceptive hormonal levels through metabolic drug interactions, and reciprocally, using the hepatic cytochrome P450 enzyme system. This is especially true of anti‐epileptic medications 5.
The aims of the present study were to (i) examine the trends in prescribing of contraception within the General Medical Service (GMS) medical card scheme in Ireland from 2008 to 2013 inclusive, (ii) explore the age‐specific patterns and types of hormonal contraceptives used by the general Irish population, (iii) determine the frequency co‐prescription of oral contraceptives with interacting medications and (iv) identify the level of contraceptive use in those co‐prescribed anti‐epileptic drugs in Ireland in 2013.
Methods
The Health Service Executive‐Primary Care Reimbursement Services (HSE‐PCRS), through the General Medical Service (GMS) scheme, provides free healthcare to approximately 40% of the Irish population (approximately 1.8 million). Eligibility is means tested and confined to persons who are unable without undue hardship to arrange General Practitioner services for themselves and their dependants. All medicines are dispensed free of charge to patients registered under this scheme. While the HSE‐PCRS population cannot be considered representative of the entire population, as the elderly, the young and the socially disadvantaged are over‐represented, it is estimated to account for approximately 70% of all medicines dispensed in primary care.
The HSE‐PCRS pharmacy claims database contains demographic details on the patients (age and gender), information related to the medicine dispensed such as the non‐proprietary drug name along with the proprietary drug name, the strength and the quantity of the drug dispensed together with the associated cost and dispensing fee. No identifiable information is available. All prescription items are coded using the World Health Organization (WHO) anatomical chemical classification (ATC) system.
Pharmacy claims data from 2008 to 2013 in women aged 16–44 years inclusive were used. For the prescribing of contraceptives we included oral contraceptives (OCs; fixed combination, sequential, classified into second, third and fourth generation OCs and progestin, ATC G03A), patches and implant formulation and IUD devices (G02BA03). Oral contraceptives were further grouped into OCs containing oestrogen greater than 50 μg and OCs containing oestrogen lower than 50 μg. In addition we considered a number of interacting co‐prescribed medicines on the same prescription claim as those receiving fixed combination oral contraceptives only in 2013. The rate of co‐prescribed interacting drug was calculated out of all claims for the fixed combination OCs.
We also examined all those women receiving at least one prescription of an anti‐epileptic drug (AED, ATC code N03) in 2013. AEDs were grouped into enzyme‐inducing AEDs and non‐enzyme‐inducing AEDs. Topiramate >200 mg and lamotrigine >300 mg were considered enzyme‐inducing AEDs. Oral contraceptives have also been shown to reduce lamotrigine levels, thereby at doses ≤300 mg thus potentially reducing seizure control if doses are not increased. Conversely, toxic levels may arise during pill‐free intervals when high doses of lamotrigine are not reduced. 6.
Statistical analysis
We calculated the prevalence of contraceptive use and frequency and percentage of different contraceptive methods among enzyme‐inducing AED users, non‐enzyme‐inducing AED users and women without AEDs. Chi‐square tests were used to compare proportions. Poisson regression with a log link function was used to examine the trends over time in the overall rates of prescribing of oral contraceptives and by specific age groups.
Analyses were carried out using SAS (V9.3) and statistical significance assumed at P < 0.05.
Results
The monthly proportion of GMS eligible women aged 16–44 years receiving any contraceptive was, on average, 17%, which remained fairly constant throughout the study period. In 2013, COCs were most frequently prescribed, followed by the progestin‐only pill (POP), patches, intrauterine devices (IUD) and implants (Figure 1). Fourth followed by second generation COCs were most popular for women aged <35 years; whereas POPs were most often prescribed for women aged ≥35 years (Figure 2). Third generation COCs steadily declined throughout the study period and across all age groups, whilst second generation COCs rose. Low uptake of long acting reversible contraceptive (LARC) methods was observed among all ages and over time (Figure 2). Total ingredient costs associated with LARCs were €1 886 300 and COCs €2 830 700 in 2013.
Figure 1.
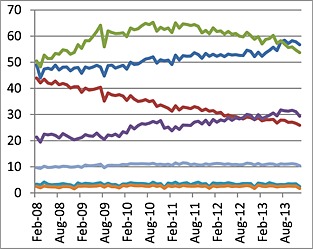
Trends in rates of prescribing (per 1000 eligible GMS female population 16–44 years) of second ( ), third (
), third ( ) and fourth (
) and fourth ( ) generation OCPs, POPs (
) generation OCPs, POPs ( ), IUD (
), IUD ( ), implant (
), implant ( ) and patch (
) and patch ( ) from 2008 to 2013
) from 2008 to 2013
Figure 2.
