Summary
Matrix metalloproteinases (MMPs), myofibroblasts (MFs) and epithelial proliferation have key roles in neoplastic progression. In this study immunoexpression of MMP‐1, MMP‐2 and MMP‐9, presence of MFs and the epithelial proliferation index were investigated in actinic cheilitis (AC), lip squamous cell carcinoma (LSCC) and mucocele (MUC). Thirty cases of AC, thirty cases of LSCC and twenty cases of MUC were selected for immunohistochemical investigation of the proteins MMP‐1, MMP‐2, MMP‐9, α‐smooth muscle actin (α‐SMA) and Ki‐67. The MMP‐1 expression in the epithelial component was higher in the AC than the MUC and LSCC. In the connective tissue, the expression was higher in the LSCC. MMP‐2 showed lower epithelial and stromal immunostaining in the LSCC when compared to the AC and MUC. The epithelial staining for MMP‐9 was higher in the AC when compared to the LSCC. However, in the connective tissue, the expression was lower in the AC compared to other lesions. The cell proliferation rate was increased in proportion to the severity of dysplasia in the AC, while in the LSCC it was higher in well‐differentiated lesions compared to moderately differentiated. There were no statistically significant differences in number of MFs present in the lesions studied. The results suggest that MMPs could affect the biological behaviour of ACs and LSCCs inasmuch as they could participate in the development and progression from premalignant lesions to malignant lesions.
Keywords: actinic cheilitis, cell proliferation, immunohistochemistry, lip cancer, matrix metalloproteinase‐1, matrix metalloproteinase‐2, matrix metalloproteinase‐9, myofibroblasts, oral cancer, squamous cell carcinoma
Actinic cheilitis (AC) is a premalignant condition resulting from long‐term or excessive exposure to ultraviolet (UV) radiation (dos Santos et al. 2003; Vieira et al. 2012). The UV exposure leads to genotypic and phenotypic alterations of the cells that can undergo malignant transformation in approximately 10–20% of cases (Krunic et al. 1998; dos Santos et al. 2003). Lip squamous cell carcinoma (LSCC) constitutes 25–30% of the malignant neoplasms that occur of the oral cavity (Luna‐Ortiz et al. 2004; Vieira et al. 2012), and is especially important in Brazil, a tropical country with high population exposure to UV rays (Sena et al. 2010).
Neoplastic progression and invasion are complex processes that involve extracellular matrix (ECM) and basement membrane (BM) degradation by matrix metalloproteinases (MMPs) and tumour cell migration into the degraded area (Amălinei et al. 2010; Vilen et al. 2013). MMPs are a family of zinc‐ and calcium‐dependent proteolytic enzymes involved in several physiological and pathological conditions such as embryonic development, wound healing, inflammation, tumour invasion and metastasis (Egeblad & Werb 2002; de Vicente et al. 2005; Amălinei et al. 2010). More than 20 different members are currently known, and they are classified according to the domain organization: collagenases (MMP‐1, ‐8, ‐13 and ‐18), gelatinases (MMP‐2 and ‐9), stromelysins (MMP‐3 and ‐10), matrilysins (MMP‐7, ‐26 and ‐11), membrane‐type MMPs (MMP‐14, ‐15, ‐16, ‐17, ‐24 and ‐25) and other MMPs (MMP‐12, ‐19, ‐20, ‐21, ‐23, ‐27 and ‐28) (Souza Freitas et al. 2011).
MMP‐1 has the ability to cleave collagens type I, II and III (Visse & Nagase 2003; Amălinei et al. 2010). MMP‐2 and ‐9 cleave collagen type IV in the BM and other ECM components such as collagens type I, V, VII and X, gelatin, fibronectin and elastin (Egeblad & Werb 2002). MMPs are overexpressed in a wide range of malignant neoplasms, with a correlation between their overexpression, tumour aggressiveness and prognosis (Ii et al. 2006). The sustained presence of these proteinases in the tumour environment is produced by the activated cells, such as fibroblasts, myofibroblasts (MFs), inflammatory and endothelial cells, and by the cancer cells and leads to destruction of normal ECMs (Bissell & Radisky 2001; Egeblad & Werb 2002).
