Summary
This project was undertaken to find ways of reducing mortalities and economic losses due to velogenic Newcastle disease (VND) in areas where the disease is enzootic. Four groups of cockerels of 44 birds each were used for this experiment. The birds in groups 1 and 2 received no dietary vitamin A supplementation, whereas groups 3 and 4 received 300 iu and 600 iu of vitamin A per kilogram of commercial feed, respectively, from 1 week of age till the end of the experiment. At 6 weeks of age, the birds in groups 2, 3 and 4 were inoculated intraocularly with a VND virus (duck/Nigeria/Plateau/Kuru/113/1991). The birds in Group 1 were given phosphate‐buffered saline intraocularly. Clinical signs appeared in Group 2 birds on day 3 PI and in groups 3 and 4 on day 5 PI. The clinical signs included a drop in feed and water consumption, depression, diarrhoea, torticollis and paralysis in all the infected groups. The average body weights of all groups were significantly different from one another on day 14 PI with Group 2 birds having the lowest body weight. Mortalities were highest in Group 2 birds (0%, 93.18%, 72.73% and 56.82% in groups 1, 2, 3 and 4 respectively). The antibody response in all the groups was significantly different from one another on days 14 and 21 PI. Group 2 birds had the lowest titres on those 2 days and showed more severe atrophy of the bursa, spleen, thymus and fibrin deposition in the spleen and thymus than the birds in groups 3 and 4. The above observations show that vitamin A dietary supplementation delayed the onset of clinical signs and significantly reduced body weight loss, atrophy of the bursa, spleen and thymus, and mortalities by 36%. It also significantly potentiated haemagglutination inhibition antibody response.
Keywords: cockerels, mortality, Newcastle disease, vitamin A
Newcastle disease (ND) is a major problematic disease of poultry worldwide. It affects over 241 avian species (Kaleta & Baldauf 1988). But since then, many more species have been found to be susceptible to the infection. It therefore appears reasonable to conclude that the vast majority of, if not all, birds are susceptible to the infection (Alexander & Senne 2008). ND produces clinical signs that range from inapparent to mild, moderate and fulminating with mortalities ranging from 0% to 100%. It is caused by virulent strains of ND virus (NDV) of the genus Avulavirus which belongs to the family Paramyxoviridae (Alexander 2001). These clinical signs and lesions may affect the respiratory, digestive, nervous and reproductive systems and can be confused with those of other diseases (Okoye et al. 2000; Ezema et al. 2009). ND has no pathognomonic signs making rapid and early diagnosis of the disease difficult, but the presence of haemorrhagic intestinal ulcers in the absence of any information on avian influenza in the area, and linear haemorrhage at the mucosa of the proventriculus–oesophagus junction, are highly suggestive of ND (Okoye et al. 2000). The disease is mainly controlled by vaccination and biosecurity. While these measures are working in the developed countries of Europe and America where velogenic Newcastle disease (VND), the most severe form of the disease, is exotic, they have not succeeded in the developing countries of Africa, Asia, Middle East and Americas where the disease is enzootic (Sun et al. 2008) due to multiple factors. Biosecurity measures in these countries are either very poor or non‐existent. The local or village chickens are not vaccinated against any disease, and they serve as reservoirs of NDV for commercial chickens (Bell & Moulous 1998). The poultry droppings, including those from infected farms, are popular farm manure. The cold chain needed for the storage of vaccines is easily broken by frequent power outages. Consequently, severe outbreaks of VND occur frequently in vaccinated commercial chickens, while large populations of village chickens are wiped out annually by this disease in developing countries. Furthermore, even in commercial chickens, LaSota vaccination can protect against the clinical signs of VND, but the lymphocidal lesions are severe (Ezema et al. 2009). It is therefore desirable to find ways of managing NDV outbreaks to reduce mortalities and economic losses in these areas where the disease is enzootic, and where a “stamping out” policy cannot be applied. Vitamin A supplementation has been demonstrated to improve specific and non‐specific immune systems (such as cytokine production, lymphocyte blastogenesis, natural killer cell activity and phagocytosis) significantly (Ross 1992). This study describes the effect of vitamin A dietary supplementation on the mortality due to NDV.
