Abstract
Aims
Inter‐individual variability in dose requirements of calcineurin inhibitors (CNI) has been linked to genetic polymorphisms of CYP3A enzymes. CYP3A5*3, CYP3A4*1B and CYP3A4*22 alleles of liver grafts may explain about one third of the inter‐individual differences in pharmacokinetics of ciclosporin and tacrolimus in recipients. However, non‐genetic factors, influencing CYP3A expression, can contribute to the variability of CYP3A function due to phenoconversion. The present study evaluated the association between CYP3A4 expression combined with CYP3A5 genotype of donor livers and recipients' CNI therapy after transplantation.
Methods
The contribution of donors' CYP3A5 genotype and CYP3A4 expression to the blood concentrations and dose requirements of CNIs was evaluated in 131 liver transplant recipients.
Results
The recipients with grafts from normal CYP3A4 expresser donors carrying CYP3A5*3/*3 required CNI maintenance doses more or less similar to the bodyweight‐controlled starting doses (9.1 mg kg−1 of ciclosporin and 0.1 mg kg−1 of tacrolimus). The patients transplanted with grafts from low CYP3A4 expressers required substantial reduction (by about 50%, 4.2 mg kg−1 of ciclosporin, 0.047 mg kg−1 of tacrolimus, P < 0.001), while the recipients with grafts from high expressers or with grafts carrying at least one copy of the functional CYP3A5*1 allele required an increase (by about 50% [12.8–13.8 mg kg−1] for ciclosporin and 100% [0.21 mg kg−1] for tacrolimus, P < 0.001) of the initial CNI dose for achieving target blood concentrations.
Conclusions
Donor livers' CYP3A‐status, taking both CYP3A5 allelic variations and CYP3A4 expression into account, can better identify the risk of CNI over‐ or underexposure, and may contribute to the avoidance of misdosing‐induced graft injury in the early post‐operative period.
Keywords: ciclosporin, CYP3A status, CYP3A4 expression, CYP3A5 genotyping, liver transplant recipients, tacrolimus
What Is Already Known About This Subject
CYP3A enzymes are the main catalysts of the metabolism of calcineurin inhibitors (CNIs).
CYP3A5*3, CYP3A4*1B and CYP3A4*22 alleles explain one third of inter‐individual differences in pharmacokinetics and dose‐requirement of ciclosporin and tacrolimus.
Hepatic CYP3A activities can be estimated by combining CYP3A5 genotyping and CYP3A4 expression analysis of leukocytes.
What This Study Adds
CYP3A4 expression rates of donors combined with CYP3A5 genotypes influenced CNI blood concentrations in recipients.
The recipients with grafts from low or high CYP3A4 expressers or with grafts carrying CYP3A5*1 required substantial modification of the initial CNI doses.
The donors' CYP3A‐status can identify the risk of CNI over or underexposure.
Introduction
The mainstay of immunosuppressive regimens for liver transplant recipients is calcineurin inhibitor (CNI) therapy with ciclosporin or tacrolimus 1, 2. Despite their effectiveness in prophylaxis of organ rejection, these drugs display a narrow therapeutic index and high inter‐ and intra‐individual variability in their pharmacokinetics requiring monitoring of blood concentrations for optimal safety and therapeutic efficacy. Underdosing increases the risk of immunological rejection of the transplanted organ, whereas overdosing leads to increasing risk of infections and hepato or nephrotoxicity 3, 4. The conventional clinical strategy for CNI treatment is based on dosage adjusted to blood concentration rather than to bodyweight. However, it does not facilitate much in achieving target blood concentrations during the critical early post‐operative days.
Genetic polymorphisms of transport proteins and drug‐metabolizing enzymes are supposed to contribute to individual differences in CNI dose‐requirement 5, 6, 7. Because of the importance of efflux transporters in absorption, distribution and elimination of drugs, they have been extensively investigated in relation to CNI pharmacokinetics. ABCB1 plays a role in in vitro expulsion of CNIs 8. However, the available clinical data for the association between ABCB1 polymorphisms and CNI pharmacokinetics are controversial, and do not confirm the influence of ABCB1 variants on CNI bioavailability 9, 10. Ciclosporin and tacrolimus undergo extensive metabolism by CYP3A enzymes. CYP3A4 activity displays more than 100‐fold inter‐individual variability 11, which is partly attributed to genetic factors. The CYP3A4*1B allele seems to result in increased transcription of CYP3A4. However, the clinical significance of CYP3A4*1B to CYP3A4 function is rather contradictory 5, 7, 12. CYP3A4*22 is associated with low hepatic CYP3A4 mRNA expression and decreased CYP3A4 activity 13. However, the association between CYP3A4*22 and pharmacokinetic behaviour of CYP3A‐substrates is suggested to be evaluated in combination with the CYP3A5 genotype 14. The CYP3A5*3 allele results in a splicing defect and non‐functional, truncated CYP3A5 protein. Those individuals who have the functional CYP3A5 enzyme (CYP3A5*1/*1 and CYP3A5*1/*3 genotypes) are presumed to metabolize some CYP3A substrates more rapidly than CYP3A5 non‐expressers. The allele frequencies of CYP3A4*22 and CYP3A5*3 (in Caucasian populations 5–7% and 90%, respectively) explain some inter‐individual differences in CNI pharmacokinetics 14.