The control in the biological process of cell proliferation is thought to be lost in cancer (Tumuluri et al. 2002). Ki‐67, a protein with a very complex and specific localization pattern within the nucleus, can be used as a prognostic indicator (Brown & Gatter 2002). In oral mucosa, overexpression of Ki‐67 antigen is associated with dysplastic changes (Kurokawa et al. 2003) and with histological grading in oral squamous cell carcinoma (OSCC) (Tumuluri et al. 2002).
MFs are modified fibroblasts with smooth muscle cell‐like features (Tomasek et al. 2002). They have key roles in wound healing, inflammatory and fibrosing conditions and contribute to neoplastic progression (Eyden et al. 2009; Vered et al. 2009). MFs can secrete growth factors, such as keratinocyte growth factor, stem cell factor, epithelial growth factor, transforming growth factor‐β and other cytokines, and MMPs, promoting carcinoma cell proliferation, invasion, metastasis and angiogenesis (Desmoulière et al. 2004; Eyden et al. 2009; Kawashiri et al. 2009). According to Kellermann et al. (2007), an abundance of MFs leads to more aggressive behaviour of OSCC and a shorter overall survival (Kellermann et al. 2007).
Despite the importance of these proteins in the neoplastic progression, there have been very few articles reporting the study of their role in lip carcinogenesis. Therefore this immunohistochemical study was designed to evaluate the association between the presence of MMP‐1, ‐2 and ‐9, MFs and the proliferation index in premalignant (AC) and malignant (LSCC) lip lesions and compare to a non‐neoplastic (MUC) lip disease.
Materials and methods
This study was reviewed and approved by the ethical board of the Federal University of Santa Catarina (protocol: CAAE 16963413.3.0000.0121). Thirty cases of LSCC, thirty cases of AC and twenty cases of mucoceles (MUC) were retrieved from the archives of the Oral Pathology Laboratory and the Anatomical Pathology Service at the Federal University of Santa Catarina (UFSC) (Florianopolis, Brazil). Mucoceles were used as a non‐neoplastic tissue because it was not possible to remove surgical samples of healthy lips. The haematoxylin and eosin slides were analysed by three calibrated observers (two pathologists and a master′s degree student in oral pathology), and the presence or absence of epithelial dysplasia criteria in the AC and the histological grade of the tumour in the LSCC were recorded according to the World Health Organization (WHO) criteria (Barnes et al. 2005).
Three‐micrometre sections from these specimens were dewaxed, rehydrated and treated in 6% H2O2 ⁄ methanol solution (1:1) for 30 min to quench the endogenous peroxidase activity. Antigen retrieval was performed with citrate buffer, pH 6.0, for 30 min in a microwave oven at 1050 W. The non‐specific binding sites were blocked with 5% skim milk in phosphate‐buffered saline solution (PBS) for 40 min. Primary antibodies were incubated at 4°C overnight, followed by incubation with LSAB. Sources, dilutions and positive controls for each antibody are shown in Table 1. Incubation with streptavidin–biotin complex (Kit LSAB Peroxidase K0690 – Dako Corp., Carpinteria, CA, USA) followed by 3‐min incubation with diaminobenzidine DAKO Liquid DAB plus (cod. K3468 – Dako Corp.) and counter staining with Mayer's haematoxylin was proceeded. Negative controls were treated as above, but omitting the primary antibody from the reaction sequence.
Table 1.