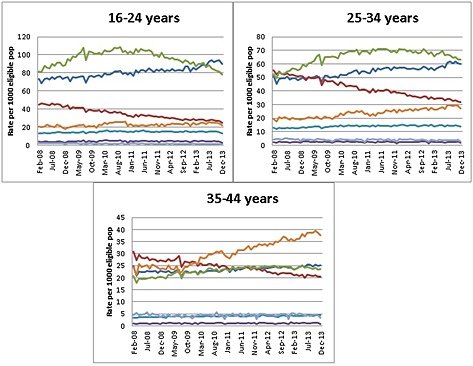
Trends in prescribing rate of second ( ), third (
), third ( ) and fourth (
) and fourth ( ) generation OCPs, implant (
) generation OCPs, implant ( ), patch (
), patch ( ), progestin (
), progestin ( ) and IUDs (
) and IUDs ( ) from 2008 to 2013 by age groups: 16‐24 years (52 573), 25‐34 years (52 499), 35‐44 years (29 352)
) from 2008 to 2013 by age groups: 16‐24 years (52 573), 25‐34 years (52 499), 35‐44 years (29 352)
Figure 1 reveals the global trends in prescribing rates by contraceptive method, including the different generations (second, third and fourth) of the combined oral contraceptives from 2008 to 2013 among eligible patients aged 16–44 years. COCs were the most frequently prescribed contraceptive method (74%), of which the fourth (drospirenone containing) followed by second generation (levonorgestrel containing) COCs were most popular. The latter showed a significant steady rise throughout the study period (P < 0.0001), whilst the former showed a significant decline since 2011 (P < 0.0001). The rates of the third generation COCs have shown a consistent and rapid significant decline from 12.6% in 2008 to 8.03% in 2013 (P < 0.0001), respectively, dropping to below the POP by late 2012, whose rates have been significantly increasing steadily throughout the study period (P < 0.0001).
Figure 2 shows the age‐specific trends in prescribing rates by contraceptive method. Of the oral methods, fourth followed by second generation COCs were most frequently prescribed for women aged <35 years. POP prescribing was lowest among women <35 years. However, it rates increased significantly over time and across all age groups (P < 0.0001 for all), being the predominant oral contraceptive method among women aged 35–44 years. The significant decline in third generation COC prescriptions was consistent across all age groups (P < 0.0001 for all age groups). Of LARC methods, patches were most frequently prescribed in all age groups, along with intra‐uterine devices (IUD) in women aged 35‐44 years.
Table 1 shows the number of prescription claims containing co‐prescribed fixed COC with important interacting medications in 2013. Co‐prescribed medications were categorized into enzyme inducers that accelerate OCP metabolism thereby reducing contraceptive effectiveness, enzyme inhibitors causing prolonged exposure to OCP thereby increasing the risk of VTE, and drugs whose therapeutic effect may either be potentiated or reduced by OCPs.
Table 1.
Interactions with the fixed combination OCs (COCs) in 2013 (total claims n = 682 784)
| Important interacting medications | Number of claims 2013 | % of all COC claims in 2013 |
|---|---|---|
| Enzyme inducers potentially leading to reduced contraceptive effectiveness | ||
| Anti‐epileptic drugs | ||
| Barbiturate/derivatives | 41 | 0.01% |
| Carbamazipine, eslicarbamazepine, oxcarbazepine, Rufinamide | 765 | 0.11% |
| Felbamate | 0 | 0.00% |
| Phenytoin | 55 | 0.01% |
| Primidone | 0 | 0.00% |
| Phenobarbital | 41 | 0.01% |
| Topiramate >200 mg | 101 | 0.01% |
| Lamotrigine >300 mg | 395 | 0.06% |
| Non antiepileptic drugs | ||
| Rifampicin | 3 | <0.01% |
| Aprepitant | 4 | <0.01% |
| Modafinil | 75 | 0.01% |
| Enzyme inhibitors that potentially increase contraceptive levels | ||
| Valproic acid | 1294 | 0.19% |
| Atrovastatin | 1144 | 0.17% |
| Rosuvastatin | 990 | 0.14% |
| Verapamil | 54 | 0.01% |
| Diltiazam | 38 | 0.01% |
| Etorcoxib | 633 | 0.09% |
| Cimetidine | 23 | <0.01% |
| Fluconazole | 1556 | 0.23% |
| Drugs whose effects may be increase by OCP potentially leading to toxicity | ||
| Diazepam + other benzodiazepines | 6044 | 0.89% |
| Prednisone | 1418 | 0.21% |
| Selegiline | 0 | 0.00% |
| Theophylline | 142 | 0.02% |
| Tizanidine | 133 | 0.02% |
| Drugs whose effects may be reduced by OCP potentially leading to reduced clinical effectiveness | ||
| Lamotrigine <300 mg | 1553 | 0.23% |
From 2008 to 2013, there were an additional 257 437 claims for COCs. Highest rates of contraceptive co‐prescriptions were seen with drugs whose effects may be potentiated by OCP: benzodiazepines,theophylline, tizandidine. Given the widespread media scrutiny on the higher risk of VTE associated with third and fourth generation COCs compared with second generation COCs, we examined their co‐prescribing patterns with the enzyme inhibitors (Table 2). Those receiving third and fourth generation COCs were more likely to be co‐prescribed enzyme inhibiting medications than those receiving second generation COCs (Table 2). Co‐prescriptions of oral contraceptives with lamotrigine at doses ≤300 mg were more frequently seen than those with higher doses (Table 1).