CYP3A4 is primarily responsible for the metabolism of ciclosporin, whereas CYP3A5 is the main catalyst of tacrolimus metabolism 15, 16. Although CYP3A genotypes of donor liver according to CYP3A4*22 and CYP3A5*3 help with identification of the risk of CNI over and underexposure, the optimization of CNI therapy in recipients is precarious and time consuming with several dose modifications. The genetically determined variance in CYP3A activities is modulated by internal factors (hormonal status, diseases, age) or environmental factors (medication, nutrition) resulting in transient poor (or extensive) metabolism. The CYP genotype determines the potential for the expression of functional or non‐functional CYP enzymes, whereas non‐genetic factors give rise to altered phenotypes. Thus, the CYP3A4*1/*1 genotype, predicted to be translated to CYP3A4 enzyme with normal function, may be switched into poor (or extensive) metabolism due to phenoconversion 17. Recipients' CNI‐metabolizing capacity can be estimated by the evaluation of the CYP3A status of donor livers. We have previously described a complex diagnostic system (CYPtest™) that determines drug‐metabolizing capacity by combining CYP3A5 genotype and current CYP3A4 expression in leukocytes 11. CYP3A4 mRNA levels in leukocytes of those subjects who do not carry CYP3A5*1 were proven to reflect hepatic CYP3A4 activities. Thus, CYP3A5 genotyping for CYP3A5*3 identifies the genetically determined CYP3A5 expresser grafts, and CYP3A4 expression in donors' leukocytes can estimate reduced or increased CYP3A4 activity of liver grafts. Recipients transplanted with liver grafts carrying CYP3A5*1 are able to metabolize tacrolimus more rapidly than others with CYP3A5 non‐expresser grafts 18. The liver grafts with the CYP3A4*22 allele are predicted to display decreased CYP3A4 mRNA levels which lead to permanent low CYP3A4 activity 14, whereas non‐genetic factors modifying the expression of a functional CYP3A4 gene in donor liver result in transient poor (or extensive) CNI metabolism.
Information on CYP3A‐status of a liver graft by estimating a donor's CYP3A4 expression combined with CYP3A5 genotypes can have predictive power regarding the recipient's medication, and may refine the immunosuppressant therapy facilitating the appropriate dosage for an individual recipient. The goals of the present work were to investigate the donors' CYP3A‐status predicting potential poor or extensive metabolism of CNIs in recipients and to analyze the potential influence of donors' CYP3A5 genotype and CYP3A4 expression on the 12 h post‐dose CNI blood concentrations and recipients' dose requirements. We attempted to provide evidence that CYP3A5 and CYP3A4 genotypes are not the only determinant factors in CYP3A metabolizer status of a graft, but the expression rate of the CYP3A4 gene can highly influence a recipient's CYP3A metabolizing capacity and his/her response to CNI therapy.
Methods
Patients and study design
The study protocol was approved by the Hungarian Committee of Science and Ethics. The study was performed under the regulation of Act CLIV of 1997 on Health and of the decree 23/2002 of the Minister of Health of Hungary, and in accordance with the declaration of Helsinki. For investigations with transplant recipients, informed consent was obtained from the participants. Liver transplant recipients (n = 131) transplanted at the Department of Transplantation and Surgery, Semmelweis University (Budapest, Hungary) were enrolled in the study. Recipients' demographic data (Table 1) as well as CNI dosage and pre‐dose blood concentrations in the early post‐operative period after transplantation (generally up to 3–4 weeks) were recorded. The liver grafts were retrieved from haemodynamically stable brain‐death donors with normal liver function (n = 130) or from a living donor (n = 1). All the donors and the recipients belonged to the Caucasian (White) population. In the early post‐operative period, the recipients' drug therapy was applied according to the conventional clinical protocol, including immunosuppressant and anti‐inflammatory agents (see below) as well as prophylactic medications, such as antibiotics (sulfamethoxazole‐trimethoprim, ciprofloxacin, meropenem), antiviral (ganciclovir, valganciclovir) and antifungal drugs (amphotericin B, fluconazole), acid‐reducing agents (famotidine, pantoprazole) and, if necessary, analgesics/anesthetics (propofol).
Table 1.
Recipients' demographic characteristics
| Recipients' demographic data | |
|---|---|
| Number | 131 |
| Gender (male, female) | Male: 54.2%, Female: 45.8% |
| Age at time of transplantation (years) | |
| median (range) | 50 (38.5–55) |
| Body weight (kg), median (range) | 74 (61.25; 87) |
| Number of transplants | |
| 1st | 126 (96.2%) |
| 2nd or more | 5 (3.8%) |
| Primary liver disease | |
| 1. Acute liver failure | |
| Mushroom poisoning | 1 (0.76%) |
| Other | 2 (1.53%) |
| 2. Chronic liver diseases | |
| Autoimmune hepatitis | 6 (4.58%) |
| Alcohol‐related liver disease | 22 (16.79%) |
| Congenital fibrosis | 3 (2.29%) |
| Hepatitis B | 7 (5.34%) |
| Hepatitis C | 51 (38.93%) |
| Primary biliary cirrhosis | 4 (3.05%) |
| Primary sclerosing cholangitis | 19 (14.50%) |
| Tumour: hepatocellular carcinoma | 3 (2.29%) |
| other | 2 (1.53%) |
| Wilson's disease | 2 (1.53%) |
| Others | 9 (6.87%) |
Immunosuppressive protocol and drug monitoring
The CNI therapy was started 6 h after liver transplantation and both ciclosporin and tacrolimus were administered twice daily. The daily dose was defined as the sum of the morning dose, given after the blood sampling for trough blood concentration measurement and the evening dose administered after 12 h. The initial CNI dose was adjusted to the recipients' bodyweight and thereafter controlled by the pre‐dose CNI blood concentrations according to the standard clinical protocol. The patients received either a 10 mg kg−1 daily dose of ciclosporin (n = 34) or a 0.1 mg kg−1 daily dose of tacrolimus (n = 97). Oral ciclosporin and tacrolimus dosage was adjusted to a target therapeutic window in the range of 200–300 ng ml−1 and of 10–15 ng ml−1, respectively. The immunosuppressant therapy based on one of the CNIs was applied in combination with mycophenolate mofetil and a steroid (methylprednisolone). Mycophenolate mofetil was applied at the daily dose of 2 g at the early post‐operative period, whereas the initial methylprednisolone dose of 1 g was administered at the time of the operation, and the subsequent doses were gradually tapered to a maintenance daily dose of 32 mg.