Primary antibodies
| Antibody | Clone | Dilution | Positive control |
|---|---|---|---|
| MMP‐1 | Policlonal | 1:300 | Placenta |
| MMP‐2 | Policlonal | 1:50 | Breast carcinoma |
| MMP‐9 | Policlonal | 1:250 | Bone marrow |
| α‐SMA | Policlonal | 1:1000 | Blood vessels |
| Ki‐67 | Monoclonal – SP6 | 1:75 | Oral squamous cell carcinoma |
Slides were examined by the three calibrated observers using an Axiostar Plus (Zeiss, Tornwood, NY, USA) to identify the presence or absence of staining and the expression pattern of each protein in the epithelial cells and connective tissue. The images of the interface between the epithelia and connective tissue (Barros et al. 2011; Souza Freitas et al. 2011) were captured at 400x magnification by an A80 camera (Cannon; Lake Success, NY, USA) and developed by the ImageJ 1.410 software (National Institute of Health, Bethesda, MD, USA). For the MMPs, the average number of positive epithelial cells and the average positive area in the connective tissue were evaluated in 5 equidistant fields per slide. For the Ki‐67, the average number of positive epithelial cells was evaluated in 5 equidistant fields per slide. For the MF, positive α‐SMA cells were counted in the connective tissue of 5 equidistant fields per slide, excluding the blood vessels. The results were analysed by the Microsoft Excel (Microsoft Office Corporation, Washington, EUA) using the t‐test. Statistical differences were defined as P < 0.05.
Results
The results are summarized in Tables 2, 3, 4. The evaluation of the presence of epithelial dysplasia criteria in the AC resulted in 9 cases of mild dysplasia (MIL), 14 cases of moderate dysplasia (MOD) and 7 cases of severe dysplasia (SEV) (Table 3). The evaluation of the histological grade of the LSCC resulted in 12 well‐differentiated (WD), 8 moderately differentiated (MD) and 10 cases of poorly differentiated (PD) LSCC (Table 4).
Table 2.
Average and standard deviation for all antibodies in the studied lesions
| Antibody | Tissue | MUC (n = 20) | AC (n = 30) | LSCC (n = 30) |
|---|---|---|---|---|
| MMP‐1 | Epithelia | 76.91% (±27.47)a | 92.26% (±11.10)a , b | 83.36% (±18.60)b |
| Connective tissue | 3.91% (±2.89)c | 4.80% (±4.24) | 7.29% (±6.21)c | |
| MMP‐2 | Epithelia | 55.83% (±27.33)c | 52.28% (±24.81)b | 33.06% (±23.33)b , c |
| Connective tissue | 2.47% (±1.65)a | 1.39% (±1.63)a | 1.91% (±1.62) | |
| MMP‐9 | Epithelia | 41.68% (±38.43) | 59.07% (±28.84)b | 35.32% (±30.67)b |
| Connective tissue | 0.58% (±0.76)a | 0.19% (±0.14)a , b | 0.84% (±0.70)b | |
| Ki‐67 | Epithelia | 21.17% (±10.90) | 19.64% (±10.87) | 24.10% (±15.52) |
| MF | Connective tissue | 8.67% (±8.27) | 13.51% (±11.43) | 11.45% (±12.25) |
Relationship between mucocele (MUC) and actinic cheilitis (AC) with P < 0.05.
Relationship between actinic cheilitis (AC) and lip squamous cell carcinoma (LSCC) with P < 0.05.
Relationship between MUC and LSCC with P < 0.05.
Table 3.
Average and standard deviation according to epithelial dysplasia degree in AC
| AC | Tissue | MIL (n = 9) | MOD (n = 14) | SEV (n = 7) |
|---|---|---|---|---|
| MMP‐1 | Epithelia | 95.19% (±4.58) | 88.87% (±14.99) | 95.25% (±5.25) |
| Connective tissue | 2.84% (±1.92) | 4.88% (±3.81) | 6.18% (±5.77) | |
| MMP‐2 | Epithelia | 45.24% (±16.12) | 58.20% (±24.47) | 48.52% (±33.10) |
| Connective tissue | 1.34% (±1.64) | 1.29% (±1.08) | 1.58% (±2.39) | |
| MMP‐9 | Epithelia | 59.11% (±28.04) | 62.39% (±31.87) | 59.11% (±28.04) |
| Connective tissue | 0.12% (±0.99) | 0.22% (±0.17) | 1.77% (±1.22) | |
| Ki‐67 | Epithelia | 15.92% (±8.97) | 19.98% (±12.56) | 22.59% (±9.78) |
| MF | Connective tissue | 9.44% (±10.14) | 16.82% (±11.96) | 12.11% (±11.45) |
MIL, mild dysplasia in AC; MOD, moderate dysplasia in AC; SEV, severe dysplasia in AC.