Materials and methods
Flock history
One hundred and seventy‐six‐day‐old cockerels obtained from Zarteck Farm Ltd. (Oyo, Ibadan, Nigeria) were used for the study. The experimental birds were not given any vaccination. Brooding was performed on deep litter. Clean water and commercial feed were given ad libitum to the birds throughout the duration of the study.
Ethical approval
Ethical approval was given on Oct. 4, 2012 by the University Committee on Medical and Scientific Research Ethics, University of Nigeria, Nsukka.
Dietary supplementation of vitamin A
At 7 days of age, the birds were randomly divided into 4 groups (1, 2, 3 and 4) of 44 birds each. The groups were housed in separate pens and started receiving different rations. Birds in groups 1 and 2 received only the commercial feed that contained 1500 iu of vitamin A/kg of feed; Group 3 the commercial feed supplemented with 300 iu of vitamin A/kg of feed; and Group 4 the commercial feed supplemented with 600 iu of vitamin A/kg of feed. The feeding was continued till the end of the experiment.
Vitamin A
The vitamin used was dry vitamin A acetate (150 mg retinol per gram) manufactured by Roche Pharmaceuticals, Basel, Switzerland.
The velogenic Newcastle disease virus inoculum
The NDV strain used was the duck/Nigeria/Plateau/Kuru/113/1991 strain, which was isolated from an apparently healthy duck in Kuru town of Plateau State in central Nigeria. The virus was purified and biologically characterized as velogenic by Echeonwu et al. (1993), while Okoye et al. (2000) reported that it was velogenic and viscerotropic.
Newcastle disease virus challenge
At six weeks of age, serum was collected from ten birds in each group and the birds were found free from NDV antibodies. One hundred microlitres of the inoculum containing a median embryo lethal dose (ELD50) of 108.46 per ml was given to each bird in each eye in groups 2, 3 and 4, while birds in Group 1 were each given 100 μl of phosphate‐buffered saline (PBS) in each eye. The groups were kept in different houses in the poultry experimental unit of the Faculty of Animal Production, and each group had its own attendants attached to it only.
Clinical examination
The birds in all the groups were observed for clinical signs from day 0 to day 21 postinfection (PI). Ten birds from each group were handpicked and weighed on days 0, 7 and 14 PI. Birds showing signs of depression were picked before others in each group. The morbidity and mortality rates were recorded.
Post‐mortem lesions
The dead birds were necropsied for gross lesions. Samples of the bursa of Fabricius, thymus, spleen, kidney and brain were collected and fixed in 10% formal saline for a minimum of 24 h, dehydrated in ascending grades of alcohol and washed in xylene. They were embedded in paraffin wax, and 5‐μm‐thick sections were cut and stained with haematoxylin and eosin (H&E) for histopathology.
Serology
On days 0, 7, 14 and 21 PI, 2 ml of blood was collected from 10 birds each from all the groups through the jugular vein. Sera were harvested and stored at −20°C until assayed for NDV haemagglutination inhibition (HI) antibody titres using the microtitre method of Beard (1989). Ten haemagglutination (HA) units of the antigen, which was a PBS suspension of LaSota vaccine, was used in the HI test. The geometrical mean titres (GMTs) of the sera were calculated using the tube method as shown in Table 3 (Villegas & Purchase 1989).
Table 3.
Geometric mean antibody titre
| Days PI | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| 0 | 0.00 ± 0.00a | 1.13 ± 0.04b | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 7 | 0.00 ± 0.00a | 3.00 ± 0.12b | 3.00 ± 0.12b | 4.50 ± 0.17c |
| 14 | 0.00 ± 0.00a | 76.80 ± 2.31b | 128.00 ± 1.15c | 135.00 ± 1.15d |
| 21 | 0.00 ± 0.00a | 207.00 ± 1.73b | 315.00 ± 2.89c | 445.70 ± 2.89d |
Means with different superscripts on the same day/row are significantly different (P < 0.05).
Statistical analysis
Data were analysed using spss 15.0 (Sax Software Corporation, Chicago, Illinois, USA). They were then subjected to one‐way anova. Differences in values less than a probability of 0.05 were considered significant.