Therapeutic drug monitoring was performed routinely (every day in the first week and every second day from the second week), and the CNI dose was modified if the exposure was out of the target range of CNI blood concentration. The 12 h post‐dose trough concentrations of CNIs (C 0) were determined in whole blood taken at 08.00 h before the patient was administered the morning dose. The blood concentrations were measured using enzyme immunoassay techniques for ciclosporin (Cyclosporine Flex on Dimension RxL HM, Dade Behring Ltd, Milton Keynes, UK) and for tacrolimus (TACR Flex Dimension, Dade Behring Inc., Newark, DE). For ciclosporin, the assay range was 80–500 ng ml−1, whereas for tacrolimus, it was 2–32 ng ml−1. The intra‐ and inter‐day variability for the quantification of CNIs was less than 10%. Pre‐dose concentrations were calculated by dividing the C 0 by the corresponding 24 h dose on a mg kg−1 bodyweight basis.
CYP3A‐status of the liver grafts
The estimation of the CYP3A‐status of 131 liver grafts was assayed in donors' peripheral blood samples obtained at the time of explantation. Genomic DNA and leukocytes were isolated from the peripheral blood samples according to the methods described by Temesvári et al. 11. CYP3A5 genotyping was carried out by hydrolysis single nucleotide polymorphism analysis for CYP3A5*3 using TaqMan probes (BioSearch Technologies, Novato CA). The CYP3A5 genotypes were distinguished by post‐PCR allelic discrimination plotting the relative fluorescence values for wild‐type and mutant alleles. The allelic content of each sample was determined by a multicomponent algorithm, yielding three allelic clusters representing the CYP genotypic constituent, homozygous wild type (CYP3A5*1/*1), homozygous mutant type (CYP3A5*3/*3) and heterozygous genotype (CYP3A5*1/*3). For CYP3A4 expression, total RNA was extracted from leukocytes, RNA (3 μg) was reverse transcribed into single‐stranded cDNA using the Maxima First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA), and then real‐time PCR with human cDNA was performed using KAPA Fast Probes Mastermix (KAPA Biosystems, Cape Town, South Africa) and UPL probe for CYP3A4 (Roche Diagnostics GmbH, Mannheim, Germany). The quantity of CYP3A4 mRNA relative to that of the housekeeping gene glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was determined. Three categories of CYP3A4 expression were applied to describe low, normal and high expressers. The cut‐off values for the CYP3A4 mRNA levels in leukocytes have been previously established on the basis of the cut‐off values for the hepatic CYP3A4 activities (nifedipine oxidation or midazolam 1′‐ and 4‐hydroxylation) 11. Low expressers displayed a CYP3A4 : GAPDH ratio less than 10−6, normal expressers a ratio between 10−6 and 10−4, whereas in high expressers the ratio was higher than 10−4.
Data analysis
The recipients were sorted by the CYP3A‐status of the donors. Donors carrying at least one CYP3A5*1 allele were considered to be CYP3A5 expressers, while donors with the CYP3A5*3/*3 genotype were CYP3A5 non‐expressers. The liver grafts from CYP3A5 non‐expressers were subdivided into low, normal and high CYP3A4 expressers by the donors' CYP3A4 mRNA levels. The serum concentration values of CNIs were normalized by the dose and the bodyweight of transplant recipients, and expressed as (μg ml−1) × (mg dose kg−1 bodyweight)−1. The data of normalized CNI blood concentrations and dose requirements for the optimal therapeutic level in the recipient groups transplanted with liver grafts in various CYP3A‐statuses were expressed as the median. It should be noted that median values did not differ much (generally by 1–2% and always under 5%) from the mean values. Between group differences were calculated by the use of Kruskal–Wallis analysis of variance followed by Dunn's multiple comparisons test. A P value of <0.05 was considered to be statistically significant.
Results
CYP3A‐status of the donors
Of 131 liver grafts for CYPtest recipients, most of the grafts (n = 106) were from CYP3A5 non‐expresser donors (CYP3A5*3/*3), and were thus expected to lack the functional CYP3A5 enzyme (Table 2). Twenty‐five liver grafts from donors carrying CYP3A5*1/*3 heterozygous (n = 24) or CYP3A5*1/*1 homozygous wild (n = 1) genotypes were considered as CYP3A5 expressers. The frequency of the CYP3A5*3 allele in the liver donors (90.1%) was similar to that in Caucasian (White) populations (88–97%) 19, 20.
Table 2.