Table 4.
Average and standard deviation according to cell differentiation degree in LSCC
| LSCC | Tissue | WD (n = 12) | MD (n = 8) | PD (n = 10) |
|---|---|---|---|---|
| MMP‐1 | Epithelia | 80.86% (±23.74) | 89.58% (±9.89) | 80.46% (±17.87) |
| Connective tissue | 6.28% (±3.88) | 10.32% (±8.90) | 5.57% (±4.99) | |
| MMP‐2 | Epithelial | 36.60% (±23.45) | 34.46% (±30.69) | 26.91% (±14.57) |
| Connective tissue | 1.91% (±1.27) | 2.40% (±2.42) | 1.41% (±0.94) | |
| MMP‐9 | Epithelial | 38.03% (±29.03) | 38.07% (±38.62) | 28.94% (±26.36) |
| Connective tissue | 0.73% (±0.33) | 1.05% (±1.01) | 0.75% (±1.74) | |
| Ki‐67 | Epithelial | 33.82% (±17.83)a | 14.71% (±5.16)a | 21.38% (±12.26) |
| MF | Connective tissue | 6.86% (±4.12) | 10.55% (±6.06) | 18.46% (±19.91) |
Relationship between well‐differentiated (WD) carcinoma and moderately differentiated (MD) with P < 0.05.
WD, well‐differentiated LSCC; MD, moderately differentiated LSCC; PD, poorly differentiated LSCC.
MMPs
The MMPs showed stronger immunostaining in the cytoplasm of the epithelial cells in the lower third of the epidermis. In the connective tissue, immunostaining was present in both the ECM (except for the basophilic degeneration areas) and cytoplasm of the individual cells. (Figure 1a–i).
Figure 1.
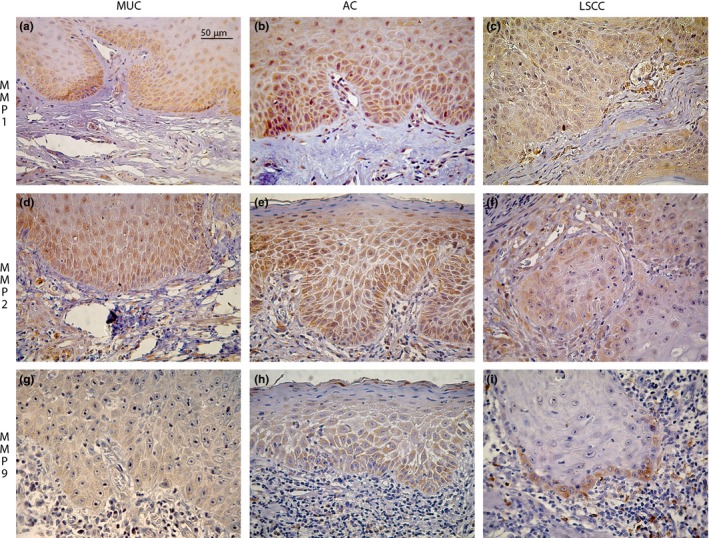
Immunohistochemical expression of MMP‐1, ‐2 and ‐9 in MUC, AC and LSCC (400X) (scale bar, 50μm). (a–i) MMPs staining stronger in the lower third of the epithelia and dispersed on connective tissue.
Epithelial immunoexpression of MMP‐1 was significantly higher in AC compared to the MUC (P = 0.023) and LSCC (P = 0.029). In the connective tissue the expression in the LSCC was significantly higher than the expression in the MUC (P = 0.013). Furthermore, MMP1 was present in a higher number of cells in the LSCC (7.29% ±6.21) than in the AC (4.80% ±4.24). There was also higher expression of MMP1 in the AC with SEV (6.18% ±5.77) when compared to the MIL (2.84% ±1.92) and MOD (4.88% ±3.81), but this was not statistically significant. MMP‐2 showed a lower epithelial immunoexpression in the LSCC than in the AC (P = 0.003) and MUC (P = 0.004). In the connective tissue, the expression was higher in the MUC when compared to the AC (P = 0.029).