Table 2.
Enzyme inhibitors by second, third and fourth generation for 2013 only (1.3%)
| Enzyme inhibitors | Number of claims 2013 | Second | Third | Fourth | Other (patch, etc.) |
|---|---|---|---|---|---|
| Valproic acid | 1294 | 429 (33.2%) | 381 (29.4%) | 473 (36.6%) | 11 (0.8%) |
| Diazepam + other benzodiazepines | 6044 | 1481 (24.5%) | 1607 (26.6%) | 2278 (37.7%) | 678 (11.2%) |
| Atrovastatin | 1144 | 249 (21.8%) | 365 (31.9) | 496 (43.4%) | 34 (3.0%) |
| Rosuvastatin | 990 | 158 (16.0%) | 293 (29.6%) | 508 (51.3%) | 31 (3.1%) |
| Verapamil | 54 | 16 (29.6%) | 27 (50%) | 5 (9.3%) | 6 (11.1%) |
| Diltiazam | 38 | 11 (28.9%) | 12 (31.6%) | 15 (39.5%) | – |
| Etorcoxib | 633 | 200 (31.6%) | 172 (27.2%) | 215 (34.0%) | 46 (7.3%) |
| Cimetidine | 23 | 0 | 9 (39.1%) | 14 (60.9%) | – |
| Fluconazole | 1556 | 506 (32.5%) | 287 (18.4%) | 622 (40.0%) | 141 (9.1%) |
The pattern of contraceptive co‐prescribing with anti‐epileptic drugs (AEDs) was further examined (Table 3). Of the prescriptions containing an anti‐epileptic medication 30.6% were co‐prescribed any contraception, with 22.0%, 34.2% and 19.2% of those containing an enzyme‐inducing AED alone, a non‐enzyme inducing AED alone, or both AED types (enzyme‐inducing and non‐inducing). These were all lower than the percentage of any contraception use in the generation population (37.7%). The most popular contraceptives across all groups were COCs followed by POP and the levonorgestrol intra‐uterine system (Mirena device). Third generation COCs were the least frequently prescribed COC among both the general population and epilepsy groups, whose uptake of the POP was highest, particularly for those on enzyme‐inducing AED (29.0%). The levonorgestrel intra‐uterine system device was most frequently used in those on an enzyme‐inducing AED (16.9%) compared with those on non‐enzyme inducing AEDs (9.0%) and the general population (6.1%). To ensure contraceptive effectiveness when co‐prescribed an enzyme‐inducing AED, the oestrogen content must be ≥50 μg. This represented 6.4% of total contraceptive‐enzyme‐inducing AED claims, which was significantly higher than those co‐prescribed non‐enzyme inducing AED (2.7%) and the non‐epilepsy population (2.3%) (χ2 = 21.0, d.f. = 2, P < 0.0001).
Table 3.