CYP3A‐status of the liver donors
| CYP3A‐status | |
|---|---|
| CYP3A5 genotype (rs776746) | |
| *1/*1 | 1 (0.76%) |
| *1/*3 | 24 (18.32%) |
| *3/*3 | 106 (80.92%) |
| CYP3A4 expression (CYP3A5 non‐expressers) | |
| High expresser | 15 (14.1%) |
| Normal expresser | 49 (46.3%) |
| Low expresser | 42 (39.6)% |
For the categorization of the liver grafts regarding CYP3A4 expression, we applied the estimation reported by Temesvári et al. 11. They measured selective CYP3A4 activities in the liver tissues of healthy organ donors (n = 164) and statistically distinguished three categories for the hepatic CYP3A4 activities (low, medium/normal and high) by calculating the quartiles of the CYP3A4 activity distributions. The cut‐off values between the CYP3A4 activity categories were set to the first and the third quartiles of the donors. The cut‐off values for the CYP3A4 mRNA levels measured in the leukocytes of the organ donors were described by the cut‐off values for the hepatic CYP3A4 activity categories. In our present study, we distinguished low, normal and high CYP3A4 expresser donors using the cut‐off values of 10−6 and 10−4 for CYP3A4 mRNA levels in leukocytes determined by Temesvári et al. 11. CYP3A4 expression assays revealed that almost half of the CYP3A5 non‐expresser donors (46.3%) expressed CYP3A4 at normal (medium) level, substantial portion (39.6%) was low CYP3A4 expressers, whereas 14.1% of the donors displayed high CYP3A4 expression (Table 2). On the basis of the donors' CYP3A‐status (CYP3A5 genotypes and CYP3A4 expression in leukocytes), the grafts were grouped into two main categories, CYP3A5 expressers and non‐expressers, and the CYP3A5 non‐expressers were subdivided into three subgroups, low, normal (medium) and high CYP3A4 expressers.
Donors' CYP3A status and recipients' CNI exposure
Since both CNIs are characterized by a narrow therapeutic window, achieving the therapeutic trough concentrations particularly in the initial period after transplantation is of high critical importance. The present study evaluated the association between the donors' CYP3A‐status and the recipients' CNI therapy after transplantation which can contribute to the improvement of personalized medication of liver transplant patients. Recipients generally receive either ciclosporin or tacrolimus to prevent rejection. However, tacrolimus appears to possess more potent immunosuppressant properties compared with ciclosporin and has been the preferred medication in liver transplantation in many centres recently. In the Budapest centre, only 34 of 131 liver transplant recipients were treated with ciclosporin after transplantation.
The statistical analysis displayed significant association between the donors' CYP3A‐status and the stable blood concentrations of ciclosporin normalized by the dose and the recipient's bodyweight. The normalized blood concentrations of ciclosporin were comparable in the patients transplanted with the liver grafts carrying the CYP3A5*1 allele and with high CYP3A4 expresser grafts from the donors with the CYP3A5*3/*3 genotype (10.8 ± 1.70 and 12.9 ± 1.95 ng ml−1 per mg kg−1 body weight, respectively) (Figure 1A). The ciclosporin blood concentrations were significantly higher in the recipients transplanted with grafts from CYP3A5 non‐expresser donors displaying low or normal CYP3A4 mRNA levels. The recipients transplanted with liver grafts from low CYP3A4 expresser donors displayed more than 5‐fold higher ciclosporin blood concentrations (66.2 ± 7.43 ng ml−1 per mg kg−1 body weight), whereas the pre‐dose concentrations in the patients with liver grafts from normal CYP3A4 expressers were about 3‐fold higher (35.0 ± 4.94 ng ml−1 per mg kg−1 body weight) than in recipients with high CYP3A4 expresser organs or with CYP3A5*1 carrier grafts (Figure 1A).
Figure 1.
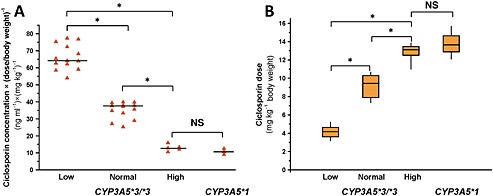
Influence of the donors' CYP3A status (CYP3A5 genotypes and CYP3A4 expression) on the blood concentrations and dose requirements of ciclosporin in liver transplant patients. (A) 12 h post‐dose trough concentrations of ciclosporin (ng ml–1 per mg kg–1 bodyweight) and (B) dose requirements (mg kg–1 bodyweight) of recipients in the course of CYP3A status of the liver donors are presented. Low, Normal, High: the levels of CYP3A4 expression; NS: not statistically significant; *: statistically significant (P < 0.001)
According to clinical practice, 250 ng ml−1 of pre‐dose ciclosporin blood concentration is considered to be optimal in liver transplant recipients in the early post‐operative period. The ciclosporin dose requirement for the target blood concentration of 250 ng ml−1 was comparable between CYP3A5 expresser and high CYP3A4 expresser groups (13.8 ± 1.77 and 12.8 ± 1.10 mg kg−1 body weight, respectively) (Figure 1B). The recipients transplanted with liver grafts from low or normal CYP3A4 expresser donors required a significantly lower dose of ciclosporin for the optimal blood concentration. The dose requirement was 30–35% lower for the patients transplanted with grafts from normal CYP3A4 expresser donors (9.1 ± 1.24 mg kg−1 body weight), and about 70% lower for the recipients transplanted with liver grafts from low CYP3A4 expresser donors (4.2 ± 0.64 mg kg−1 body weight) than for the patients in the high CYP3A4 expresser or CYP3A5 expresser groups (Figure 1B). Multiple comparison analysis showed that both the CYP3A5 genotypes and CYP3A4 expression influenced the pre‐dose blood concentrations or the dose requirements of ciclosporin for the optimal blood concentration in the recipients. However, the functional CYP3A5 expression in the liver grafts seemed to display similar effects on ciclosporin exposure and dose requirement in recipients to the high CYP3A4 expression. Consistently, the blood concentration and dose requirement of ciclosporin in the recipients transplanted with CYP3A5 non‐expresser liver grafts were found to be influenced by CYP3A4 expression.