Finally, in the case of MMP‐9, the epithelial staining was higher in the AC when compared to the LSCC (P = 0.003). This trend was also seen in the comparison between AC and MUC but there was much wider variation in the MUC expression, and hence the results were not statistically significant. (59.07% ±28.84 for the AC and 41.68% ±38.43 for the MUC). When the expression in the connective tissue was analysed, expression in LSCC was higher than in AC (P < 0.001) and MUC (P = 0.033) expression. Therefore, the MMPs studied showed predominance of epithelial expression in premalignant disease (AC) and predominance of connective tissue expression in the malignant disease (LSCC).
Cell proliferation (Ki‐67)
Ki‐67 epithelial immunostaining was observed in the nuclei of the cells of the basal and parabasal layers in the AC and MUC and in the cells of the invasion areas of the LSCC (Figure 2a–c). There were no statistical differences between the AC, MUC and LSCC; however, the average of positive cells was higher in the LSCC. Furthermore, the proliferation index was higher according to the severity of dysplasia in the AC. In the LSCC, there was higher expression in the WD when compared to the MD (P = 0.004).
Figure 2.
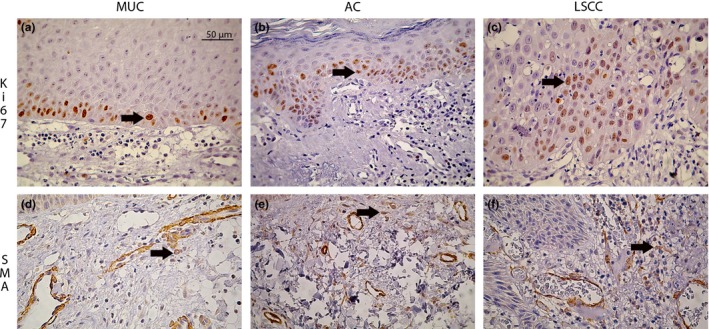
Immunohistochemical expression of Ki‐67 and α‐SMA in MUC, AC and LSCC (400X) (scale bar, 50μm). (a–c) Ki‐67 nuclear immunostaining concentrated in the basal and parabasal layers of the epithelial tissue in MUC and AC and dispersed in the invasion areas of LSCC (arrow). (d–f) α‐SMA staining MFs in the connective tissue (arrow).
MFs (α‐SMA)
MFs were observed in the connective tissue of all lesions (Figure 2d–f) without a statistical difference in the sample studied; however, the presence of MFs was higher in premalignant (AC) and malignant disease (LSCC) when compared to non‐neoplastic disease (MUC).
Discussion
There have been few articles which have studied the relationship between MMPs and AC and between MMPs and OSCC (de Vicente et al. 2005; Barros et al. 2011; Mohtasham et al. 2013; Poulopoulos et al. 2013), and these have focussed primarily on the correlation of MMPs' expression between AC and LSCC (Souza Freitas et al. 2011). We evaluated the immunohistochemical expression of MMP‐1, ‐2, and ‐9, Ki‐67 and α‐SMA in the epithelial component and connective tissue of LSCC, an invasive malignant neoplasia, as well as in AC, a premalignant lesion. As a benchmark, we evaluated the same proteins in the MUC, a non‐neoplastic lip disease. It is noteworthy that MUC was not the best lesion for this comparison because it has an inflammatory component and several inflammatory cytokines such as tumour necrosis factor‐alpha (TNF‐α) and interleukin‐1 beta (IL‐1β) that can stimulate the production of MMPs (Hoque et al. 1998). Nevertheless, in the face of the absence of healthy lip samples in the pathology archives and the ethical conflicts involved in surgical acquisition of samples of healthy lips tissue, MUC was the more suitable source of non‐neoplastic epithelia.