Contraceptive use in 2013 in women aged 16–45 years – comparing the general population (not on any AED) with those on enzyme inducing, non‐enzyme inducing or both types of AED
| General GMS population † | % | Enzyme inducing AED only (n = 3385) | % | Non‐enzyme inducing AED only (n = 14 140) | % | Both types of AED (n = 1836) | % | |
|---|---|---|---|---|---|---|---|---|
| Any contraceptive methods† | 128 493 | 37.7%* | 745 | 22.0% | 4834 | 34.2% | 352 | 19.2% |
| Second generation | 28 995 | 22.6% | 84 | 11.3% | 771 | 16.0% | 38 | 10.8% |
| Third generation | 12 865 | 10.0% | 55 | 7.4% | 504 | 10.4% | 37 | 10.5% |
| Fourth generation | 27 888 | 21.7% | 77 | 10.3% | 908 | 18.8% | 38 | 10.8% |
| Progestin‐only pill | 17 018 | 13.2% | 216 | 29.0% | 919 | 19.0% | 95 | 27.0% |
| Mirena device | 7793 | 6.1% | 126 | 16.9% | 435 | 9.0% | 63 | 17.9% |
| Patch/implant | 5628 | 4.4% | 24 | 3.2% | 155 | 3.2% | 9 | 2.6% |
| Combination of above | 28 306 | 22.0% | 163 | 21.9% | 1142 | 23.6% | 72 | 20.5% |
| With oestrogen content < 50 μg | 82 071 | 97.7% | 263 | 93.6% | 2700 | 97.4% | 131 | 95.6% |
| With oestrogen content > 50 μg | 1951 | 2.3% | 18 | 6.4 | 71 | 2.6% | 6 | 4.4% |
excluding AED users; assumed denominator (n = 340 981) for eligible population in December 2013, likely to be an under‐estimate for the number over the whole year.
P < 0.001 comparing any contraceptive in general population vs. enzyme‐inducing AED and vs. non‐enzyme‐inducing AED (chi‐square test); ~ comparison of enzyme‐inducing AED vs. general population for oestrogen content P < 0.001 (Fisher’s exact test).
Discussion
In Ireland, the oral contraceptive pill remains the most commonly prescribed form of contraception to date, representing 74% of total contraceptive use in 2013. LARC methods accounted for 7.5% of total uptake. Women aged less than 35 years were most frequently prescribed combined oral contraceptives while the progestin‐only pill for women 35 years or older. Fourth generation (drosperinone containing) COC use prevailed throughout 2008–2013, although a significant small decline was noted since 2011 following new evidence of an increased risk of VTE compared with second generation COCs, whose uptake continued to rise since the British media contraceptive controversy in 1995, whilst third generation COCs continued their downward decline to below the POP, the latter increasing slightly but significantly over time and with age, representing 17% of total contraceptive uptake in 2013.
Due to their widespread use, only COC co‐prescribing patterns with important interacting medications were examined in this study. Low co‐prescribing rates were observed overall (16 502 of total co‐prescription claims in 2013), with benzodiazepines and prednisone being the highest co‐prescribed medicines, followed by enzyme inhibiting drugs, lamotrigine and, less frequently prescribed, enzyme‐inducing anti‐epileptic medications. Enzyme inhibiting drugs were more frequently co‐prescribed with third or fourth generation COCs than with second generation COCs. The dual pharmacologic complexities of hormonal contraception and anti‐epileptic drugs is highlighted in this study, as high rates of ineffective contraceptive methods were co‐prescribed with enzyme‐inducing anti‐epileptic medications, of which 93.6% of contraceptives contained <50 μg oestrogen.
Our study affirms the ongoing popularity of the oral contraceptive pill in Ireland, as also demonstrated by the Irish Contraception and Crisis Pregnancy (ICCP) surveys which reported a 38% and 43% OCP uptake in 2004 and 2010, respectively, increasing from 27% in 2002 of women surveyed by the Irish Survey on Lifestyle and Attitudes to Nutrition (SLAN) survey. 7, 8, 9 Across Europe, the NHS contraceptive service in the UK reported a 47% OCP uptake in those aged 16–49 years, with the majority of users being ≤35 years (2013–2014), slightly higher than in 2008–2009 (44%). 10, 11 In France, 55% of women aged 15–49 years were OCP users in 2010, with higher prevalence among those aged 15–34 years. 12 Similarly, in the United States, 16% of all women on contraception aged 15–44 years were OCP users in 2011–2013, the majority of whom were aged 15–24 years (22.4%). Female sterilization accounted for 15.5% and male condoms 9.4%. 13.