Results
Clinical signs
Clinical signs first appeared in Group 2 cockerels on day 3 PI and included a drop in feed and water consumption, ruffled feathers and sleepiness. By day 6 PI, 50% of the birds were affected and additionally, greenish yellow diarrhoea, dyspnoea and cough were observed. All the birds were affected on day 9 PI. Torticollis, incoordination, and leg and wing paralysis appeared between days 10 and 13 PI and continued throughout the experiment. Group 3 cockerels started showing clinical signs on day 5 PI which included a drop in feed and water consumption, sleepiness, ruffled feathers and yellowish green diarrhoea. By day 7 PI, cough appeared. All the birds were affected by day 12 PI. There was incoordination, and signs of recovery were noticed on day 19 PI. Clinical signs appeared in Group 4 birds on day 5 PI and included a drop in feed and water consumption, ruffled feathers and sleepiness in some birds. By day 10 PI, half of the birds were affected and greenish diarrhoea was present. All the birds were down on day 13 PI when cough was first observed. Recovery started on day 19 PI with the nervous signs subsiding. Weight loss was significantly most severe in Group 2 birds (Table 1) and reduced with increased levels of vitamin A supplementation in groups 3 and 4. Total mortalities observed were as follows: Group 1, 0.00; Group 2, 93.18%; Group 3, 72.73%; and Group 4, 56.82% (Table 2). Birds in Group 1 did not show clinical signs.
Table 1.
Mean body weight of birds (g) ± SEM
| Days post infection | Groups | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 0 | 253.30 ± 2.56a | 248.20 ± 2.10a | 251.80 ± 1.91a | 249.90 ± 2.9a |
| 4 | 255.80 ± 2.6a | 243.70 ± 4.10b | 250.60 ± 2.23ab | 251.30 ± 2.79ab |
| 7 | 256.30 ± 4.90a | 226.50 ± 5.16b | 253.70 ± 2.65ac | 255.30 ± 2.72ac |
| 14 | 269.40 ± 3.53a | 230.10 ± 2.01b | 247.80 ± 2.70c | 258.10 ± 1.88d |
Means with different superscript on the same day/row are significantly different (P < 0.05).
Table 2.
Daily mortality
| Days PI | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| No. dead | No. dead | No. dead | No. dead | |
| 5 | 0 | 3 | 0 | 0 |
| 6 | 0 | 4 | 0 | 0 |
| 7 | 0 | 4 | 3 | 3 |
| 8 | 0 | 4 | 4 | 2 |
| 9 | 0 | 6 | 4 | 3 |
| 11 | 0 | 4 | 4 | 2 |
| 12 | 0 | 5 | 6 | 2 |
| 13 | 0 | 3 | 4 | 5 |
| 15 | 0 | 5 | 4 | 6 |
| 18 | 0 | 3 | 3 | 2 |
| 21 | 0 | 0 | 0 | 0 |
| Mortality rate (%) | 0a | 93.18b | 72.73c | 56.82d |
Means with different superscripts on the same day/row are significantly different (P < 0.05).
Lesions
Group 1 birds showed no lesions. The lesions in the infected groups were similar but appeared most severe in Group 2 birds. Removal of the skin showed congested muscles of the breast, thigh and legs. There were haemorrhages on the proventricular mucosa, some at the tips of the proventricular glands. The proventriculus–oesophageal junction showed linear haemorrhages. Haemorrhagic ulcers were observed in the intestines especially at the ileum. The caecal tonsils were swollen and haemorrhagic and contained cheesy materials. The bursa was atrophic by day 5 PI in groups 2, 3 and 4. The atrophy was most severe in Group 2, while the bursa of groups 3 and 4 appeared to be the same size. By day 9 PI, the atrophy was more severe in Group 2 birds (Figure 1). By day 21 PI, there was marked increase in the size of the bursa in the three infected groups and the sizes were close to those of the control group. But two samples in Group 2 were mildly atrophic. The spleen in the infected groups was swollen on day 5 P1. The swelling was more severe in Group 2 than in groups 3 and 4. But by day 14 PI, the Group 2 birds showed more severe atrophy of the spleen than the birds in groups 3 and 4 (Figure 2). The spleen of all the infected groups was still atrophic on day 21 PI. The atrophy of the thymus (data not shown) followed the same pattern of severity as that of the spleen and bursa, but by day 21 PI, the lesion was still moderately severe. Kidneys were swollen, congested and often haemorrhagic. The brain showed no gross lesions. Microscopically, there was severe depletion of lymphocytes in the bursa (Figure 3), spleen and thymus. Large deposits of fibrin were observed around the sheathed arterioles in the spleen (Figure 4). The thymus also showed fibrin deposition (Figure 5). There was severe congestion of the peritubular blood vessels in the kidney. Sections of the brain showed congestion of the blood vessels in the cerebrum and cerebellum with demyelination of the white matter of the cerebellum (data not shown). Group 1 birds showed no lesions (Figures 6, 7, 8).