For tacrolimus, the blood concentration range of 7.5–15 ng ml−1 was targeted, whereas 11 ng ml−1 of pre‐dose tacrolimus blood concentration was considered to be optimal in liver transplant recipients in the early post‐operative period. The majority (n = 97) of the 131 recipients were treated with tacrolimus after liver transplantation. A similar trend in trough concentrations and dose requirements was observed in tacrolimus treated recipients to ciclosporin treated patients. No significant differences in pre‐dose concentrations and dose‐requirement of tacrolimus were found between the recipients transplanted with CYP3A5 expresser grafts and those with grafts from high CYP3A4 expressers carrying the CYP3A5*3/*3 genotype (32.0 ± 10.91 and 52.3 ± 7.00 ng ml−1 per mg kg−1 body weight, respectively; Figure 2A) (0.204 ± 0.028 and 0.213 ± 0.037 mg kg−1 body weight, respectively; Figure 2B). Normal CYP3A4 expression resulted in some increase in pre‐dose concentrations of tacrolimus (91.05 ± 6.65 ng ml−1 per mg kg−1 body weight) in recipients, while the liver grafts from donors expressing CYP3A4 at low levels drastically increased the trough blood concentrations of tacrolimus (235.4 ± 25.92 ng ml−1 per mg kg−1 body weight) (Figure 2A). Consequently, the dose requirements of tacrolimus were about 50% lower in the normal CYP3A4 expresser group (0.109 ± 0.009 mg kg−1 body weight), and about 80% lower in recipients transplanted with liver grafts from low CYP3A4 expresser donors (0.047 ± 0.011 mg kg−1 body weight) than in those with grafts from donors expressing high CYP3A4 mRNA levels or in those with grafts carrying the CYP3A5*1 allele (Figure 2B). Taken together, the CYP3A‐status of the liver donors was demonstrated to be in close association with tacrolimus exposure in recipients similar to ciclosporin exposure. Furthermore, the presence of the CYP3A5*1 allele had the same effect on trough concentrations and dose requirements of tacrolimus as high CYP3A4 expression.
Figure 2.
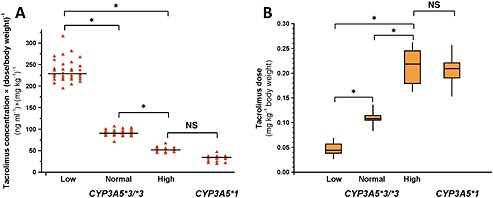
Influence of the donors' CYP3A status (CYP3A5 genotypes and CYP3A4 expression) on the blood concentrations and dose requirements of tacrolimus in liver transplant patients. (A) 12 h post‐dose trough concentrations of tacrolimus (ng ml–1 per mg kg–1 bodyweight) and (B) dose requirements (mg kg–1 bodyweight) of recipients in the course of CYP3A status of the liver donors are presented. Low, Normal, High: the levels of CYP3A4 expression; NS: not statistically significant; *: statistically significant (P < 0.001)
Discussion
The blood concentrations of tacrolimus and ciclosporin in recipients are strongly influenced by the CNI metabolizing capacity of the liver grafts and are also critical to avoid the therapeutic failure or toxicity of CNIs. Considering the primary role of CYP3A enzymes in the metabolism of ciclosporin and tacrolimus, several authors have attempted to display association between CYP3A genotypes and pharmacokinetics of orally administered CNIs 6, 7, 9. CYP3A5*3 and CYP3A4*22 have been demonstrated to influence tacrolimus dose requirements of transplant patients, whereas there has been much controversy about the association with ciclosporin pharmacokinetics 5. Most of the subjects in White (Caucasian) populations are homozygous for the non‐functional CYP3A5*3, and only 7–10% of the population are CYP3A5 expressers, carrying CYP3A5*1/*3 or CYP3A5*1/*1 genotypes 19, 20. Tacrolimus has been reported to be extensively metabolized in recipients transplanted with liver grafts from CYP3A5 expresser donors 18, 21. The CYP3A4*22 decrease‐of‐function allele has been linked to a decrease in CYP3A4 expression (mRNA and enzyme protein) and to reduced metabolism of both tacrolimus and ciclosporin 14, 22. Nonetheless, screening of CYP3A5*3 and CYP3A4*22 can explain less than 20% of altered CNI dose requirements of transplant recipients 23, 24, likely due to the relatively low prevalence of CYP3A5*1 and CYP3A4*22 alleles in European White populations. According to our hypothesis, the CYP3A‐status of the liver donors, taking both CYP3A5 allelic variations and CYP3A4 expression into account, may better identify the risk of CNI over or underexposure, and may optimize CNI therapy particularly in the early post‐operative period after transplantation.