In Ireland, the combined oral contraceptive pill, most notably the fourth generation COCs, proved most popular amongst women aged <35 years and POPs for women ≥35 years (17% in 2013), consistent with UK Medical Eligibility Criteria guidelines 14, uptake of which appears variable across countries. For POPs, high uptake was noted in France (16%) and Sweden (24.4%), while low rates were observed in Denmark (4.5%), UK (5.6%) and United States (0.4%). In the latter, POP use did not vary with age, across BMI or diabetic groups. Rather, these were most frequently used among multiparous and post‐partum women, who tended to smoke ≥1 pack of cigarettes per day 2, 15, 16, 17, 18. For fourth generation COCs, these were most popular in Spain (52.4%) and Germany (44.3%). In Italy, these were second only to the third generation COCs. In France, they were third most popular and in the UK, the least favourite oral combined contraceptive method 19. Following new evidence of an increased risk of VTE compared with second generation COCs 20, Irish uptake of fourth generation COCs has been declining since 2011 (34.5% to 30.6% from 2011 to 2013, respectively), similar to the decline of third generation COCs and rise of the second generations since 1995, when the British media contraceptive controversy caused second generation COC prescriptions to rise from 31% to 43% and third generation COCs to fall from 53% to 30% between 1995 and 1996, respectively 21. Similarly, across Europe, 17% of first time users in The Netherlands were prescribed third generation COCs by 2000 compared with 59% in 1995 22. In Germany, similar trends were observed for adolescents, 46% of whom were prescribed a second generation COC in 2011 compared with 25% in 2007, whilst third generation COC prescriptions dropped (15% to 10%) 23. Recent media attention in France in 2012 following legal initiatives taken by a women who had suffered a disabling stroke whilst on a third generation COC, led to immediate changes in prescribing behaviour which resulted in a 45% drop in sales of third and fourth generation COCs and a 30% rise in first and second generation COC sales, with preference toward low dose oestrogen‐containing COCs. 12 Similarly, Denmark followed suit, reducing their sales of third and fourth generation COCs, 24 which had previously represented over 80% of total OCP sales in 2010 16. Although oral contraceptives are still the leading contraceptive method in France, their sales have been declining since 2000, from 55% to 41% between 2010 and 2013, respectively, among women aged 15–49 years. Rather, their attention has been drawn to the to long acting reversible contraceptive methods, uptake of which have increased from 2010 to 2013, especially among women aged 20–24 years (rising from 2% to 5%, respectively) and those aged 25–29 years (from 8% to 16%, respectively) 2.
LARCs are currently more cost effective than oral contraceptives and have been shown to reduce pregnancy rates, birth and abortion rates 25, 26, 27. Although uptake rates are increasing slightly over time, these remain underutilized in Ireland (7.5% in 2013), which has been recently attributed to concerns regarding insertion difficulty, particularly in nulliparous women 28. Compared with oral contraception, LARC provision services are more time consuming and labour intensive. This, together with financial and workforce challenges faced by GPs in the current climate of increasing GMS service demands, by 27% reported between 2009 and 2012, is likely to be contributory to the low LARC uptake rate identified in this study 29, despite high rates of GPs holding advanced certification in LARC methods and use. Approximately 20% of an estimated 2954 GPs in Ireland to date are currently certified by the Irish College of General Practitioners since 2009.
The low, but increasing, uptake rate of LARCs in Ireland reflects similar trends from the SLAN surveys which reported an uptake of 2.5% to 6.0% from 1998 to 2002, respectively, with 67.2% of LARC users being ≥35 years of age. Additionally, the ICCP surveys reported a 6% IUD and 3% non‐IUD (patch, implant and injection) uptake in 2003, which rose to 11% and 8% by 2010, respectively, with the biggest increase seen in women aged 18–25 years (4% to 12%) among the non‐IUD LARC group 7, 8, 9. Although still underutilized in many other countries, LARC uptake is increasing in the UK (31%), Norway (12%), United States (7.2%) and Australia (15.4%) 10, 34, 35, 36, 37. International programmes to increase uptake of LARCs in developing countries in an attempt to address the vast unmet contraceptive needs of these women have been promising 10, 30, 31, 32, 33, 34.
Further examination of contraceptive co‐prescribing practices with important interacting medication revealed an overall low co‐prescribing rate (2.4% in 2013). Polypharmacy and oral contraceptive use are age‐related, which may suggest an older cohort of GMS patients on these drugs. From most to least frequently co‐prescribed, interacting drug categories are 1) those whose effects may be potentiated by hormonal contraception, such as prednisone and benzodiazepines, 2) enzyme inhibitors (statin therapy, fluconazole, valproic acid) which may potentiate oestrogenic effect and VTE risk, 3) lamotrigine and 4) enzyme‐inducing antiepileptic drugs. Enzyme inhibitors were also found co‐prescribed with a concurrent third or fourth generation COC rather than with a second generation COC. Given the evidence to suggest that third and fourth generation COCs carry a higher risk of VTE, this interaction could theoretically potentiate this VTE risk, in particular when higher oestrogen‐containing COCs are contemplated.