Figure 1.

Severe atrophy of the bursa of Group 2 cockerels on day 9 PI.
Figure 2.

Atrophy of the spleen in cockerels of groups 2, 3 and 4 on day 14 PI.
Figure 3.
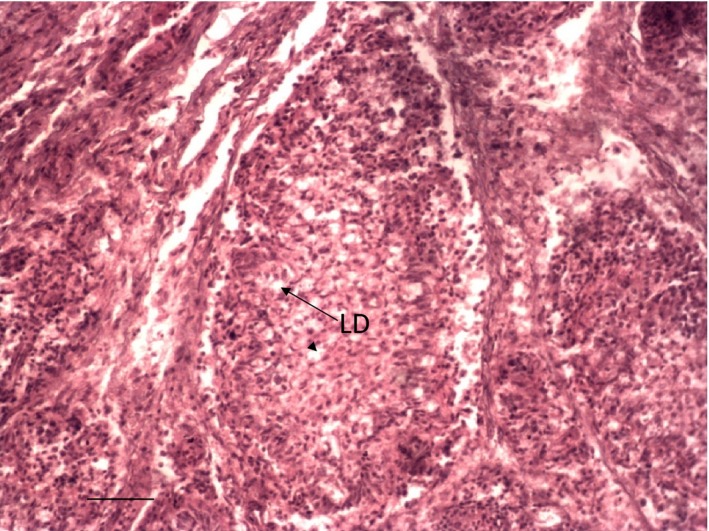
Depletion of lymphocytes (LD) in the follicles of the bursa of Group 2 cockerel on day 6 PI. H & E ×200.
Figure 4.
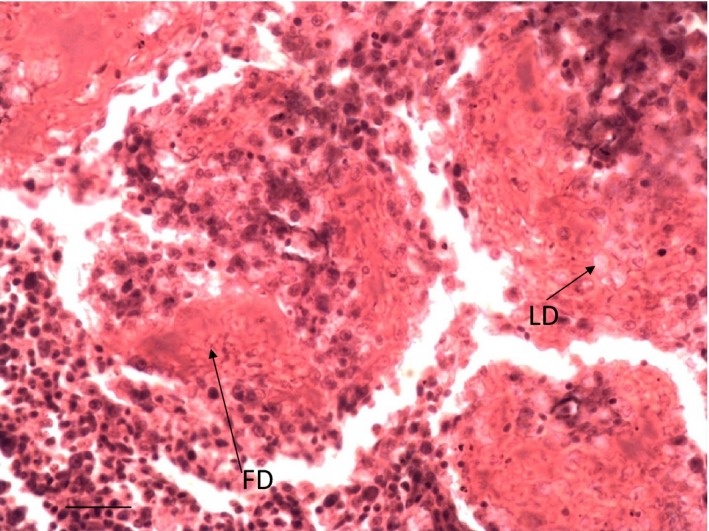
Depletion of lymphocytes (LD) and deposition of fibrin (FD) around the sheathed arterioles of the spleen of Group 2 cockerel on day 6 PI. H & E ×400.
Figure 5.