Our present work is the first attempt to provide evidence for that inter‐individual variability in CNI metabolism can be estimated by combining CYP3A5 genotyping and CYP3A4 expression analysis of the liver donors. The recipients transplanted with grafts from normal CYP3A4 expresser donors carrying CYP3A5*3/*3 required a maintenance dose more or less similar to the starting dose which was calculated from the bodyweight (9.1 mg kg−1 of ciclosporin and 0.1 mg kg−1 of tacrolimus). However, the CNI dosing for the recipients with grafts from low or high CYP3A4 expresser donors as well as with grafts carrying at least one copy of the functional CYP3A5*1 allele had to be modified. More than 60% of the recipients required an increase or decrease of the initial CNI dose for achieving the desired target blood concentrations. For liver transplant recipients, the primary importance of the donors' CYP3A5 genotype in tacrolimus clearance has been reported previously, and pharmacogenetic analysis of CYP3A5 in donors has been proposed for the evaluation of the appropriate initial dosage 18, 21. Our results confirmed that the expression of functional CYP3A5 in the liver grafts substantially increased the oral clearance of both ciclosporin and tacrolimus. Thus, higher doses (50% higher for ciclosporin and 100% higher for tacrolimus) are needed to maintain the target trough concentrations in the recipients with grafts carrying the CYP3A5*1 allele than the bodyweight controlled clinical practice would have proposed. These findings are consistent with the majority of previous reports on liver transplants that CYP3A5 genotype has a significant effect on the pharmacokinetics of tacrolimus and a lesser influence on that of ciclosporin 5, 6, 7, 10, 15. However, CYP3A5 genotyping of the donors and tailoring of recipient CNI therapy could prevent only about 30% of misdosing events (25 of 82 misdosed recipients). It should be mentioned that several authors have indicated the potential influence of recipients' CYP3A5 genotype on tacrolimus blood concentrations and dose requirements in liver transplant patients 25, 26, 27, 28. Both the recipient's intestinal genotype and the donor liver genotype are suggested to contribute to the overall tacrolimus disposition. Nevertheless, other authors have reported that the donor CYP3A5 genotype has a more dominant effect on tacrolimus pharmacokinetics than the recipient genotype or have found no association between recipient CYP3A5 genotype and tacrolimus clearance 18, 21, 29, 30, 31, 32. The low frequency of the CYP3A5*1 allele in the Caucasian population can render it more difficult to show a statistically significant influence of recipient genotype on tacrolimus blood concentrations and dose requirements of liver transplant patients 18, 21, 29, 31, 32. Furthermore, the higher relative graft size in children than in adults may decrease the contribution of intestinal CYP3A5 to tacrolimus clearance 30. One of the limitations of our present study is that the confounding effect of the recipient's CYP3A5 genotype was not examined.
In CYP3A5 non‐expressers, the variability in CYP3A4 expression can obscure the effect of CYP3A4 genetic polymorphisms on CYP3A substrates 7, 33. Thus, CYP3A4 phenotyping of the liver grafts advances the rationalization of CNI medication after liver transplantation. The CYP3A4*1B allele has been reported to be associated with increased promoter activity in vitro and consequently with increased transcription, whereas for the CYP3A4*22 allele, a decrease in CYP3A4 mRNA level has been demonstrated 12, 13. Since CYP3A4*1B and CYP3A4*22 alleles manifest altered CYP3A4 mRNA levels, the CYP3A4 expression comprises not only the effects of the non‐genetic but also of these genetic factors. This means that increased CYP3A4 mRNA levels can indicate enhanced transcription as a consequence of CYP3A4*1B and/or of CYP3A4 induction, whereas decreased CYP3A4 mRNA concentrations can be the result of CYP3A4 suppression and/or of reduced transcription of CYP3A4*22. The CYP3A4 mRNA levels in leukocytes have been proven to reflect the hepatic CYP3A4 activities in CYP3A5 non‐expressers 11. Thus, the drug metabolizing capacity of the liver grafts at 0 time point (at the time of transplantation) was estimated by the CYP3A4 expression determined in the donors' leukocytes taken at the time of organ procurement. It might be assumed that CYP3A4 activities or CNI metabolizing activities of the liver grafts can be modified in the post‐operative period. The blood concentrations of ciclosporin and tacrolimus were monitored in recipients up to 4 weeks after transplantation. The stable concentrations were generally achieved within 8–12 days, and the recipients rarely required dose‐modifications thereafter. The CYP3A4 groups classified by the donors' CYP3A4 expression displayed distinct and significantly different stable blood concentrations of CNIs throughout the monitoring period. Thus, we may conclude that CYP3A4 activities estimated just before the transplantation were retained at least up to 4 weeks. The recipients transplanted with liver grafts from high CYP3A4 expresser donors carrying CYP3A5*3/*3 required more or less the same dose of ciclosporin or tacrolimus as patients transplanted with CYP3A5 expresser livers. On the other hand, the dose requirement of the recipients with grafts from low CYP3A4 expresser donors was about 50% lower than that of the patients with grafts from donors expressing CYP3A4 at the normal level.
Although assaying the CYP3A‐status of donors cannot substitute the monitoring of CNI blood concentrations, pre‐transplantation screening of CYP3A5 genotype and CYP3A4 expression can provide a tool for fast and better evaluation of CNI metabolizing capacity of liver grafts. The donor's peripheral blood taken during liver procurement is an appropriate biological sample and can be used for assaying CYP3A‐status in parallel with the graft implantation. The knowledge of the liver donors' CYP3A‐status can guide the optimization of the initial CNI dose for the recipients. CYP3A‐status controlled treatment of liver recipients may substantially reduce the time for achieving the optimal CNI blood concentration. Prospective investigation of the donors' genetic and non‐genetic variations in CYP3A can improve not only the optimization of the initial dose of tacrolimus or ciclosporin for transplant recipients, but may also contribute to the avoidance of misdosing induced graft injury in the early post‐operative period. Tailored CNI medication can eventually contribute to the improvement of the recipients' recovery.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work.