Reciprocal pharmacokinetic interactions involving anti‐epileptic medications and hormonal contraceptives are well recognized 35. However, ensuring effective contraception and optimal seizure control remains an ongoing challenge, as can be demonstrated by the use lamotrigine, an enzyme‐inducer with the potential to reduce the contraceptive effect, but whose metabolism can be compromised by OCPs. Although such combination is currently not advised by the Royal College of Obstetricians and Gynaecologists, these were identified in 1948 prescription claims in 2013. Enzyme‐inducing anti‐epileptic medications have the potential to render POPs, implant/patches and COCs containing <50 μg of oestrogen ineffective contraceptive methods 36. In this study, these were the main contraceptive methods co‐prescribed with enzyme‐inducing anti‐epileptic drugs. In addition, in those prescribed an enzyme‐inducing AED most were co‐prescribed COCs containing <50μg of oestrogen. The UK and The Netherlands reported equally high proportions of women with epilepsy being prescribed <50 μg oestrogen containing COCs whilst on an enzyme‐inducing AED (56% and 43.5%, respectively) 37, 38, thereby inevitably placing these women at an increased risk of unintended pregnancies. A recent Epilepsy Birth Control Registry (EBCR) survey of women with epilepsy aged 18–47 years in the United States effectively revealed that, of the women who had experienced pregnancies, 60% were unintended compared with a rate of 49% in the general population. 39.
Thus, such knowledge of the intricate dual pharmacology of anti‐epileptic drugs and hormonal contraceptives is essential to ensure therapeutic and clinical effectiveness. This current study highlights the possibility of poor levels of pharmacological knowledge, as can also be demonstrated in a survey of hospital residents in the United States, whereby, despite knowing that phenytoin and carbamazepine can lower OCP levels, neurology and obstetrics and gynaecology (OBGYN) residents were not aware that carbamazepine can also interact with other forms of hormonal contraception; 85% of OBGYN and 63% of neurology residents were not aware that topiramate may decrease the efficacy of OCPs and 65% and 47% of these residents, respectively, were also unaware that oral contraception can additionally lower lamotrigine levels 40.
Strengths of the study were that data from the GMS population were used. Although they are not representative of the entire Irish population, they do comprise of a large cohort of patients for whom all prescriptions are identified.
A limitation of the HSE‐PCRS database is the lack of clinical information to help determine prescribing adherence level according to the UKMEC criteria. In addition, no over the counter product information was available. However, all of the drugs considered are prescription only and, therefore, this is not likely to have had a significant effect.
In the cases of inappropriate prescribing, we did not examine for evidence that could indicate appropriate dose adjustments or changes in contraceptive method to ensure contraceptive effectiveness because of the overall low frequencies of co‐prescribing practices identified in this study, in addition to the lack of clinical information.
Since their approval for over the counter sales in 2011, emergency contraceptive use in Ireland cannot be fully captured for the purpose of this study and therefore was not included in this study.
Other important interacting medications that were not included in this study were antiviral medications, which are not included in the HSE‐PCRS database.
This study emphasizes the need to optimise co‐prescribing practices involving hormonal contraceptives and anti‐epileptic medications and highlights the need to address the barriers to the currently low uptake of LARC methods in Ireland.
Copyright Statement
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide licence to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to i) publish, reproduce, distribute, display and store the Contribution, ii) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the Contribution, iii) create any other derivative work(s) based on the Contribution, iv) to exploit all subsidiary rights in the Contribution, v) the inclusion of electronic links from the Contribution to third party material where‐ever it may be located and, vi) licence any third party to do any or all of the above.
Competing Interests
All authors have completed the Unified Competing Interest form and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Contributors
LOM drafted and revised the manuscript, AML and MB reviewed the drafted manuscript, KB obtained and analyzed the data and reviewed the manuscript.
Transparency Declaration
This manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
We thank the HSE‐PCRS for supplying the data on which the study was based and the ICGP for releasing their data on LARC course uptake by GPs.
O'Mahony, L. , Liddy, A.‐M. , Barry, M. , and Bennett, K. (2015) Hormonal contraceptive use in Ireland: trends and co‐prescribing practices. Br J Clin Pharmacol, 80: 1315–1323. doi: 10.1111/bcp.12755.
References
- 1. Szarewski A, Mansour D. The ‘pill scare’: the responses of authorities, doctors and patients using oral contraception. Hum Reprod Update 1999; 5: 627–32. [DOI] [PubMed] [Google Scholar]
- 2. Bajos N, Rouzaud‐Cornabas M, Panjo H, Bohet A, Moreau C and the Fecond team . The French pill scare: towards a new contraceptive model? Popul Soc 2014;511. [Google Scholar]
- 3.European Medicines Agency: EMA/739865/2013 Assessment report for combined hormonal contraceptives containing medicine products 2014.