It is known that the AC and LSCC both result from long‐term or excessive exposure to UV radiation (dos Santos et al. 2003; Vieira et al. 2012). UV irradiation initiates and activates a complex cascade of biochemical reactions that can activate MMPs leading to ECM degradation (Pillai et al. 2005). The MMPs immunostaining pattern in the epithelial component of all lesions studied showed a higher expression in the lower third of the surface epithelium. In the connective tissue the staining was in both the ECM and the cytoplasm of individual cells. Since the relationship between epithelial cells and connective tissue is crucial to neoplastic progression (Desmoulière et al. 2004; Kellermann et al. 2007), this staining pattern, mainly in AC and LSCC, is compatible with MMPs functions in neoplastic progression and invasion (de Vicente et al. 2005; Kellermann et al. 2008; Pietras & Ostman 2010).
MMP‐1 has a key role in ECM remodelling, degrading several ECM components, mainly the collagen I, and indirectly activating MMP‐2, a protagonist enzyme in neoplastic invasion (de Vicente et al. 2005; Cao et al. 2009). Therefore, MMP‐1 seems to have important roles in premalignant and in situ lesions, which matches with its higher expression in the AC, mainly in SEV. On the other hand, the connective tissue expression of MMP‐1 was higher in the LSCC, and this could be explained by its specific ability to degrade type‐I collagen, which is the most abundant substrate in the tumour surrounding stroma (Shimizu et al. 2008). Therefore, MMP‐1 takes part in the invasion phenomena, one of the most important characteristics of the malignant neoplasia. Barros et al. (2011) suggested that the overexpression of MMP‐1 in the stroma of oral SCCs is due to the production of MMPs by the stroma under parenchymal stimulation (Barros et al. 2011).
The expression of MMP‐2 and MMP‐9 was lower in the epithelia of LSCC when compared to AC and MUC. Both MMPs cleave BM and ECM components allowing the penetration of transformed cells in the connective tissue (de Vicente et al. 2005; Pietras & Ostman 2010). This could justify the higher presence of these enzymes in AC, a premalignant lesion without BM destruction, in comparison with LSCC, an invasive disease. Nevertheless, the same enzymes were more abundant in the connective tissue of LSCC when compared to AC. This expression is compatible with the MMP‐1 expression, as MMP‐1 transforms the collagen type 1 in gelatin, the main substrate of MMP‐2 and ‐9, and this sequence is essential to neoplastic progression and invasion (Cotrim et al. 2002; de Vicente et al. 2005; Pietras & Ostman 2010).
Finally, the MMPs studied showed predominance of epithelial expression in premalignant disease (AC) and predominance of connective tissue expression in the malignant disease (LSCC). This fact suggests that in the non‐invasive premalignant disease, MMPs would be one of the factors involved in the process of epithelial malignant transformation, probably by their capacity of microenvironment regulation, cell proliferation and BM degradation (Bissell & Radisky 2001; Egeblad & Werb 2002; Visse & Nagase 2003; de Vicente et al. 2005; Amălinei et al. 2010). In malignant disease, MMPs would be more involved in the tumour cells invasion, mainly by the production of MMPs by the stroma under parenchymal stimulation (Ziober et al. 2000; de Vicente et al. 2005; Barros et al. 2011). The remarkable presence of the MMPs studied in the MUC could be explained by the presence of a persistent inflammation in its connective tissue. It is known that in tissues under persistent inflammatory conditions, continual upregulation of MMPs by stromal fibroblasts can disrupt the ECM (Bissell & Radisky 2001). Moreover, macrophages can express MMP‐1 and MMP‐9 (Hoque et al. 1998; Pesce et al. 2003).