Author contributions
KM: research design, interpretation of data, manuscript writing.
KT: assaying CYP3A4 expression.
AK: CYP3A5 genotyping.
EH: collecting blood samples, isolation DNA and RNA from leukocytes.
NC: collecting patients' clinical data.
JP: contributed new analytical tools.
ES: measuring blood concentrations of calcineurin inhibitors.
LK: research design, coordination of the clinical part.
Acknowledgements
The study was supported by the National Development Agency and the European Union (Grants GOP‐1.3.1–11/B‐2011–0042 and GOP‐1.1.1–11–2012–0027). The authors would like to thank Maria Mérey for the access to patients' data at the Department of Transplantation and Surgery, Semmelweis University (Budapest, Hungary).
Monostory, K. , Tóth, K. , Kiss, Á. , Háfra, E. , Csikány, N. , Paulik, J. , Sárváry, E. , and Kóbori, L. (2015) Personalizing initial calcineurin inhibitor dosing by adjusting to donor CYP3A‐status in liver transplant patients. Br J Clin Pharmacol, 80: 1429–1437. doi: 10.1111/bcp.12747.
Principal investigators:
Katalin Monostory: responsible for the issues of CYP testing and laboratory testing as well as for the evaluation of the results.
László Kóbori: responsible for the clinical part.
References
- 1. Penninga L, Wettergren A, Chan AW, Steinbrüchel DA, Gluud C. Calcineurin inhibitor minimisation versus continuation of calcineurin inhibitor treatment for liver transplant recipients. Cochrane Database Syst Rev 2012; 3: CD008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choudhary NS, Saigal S, Shukla R, Kotecha H, Saraf N, Soin AS. Current status of immunosuppression in liver transplantation. J Clin Exp Hepatol 2013; 3: 150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pallet N, Legendre C. Deciphering calcineurin inhibitor nephrotoxicity: a pharmacological approach. Pharmacogenomics 2010; 11: 1491–501. [DOI] [PubMed] [Google Scholar]
- 4. Beckebaum S, Cicinnati VR, Radtke A, Kabar I. Calcineurin inhibitors in liver transplantation ‐ still champions or threatened by serious competitors? Liver Int 2013; 33: 656–65. [DOI] [PubMed] [Google Scholar]
- 5. Kurzawski M, Drozdzik M. Pharmacogenetics in solid organ transplantation: genes involved in mechanism of action and pharmacokinetics of immunosuppressive drugs. Pharmacogenomics 2013; 14: 1099–118. [DOI] [PubMed] [Google Scholar]
- 6. Elens L, Hesselink DA, van Schaik RH, van Gelder T. Pharmacogenetics in kidney transplantation: recent updates and potential clinical applications. Mol Diagn Ther 2012; 16: 331–45. [DOI] [PubMed] [Google Scholar]
- 7. Hesselink DA, Bouamar R, Elens L, van Schaik RH, van Gelder T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet 2014; 53: 123–39. [DOI] [PubMed] [Google Scholar]
- 8. Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. Human P‐glycoprotein transports cyclosporin A and FK506. J Biol Chem 1993; 268: 6077–80. [PubMed] [Google Scholar]
- 9. Provenzani A, Santeusanio A, Mathis E, Notarbartolo M, Labbozzetta M, Poma P, Provenzani A, Polidori C, Vizzini G, Polidori P, D'Alessandro N. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J Gastroenterol 2013; 19: 9156–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elens L, Bouamar R, Shuker N, Hesselink DA, van Gelder T, van Schaik RH. Clinical implementation of pharmacogenetics in kidney transplantation: calcineurin inhibitors in the starting blocks. Br J Clin Pharmacol 2014; 77: 715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Temesvári M, Kóbori L, Paulik J, Sárváry E, Belic A, Monostory K. Estimation of drug‐metabolizing capacity by cytochrome P450 genotyping and expression. J Pharmacol Exp Ther 2012; 341: 294–305. [DOI] [PubMed] [Google Scholar]
- 12. Amirimani B, Ning B, Deitz AC, Weber BL, Kadlubar FF, Rebbeck TR. Increased transcriptional activity of the CYP3A4*1B promoter variant. Environ Mol Mutagen 2003; 42: 299–305. [DOI] [PubMed] [Google Scholar]
- 13. Okubo M, Murayama N, Shimizu M, Shimada T, Guengerich FP, Yamazaki H. CYP3A4 intron 6 C > T polymorphism (CYP3A4*22) is associated with reduced CYP3A4 protein level and function in human liver microsomes. J Toxicol Sci 2013; 38: 349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elens L, van Gelder T, Hesselink DA, Haufroid V, van Schaik RH. CYP3A4*22: promising newly identified CYP3A4 variant allele for personalizing pharmacotherapy. Pharmacogenomics 2013; 14: 47–62. [DOI] [PubMed] [Google Scholar]
- 15. Thervet E, Legendre C, Beaune P, Anglicheau D. Cytochrome P450 3A polymorphisms and immunosuppressive drugs. Pharmacogenomics 2005; 6: 37–47. [DOI] [PubMed] [Google Scholar]
- 16. Kamdem LK, Streit F, Zanger UM, Brockmöller J, Oellerich M, Armstrong VW, Wojnowski L. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem 2005; 51: 1374–81. [DOI] [PubMed] [Google Scholar]