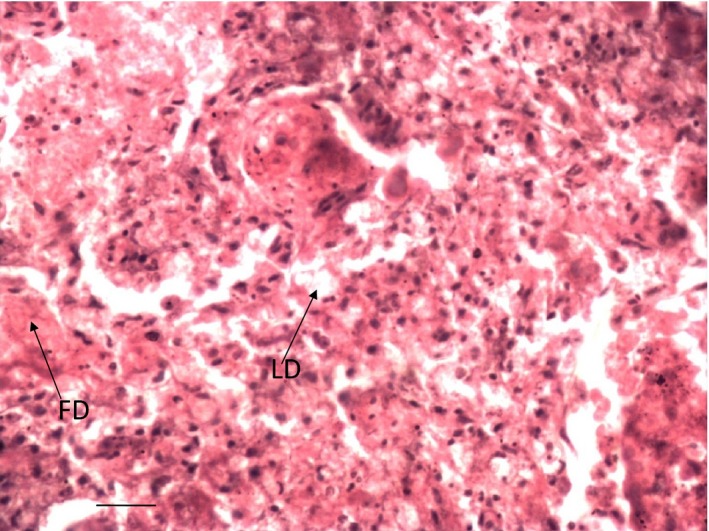
Depletion of lymphocytes (LD) and fibrin deposition (FD) in the thymus of Group 2 cockerel on day 6 PI. H & E ×400.
Figure 6.
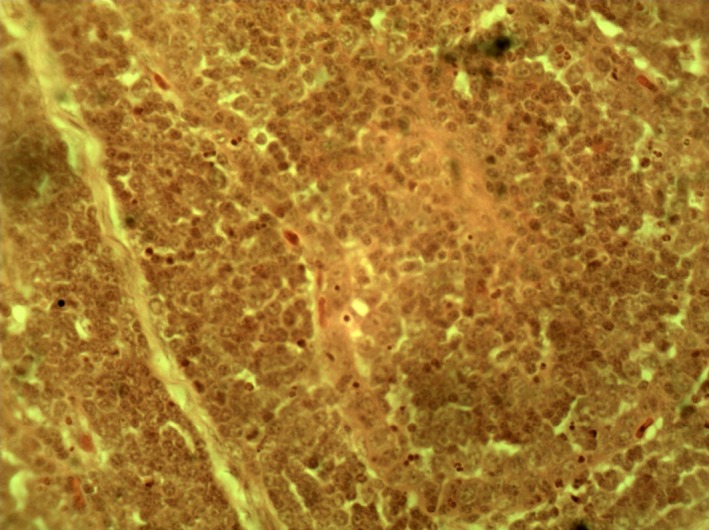
Bursa of Group 1 cockerel (normal bursa) on day 3 PI. H & E ×400.
Figure 7.
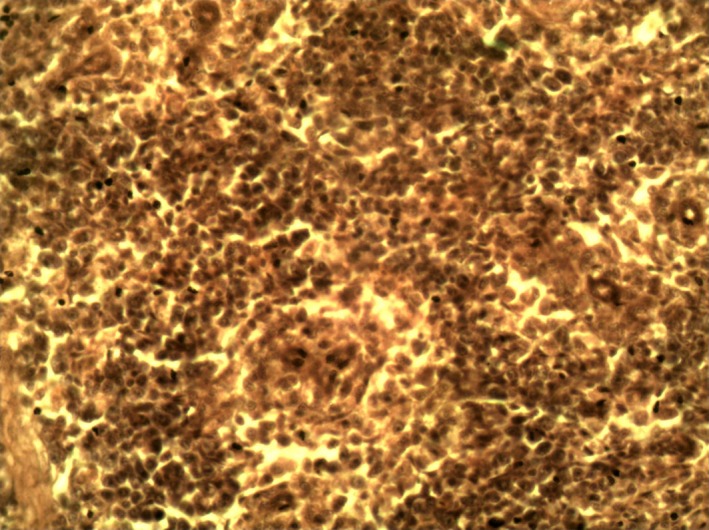
Spleen of Group 1 cockerel (normal spleen) on day 3 PI. H & E ×400.
Figure 8.
Thymus of Group 1 cockerel (normal thymus) on day 3 PI. H & E ×200.
Serology
All the groups had no NDV HI antibodies on the day of inoculation, day 0 PI. The GMT was significantly lowest in Group 2, lower in Group 3 and highest in Group 4 birds on days 14 and 21 PI (Table 3).