- 4. Williams D, Kelly A, Carvalho M, Feely J. Effect of the British warning on contraceptive use in the general medical service in Ireland . Irish Med J 1998; 91: 202–3. [PubMed] [Google Scholar]
- 5. Back DJ, Orme ML. Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokinet 1990; 18: 472–84. [DOI] [PubMed] [Google Scholar]
- 6. Williams D. Antiepileptic drugs and contraception. US Pharm 2014; 39: 39–42. [Google Scholar]
- 7. Shiely F, Kelleher C, Galvin M. Sexual health of the Irish adult population: Findings from SLAN. Crisis Pregnancy Agency Report no.11 2004;1–60.
- 8. Rundle K, Leigh C, McGee H, Layte R. Irish contraception and crisis pregnancy study: A survey of the general population. Crisis Pregnancy Agency Report no.7 2004; 1–179.
- 9. McBride O, Morgan K, McGee H. Irish contraception and crisis pregnancy study: A survey of the general population. Crisis Pregnancy Agency Report no.24 2010; 1–176.
- 10.NHS Contraceptive Services: England, Community Contraceptive Clinics, Statistics from 2013‐2014. Health and Social Care Information Centre 2014; 1‐30.
- 11.NHS Contraceptive Services: England 2008–2009, Health and Social Care Information Centre, Lifestyle Statistics 2009; 2: 1–52 [Google Scholar]
- 12.Agence National de Securité du Médicament et des produits de santé. Evolution recente de l'utilisation des contraceptifs en France: Contraceptifs oraux combinés et autres contraceptifs. Bilan à un an 2014. Available at http://ansm.sante.fr/content/download/58585/751403/version/1/file/Contraceptifs‐Oraux_Evolution‐consommation‐1an‐fevrier2014.pdf (last accessed 24 September 2005).
- 13. Daniels K, Daughtery J, Jones J. Current contraceptive states among women aged 15–44: United States, 2011–2013. NCHS Data Brief 2014; 173: 1–8. [PubMed] [Google Scholar]
- 14. Faculty of sexual & reproductive healthcare . Royal College of Obstetricians & Gynaecologists. Available at http://www.fsrh.org/pdfs/UKMEC2009.pdf (last accessed 24 September 2015).
- 15. Josefsson A, Wirehn AB, Lindberg M, Foldemo A, Brynhidsen J. Continuation rates of oral hormonal contraceptives in a cohort of first‐time users: a population‐based registry study, Sweden 2005–2010. BMJ Open 2013; 3: e003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson NM, Laursen M, Lidegaard Ø. Oral contraception in Denmark 1998–2010. Acta Obstet Gynecol Scand 2012; 91: 810–5. [DOI] [PubMed] [Google Scholar]
- 17. Cea‐Soriano L, Barcia Rodriguez LA, Machlitt A, Wallander MA. Use of prescription contraceptive methods in the UK general population: a primary care study. BJOG 2014; 121: 53–60. [DOI] [PubMed] [Google Scholar]
- 18. Hall KS, Trussel J, Schwartz EB. Progestin‐only contraceptive pill use among women in the United States . Contraception 2012; 86: 653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agence National de Securité du Médicament et des produits de santé . L'utilisation des contraceptifs depuis 1 an et les actions mises en place 2013–2014. Available at http://ansm.sante.fr/content/download/58627/751857/version/3/file/Contraceptifs‐oraux_utilisation‐fevrier 2014‐presentation.pdf (last accessed 24 September 2015)
- 20.Available at https://www.hpra.ie/docs/default‐source/Safety‐Notices/imb‐mims‐july‐2011‐cocs‐hyperlinked.pdf (last accessed 24 September 2015).
- 21. Williams D, Kelly A, Carvalho M, Feely J. Effect of the British warning on contraceptive use in the general medical service in Ireland . Ir Med J 1998; 91: 202–3. [PubMed] [Google Scholar]
- 22. De Jong‐van den Berg L, Tobi H, Bijker B, Van den Berg P. Influence of the third generation pill controversy on prescriptions for oral contraceptives among first time users: population based study. BMJ 2003; 326: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ziller M, Rashed AN, Ziller V, Kostev K. The prescribing of contraceptives for adolescents in German gynaecologic practices in 2007 and 2011: a retrospective database analysis. J Paediatr Adolesc Gynaecol 2013; 26: 261–4. [DOI] [PubMed] [Google Scholar]
- 24. Available at https://sundhedsstyrelsen.dk/en/news/2012/doctors‐in‐denmark‐follow‐new‐recommendations‐for‐contraceptive‐pills (last accessed 24 September 2015).