- 17. Shah RR, Smith RL. Addressing phenoconversion: the Achilles' heel of personalized medicine. Br J Clin Pharmacol 2015; 79: 222–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Provenzani A, Notarbartolo M, Labbozzetta M, Poma P, Vizzini G, Salis P, Caccamo C, Bertani T, Palazzo U, Polidori P, Gridelli B, D'Alessandro N. Influence of CYP3A5 and ABCB1 gene polymorphisms and other factors on tacrolimus dosing in Caucasian liver and kidney transplant patients. Int J Mol Med 2011; 28: 1093–102. [DOI] [PubMed] [Google Scholar]
- 19. Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics‐related genes in Eastern Asians and Europeans: implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet 2012; 27: 9–54. [DOI] [PubMed] [Google Scholar]
- 20. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013; 138: 103–41. [DOI] [PubMed] [Google Scholar]
- 21. Provenzani A, Notarbartolo M, Labbozzetta M, Poma P, Biondi F, Sanguedolce R, Vizzini G, Palazzo U, Polidori P, Triolo F, Gridelli B, D'Alessandro N. The effect of CYP3A5 and ABCB1 single nucleotide polymorphisms on tacrolimus dose requirements in Caucasian liver transplant patients. Ann Transplant 2009; 14: 23–31. [PubMed] [Google Scholar]
- 22. Gijsen VM, van Schaik RH, Elens L, Soldin OP, Soldin SJ, Koren G, de Wildt SN. CYP3A4*22 and CYP3A combined genotypes both correlate with tacrolimus disposition in pediatric heart transplant recipients. Pharmacogenomics 2013; 14: 1027–36. [DOI] [PubMed] [Google Scholar]
- 23. Elens L, van Schaik RH, Panin N, de Meyer M, Wallemacq P, Lison D, Mourad M, Haufroid V. Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors' dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics 2011; 12: 1383–96. [DOI] [PubMed] [Google Scholar]
- 24. Bruckmueller H, Werk AN, Renders L, Feldkamp T, Tepel M, Borst C, Caliebe A, Kunzendorf U, Cascorbi I. Which genetic determinants should be considered for tacrolimus dose optimization in kidney transplantation? A combined analysis of genes affecting the CYP3A locus. Ther Drug Monit 2015; 37: 288–95. [DOI] [PubMed] [Google Scholar]
- 25. Uesugi M, Masuda S, Katsura T, Oike F, Takada Y, Inui K. Effect of intestinal CYP3A5 on postoperative tacrolimus trough levels in living‐donor liver transplant recipients. Pharmacogenet Genomics 2006; 16: 119–27. [DOI] [PubMed] [Google Scholar]
- 26. Li D, Lu W, Zhu JY, Gao J, Lou YQ, Zhang GL. Population pharmacokinetics of tacrolimus and CYP3A5, MDR1 and IL‐10 polymorphisms in adult liver transplant patients. J Clin Pharm Ther 2007; 32: 505–15. [DOI] [PubMed] [Google Scholar]
- 27. Muraki Y, Usui M, Isaji S, Mizuno S, Nakatani K, Yamada T, Iwamoto T, Uemoto S, Nobori T, Okuda M. Impact of CYP3A5 genotype of recipients as well as donors on the tacrolimus pharmacokinetics and infectious complications after living‐donor liver transplantation for Japanese adult recipients. Ann Transplant 2011; 16: 55–62. [DOI] [PubMed] [Google Scholar]
- 28. Chen YK, Han LZ, Xue F, Shen CH, Lu J, Yang TH, Zhang JJ, Xia Q. Personalized tacrolimus dose requirement by CYP3A5 but not ABCB1 or ACE genotyping in both recipient and donor after pediatric liver transplantation. PLoS One 2014; 9: e109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu S, Wu L, Jin J, Yan S, Jiang G, Xie H, Zheng S. Influence of CYP3A5 gene polymorphisms of donor rather than recipient to tacrolimus individual dose requirement in liver transplantation. Transplantation 2006; 81: 46–51. [DOI] [PubMed] [Google Scholar]
- 30. Fukudo M, Yano I, Masuda S, Goto M, Uesugi M, Katsura T, Ogura Y, Oike F, Takada Y, Egawa H, Uemoto S, Inui K. Population pharmacokinetic and pharmacogenomic analysis of tacrolimus in pediatric living‐donor liver transplant recipients. Clin Pharmacol Ther 2006; 80: 331–45. [DOI] [PubMed] [Google Scholar]
- 31. Wei‐lin W, Jing J, Shu‐sen Z, Li‐hua W, Ting‐bo L, Song‐feng Y, Sheng Y. Tacrolimus dose requirement in relation to donor and recipient ABCB1 and CYP3A5 gene polymorphisms in Chinese liver transplant patients. Liver Transpl 2006; 12: 775–80. [DOI] [PubMed] [Google Scholar]
- 32. Barrera‐Pulido L, Aguilera‐García I, Docobo‐Pérez F, Alamo‐Martínez JM, Pareja‐Ciuró F, Nuñez‐Roldán A, Gómez‐Bravo MA, Bernardos‐Rodríguez A. Clinical relevance and prevalence of polymorphisms in CYP3A5 and MDR1 genes that encode tacrolimus biotransformation enzymes in liver transplant recipients. Transplant Proc 2008; 40: 2949–51. [DOI] [PubMed] [Google Scholar]
- 33. Miao J, Jin Y, Marunde RL, Gorski CJ, Kim S, Quinney S, Radovich M, Li L, Hall SD. Association of genotypes of the CYP3A cluster with midazolam disposition in vivo . Pharmacogenomics J 2009; 9: 319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