Discussion
The above results show that vitamin A dietary supplementation reduced NDV mortality by 36% in Group 4 that received 600 iu of the vitamin/kg of feed and 21% in Group 3 cockerels that received 300 iu. This reduction appears to be directly proportional to the level of vitamin A supplementation. The same trend was observed in other parameters. The weight loss was highest in Group 2 and lowest in Group 4. It seems that the biggest effect of the vitamin A on body weight occurred between days 4 and 7 PI: Group 2 birds lost 17 g, but the birds in groups 3 and 4 gained 3 and 4 g respectively. However, the differences between days 7 and 14 PI in groups 3 and 4 were not marked compared to those seen in Group 2. In fact, Group 2 birds gained 4 g, but Group 3 lost 6 g and Group 4 gained 3 g. It is difficult to explain the weight loss in Group 3. The HI antibody response was highest in Group 4 and lowest in Group 2 among the infected groups. These beneficial effects of vitamin A on reduction in mortality, weight loss and potentiating antibody response are in agreement with some earlier reports, but the exact mechanism through which vitamin A influences the pathogenicity of infectious agents is not clearly known. It could be either systemic, i.e. not specifically immune response related, or immune system specific (Shapiro & Edelson 1985). Friedman and Sklan (1985) were of the opinion that the vitamin could have a direct effect on the metabolic ability of B lymphocytes to differentiate into plasma cells and antibody production by plasma cells. Other workers have shown that vitamin A influences the differentiation of B lymphocyte hybridomas (Sherr et al. 1988). Vitamin A in the diet influences not only the activity of one cell type but also the activity of several other immune cells (Friedman et al. 1991). The level of vitamin A in the diet has been shown to have a modulating effect on the general immune response of chicken (Lessard et al. 1996). Similarly, there are reports that increasing the level of vitamin A in the diet led to increased antibody production against pox and NDV (Sklan et al.1994, 1995). Antibodies constitute an essential component of the protection against NDV, and are important for the clearance and neutralization of the pathogen in two ways: (i) by binding to infected cells and thereby reducing the production of progeny virus, (ii) by binding to released progeny virus and thereby inhibiting its spread (Al‐Garib et al. 2003). Morbidity and mortality to Eschericia coli infection were observed to be drastically reduced in chickens that received dietary vitamin A supplementation. Clinical signs appeared in Group 2 birds on day 3 PI and in groups 3 and 4 on day 5 PI. The delayed onset of the disease in the groups that received vitamin A may be due to mucosal immunity resisting the entry and establishment of the infection. Mucosal immunity plays a vital role in the resistance of the host to many infectious agents including NDV, as many of these agents invade the host through the mucosal surface (Scicchitano et al. 1988). Vitamin A has been described as an infection‐resisting vitamin because proper development of the epithelium is dependent on vitamin A status (Chauhan 1993). The severity of the lesions to a large extent followed the same pattern as the weight loss, mortality and antibody response. The atrophy of the spleen, bursa, thymus and fibrin deposition in the spleen was most severe in Group 2 birds. It is recommended that while biosecurity and vaccination are still used as the cheapest and effective means of ND control in enzootic areas, vitamin A supplementation should be used in reducing mortality when outbreaks occur. Prophylactic supplementation should be recommended for expensive flocks like parent stock and layers at high‐risk periods.
Conflict of interest
Authors do not have any conflicts of interest to report.