- 25. Secura GM, Madden T, McNicholas C, Mullersman J, Buckel CM, Zhao Q, Peipert JF. Provision of no‐cost, long‐acting contraception and teenage pregnancy. NEJM 2014; 371: 1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Connolly A, Pietri G, Yu J, Humphreys S. Association between long‐acting reversible contraceptive use, teenage pregnancy, and abortion rates in England . Int J Wom Health 2014; 6: 961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Birbala Dixit, Anjali C. Awareness and impact of long‐acting reversible contraception in the community general practice. RCOG poster 2014. Available at http://www.epostersonline.com/rcog2014/?q=node/427 (last accessed 24 September 2015).
- 28. Buhling KJ, Hauck B, Dermout S, Ardaens K, Marions L. Understanding the barriers and myths limiting the use of intrauterine contraception in nulliparous women: results of a survey of European/Canadian healthcare providers. Eur J Obstet Gynaecol Reprod Biol 2014;183:146‐54. [DOI] [PubMed] [Google Scholar]
- 29.Irish Medical Organisation on the review of the operation of cuts under the Financial Emergency Measures in the Public Interest Act 2009 as covered by S.I No.638/2010‐Health Professionals (Reduction of payments to General Practitioners) Regulations 2010. 2013. Available at http://www.imo.ie/national‐professionwide‐i/fempi‐submissions‐2013/GPs‐FEMPI‐SUBMISSION.pdf (last accessed 24 September 2015).
- 30. Bratlie M, Aarvold T, Skårn ES, Lundekvam JA, Nesheim BI, Askevold ET. Long‐acting reversible contraception for adolescents and young adults – a cross‐sectional study of women and general practitioners in Oslo, Norway. Eur J Contracept Reprod Health Care 2014. Jun; 9(3): 194–202. [DOI] [PubMed] [Google Scholar]
- 31. Branum AM, Jones J. Trends in long‐acting reversible contraception use among U.S. women aged 15–44. NCHS Data Brief, 2015; 188: 1–8. [PubMed] [Google Scholar]
- 32. Mazza D, Harrison C, Taft A, Brijnath B, Britt H, Hobbs M, Stewart K, Hussainy S. Current contraceptive management in Australian general practice: an analysis of BEACH data. Med J Aust 2012; 197: 110–4. [DOI] [PubMed] [Google Scholar]
- 33. Ngo TD, Nuccio O, Reiss K, Pereira S. Expanding long‐acting and permanent contraceptive use in sub‐Saharan Africa to meet FP2020 goals. London: Marie Stopes International, Research brief series, 2013. [Google Scholar]
- 34. Curry DW, Rattan J, Huang S, Noznesky E. Delivering high‐quality family planning services in crisis‐affected settings II: results. Glob Health Sci Pract 2015; 3: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol 2010; 3: 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Epilepsy Ireland . Contraception for women with epilepsy 2013. Available at http://www.epilepsy.ie/assets/78/278BFE75‐A681‐8F2B‐037F919995FFDD3E_document/Contraception_for_women_with_epilepsy.pdf 2015 (last accessed (last accessed 24 September 2015).
- 37. Shorvon SD, Tallis RC, Wallace HK. Antiepileptic drugs: coprescription of proconvulsant drugs and oral contraceptives: a national study of antiepileptic drug prescribing practice. J Neurol Neurosurg Psychiatry 2002; 72: 114–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang H, Bos JH, de Jong‐van den Berg LT. Co‐prescription of antiepileptic drugs and contraceptives. Contraception 2012; 85: 28–31. [DOI] [PubMed] [Google Scholar]
- 39. Herzog AG, Davis AR, Allen Hauser A. Epilepsy Birth Control Registry. Available at http://www.epilepsybirthcontrolregistry.org/ (last accessed (last accessed 24 September 2015).
- 40. Sahay M, Garic I, Gawron L, Hammond C, Kennedy J, Macken M, Schuele S, Stika C, York S, Gerard E. Awareness of drug‐drug interactions between synthetic hormones and antiepileptic medications: a survey of neurology, obstetrics and gynaecology, internal medicine and psychiatry residents. Neurology 2013; 80: (Meeting Abstract 1):P01.034. [Google Scholar]