References
- Alexander D.J. (2001) Newcastle disease. Br. Poult. Sci. 42, 5–22. [DOI] [PubMed] [Google Scholar]
- Alexander D.J., Senne D.A. (2008) Newcastle disease In: Diseases of Poultry, 12th edn, pp. 75–100 (eds Saif Y.M. (Ed in Chief), Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E. (Assoc. Eds)), Ames: Iowa State University Press; [Google Scholar]
- Al‐Garib S.O., Gielkens A.L.J., Gruys E. & Koch G. (2003) Review of Newcastle disease virus with particular reference to immunity and vaccination. World Poult. Sci. J. 59, 185–200. [Google Scholar]
- Beard C.W. (1989) Serological procedures In: A Laboratory Manual for Isolation and Identification of Avian Pathogens, 3rd edn, pp. 192–200 (eds Purchase H.G., Arp L.H., Domermuth C.H., Pearson J.E.), Keneth Square, PA: American Association of Avian Pathologists. [Google Scholar]
- Bell J.G. & Moulous S. (1998) A reservoir of virulent Newcastle disease virus in village chickenflocks. Prev. Vet. Med. 6, 37–42. [Google Scholar]
- Chauhan H.V.S. (1993) Nutritional diseases In: Poultry Diseases, Diagnosis and Treatment, 1st edn, pp. 120 Wiley Eastern Limited, Somerset, New York. [Google Scholar]
- Echeonwu G.O.N., Ireogbu C.I. & Emeruwa A.C. (1993) Recovery of velogenic Newcastle disease virus from dead and healthy free roaming birds in Nigeria. Avian Pathol. 22, 383–387. [DOI] [PubMed] [Google Scholar]
- Ezema W.S., Okoye J.O.A. & Nwanta J.A. (2009) Lasota vaccination may not protect against the lesions of velogenic Newcastle disease in chickens. Trop. Anim. Health Prod. 41, 477–484. [DOI] [PubMed] [Google Scholar]
- Friedman A., Sklan D. (1985) Antigen‐specific Immune Response Impairment in the Chick as Influenced by Dietary Vitamin A. American Institute of Nutrition, Bethesda, MD. [DOI] [PubMed] [Google Scholar]
- Friedman A., Meidovsky A., Leitner G. & Sklan D. (1991) Decreased resistance and immune response to Escherichia coli infection in chicks with low or high intake of vitamin A. J. Nutr. 121, 395–400. [DOI] [PubMed] [Google Scholar]
- Kaleta E.F. & Baldauf C. (1988) Newcastle disease in free living and pet birds In: Newcastle Disease, pp. 197–246 (ed. Alexander D.J.), Boston, MA: Kluwer Academic Publishers. [Google Scholar]
- Lessard M., Hutchings D. & Cave A.N. (1996) Cell‐mediated and humoral immune response in broiler chickens maintained on diets containing different levels of vitamin A Poultry . Science 76, 1368–1378. [DOI] [PubMed] [Google Scholar]
- Okoye J.O.A., Agu A.O., Chineme C.N. & Echeonwu G.O.N. (2000) Pathological characterization in chickens of a velogenic Newcastle disease virus isolated from Guinea fowl. Revue d'Elevage et de Medicine veterinaries des pays Tropicaux 53, 325–330. [Google Scholar]
- Ross A.C. (1992) Vitamin A and protective immunity. Nutr. Today 27, 18–26. [Google Scholar]
- Scicchitano R., Stanisz A., Ernst P., Bienenstock J. (1988) A common mucosal immune system revisited In: Migration and Homing of Lymphoid Cells, 2, pp. 1–34 (ed. Husband A.J.), Boca Raton, FL: CRC Press; [Google Scholar]
- Shapiro P.E., Edelson R.L. (1985) Effects of retinoids on the immune system. Retinoids: New Trends in Research and Therapy. Retinoid Symposium, Geneva. A.H.Saurat, (ed). Switzerland, Karger, Basel, pp. 225–235
- Sherr B., Adelman D.C., Saxon A., Gilly M., Wall R. & Sidell N. (1988) Retininoic acid induces the differentiation of B cell hybridomas from patients with common variable immunodeficiency. J. Exp. Med. 168, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklan D., Melamed D. & Friedman A. (1994) The effect of varying levels of dietary vitamin A on immune response in the chick. Poult. Sci. 73, 843–847. [DOI] [PubMed] [Google Scholar]
- Sklan D., Melamed D., Friedman A. (1995) The effect of varying concentrations of vitamin A on immune response in turkey. Br. Poult. Sci. 36, 385. [DOI] [PubMed] [Google Scholar]
- Sun Q., Wang D., She R. et al (2008) Increased mast cell density during the infection with velogenic Newcastle disease virus in chickens. Avian Pathol. 37, 579–585. [DOI] [PubMed] [Google Scholar]
- Villegas P., Purchase H.P. (1989) Titration of biological suspensions In: A Laboratory Manual for Isolation and Identification of Avian Pathogens, 3rd edn, pp. 186–191, (eds Purchase H.G., Arp L.H., Domermuth C.H., Pearson J.E.), Kennett Square, PA: American Association of Avian Pathologists. [Google Scholar]