The evaluation of cell proliferation index did not show statistical differences in the samples studied. Despite this, the average of positive cells was higher in the LSCC. Considering that the process of OSCC development has multiple steps such as initiation, promotion and progression, the loss of the control of cell proliferation is one of the most important phenomena (Tumuluri et al. 2002; Gonzalez‐Moles et al. 2010; Chen et al. 2011). The high presence of Ki‐67 in the MUC can be explained by the inflammation again, because in tissues under persistent inflammatory conditions, immune cells can overproduce factors that promote abnormal epithelial proliferation (Bissell & Radisky 2001; Lin et al. 2007).
Moreover, the proliferation index was higher according to the severity of dysplasia in the AC, data observed before in the AC and intra‐oral premalignant lesions (Kurokawa et al. 2003; Birajdar et al. 2014; Salvadori et al. 2014). In the case of LSCC, proliferation was higher in the WD than in the MD and PD. This result, which is in disagreement with the literature (Tumuluri et al. 2002; Kurokawa et al. 2003; Birajdar et al. 2014; Salvadori et al. 2014), could be due to the low number of cases in this comparison: 12 cases of WD, 8 cases of MD and 10 cases of PD.
The absence of significance in the comparison of MFs in the studied lesions could be explained by the basophilic degeneration of the connective tissue present in AC and LSCC. It is known that the excessive exposure to UV radiation leads to a degradation of connective tissue by quantitative and qualitative alterations of cells and ECM proteins, with ultrastructural alterations and deposition of exogenous substances (Suwabe et al. 1999; Ma et al. 2008). Furthermore, UV‐exposed fibroblasts showed phenotypic changes, and the fibroblasts are one of the main origin sources of MFs (Tomasek et al. 2002; Ma et al. 2008). It is not clear whether the UV alterations in the connective tissue directly affected the presence and function of MFs, but this hypothesis should be considered in future studies. The presence of MFs in the MUC could be explained because the earlier events in the differentiation of fibroblasts in MFs are influenced by the inflammatory cytokines (Vered et al. 2009).
In conclusion, it is believed that MMP‐1, ‐2 and ‐9 have key roles in the biological behaviour of AC and LSCC and they could be involved in the development and progression from premalignant lesion to malignant lesion in the lips. The presence of MFs and the proliferation index did not have a significant influence in the sample studied.
Conflict of interests' statement
None of the authors declared that they have conflict(s) of interests. There is no financial or personal relationship with people or organization that could inappropriately influence or benefit from this work.
Acknowledgements
We thank the National Council for the Improvement of Higher Education (CAPES) for their financial support.
References
- Amălinei C., Căruntu I.D., Giuşcă S.E. & Bălan R.A. (2010) Matrix metalloproteinases involvement in pathologic conditions. Rom. J. Morphol. Embryol. 51, 215–228. [PubMed] [Google Scholar]
- Barnes L., Everson J.W., Reichart P., Sidransky D. (2005) Pathology and genetics of head and neck tumours. Lyon: IARC Press. [Google Scholar]
- Barros S.S., Henriques Á., Pereira K.M., de Medeiros A.M., Galvão H.C., Freitas R.e.A. (2011) Immunohistochemical expression of matrix metalloproteinases in squamous cell carcinoma of the tongue and lower lip. Arch. Oral Biol. 56, 752–760. [DOI] [PubMed] [Google Scholar]
- Birajdar S.S., Radhika M., Paremala K., Sudhakara M., Soumya M. & Gadivan M. (2014) Expression of Ki‐67 in normal oral epithelium, leukoplakic oral epithelium and oral squamous cell carcinoma. J Oral Maxillofac Pathol 18, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M.J. & Radisky D. (2001) Putting tumours in context. Nat. Rev. Cancer 1, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.C. & Gatter K.C. (2002) Ki67 protein: the immaculate deception? Histopathology 40, 2–11. [DOI] [PubMed] [Google Scholar]
- Cao Z., Xiang J. & Li C. (2009) Expression of extracellular matrix metalloproteinase inducer and enhancement of the production of matrix metalloproteinase‐1 in tongue squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 38, 880–885. [DOI] [PubMed] [Google Scholar]
- Chen Y.K., Yang S.H., Huang A.H., Hsue S.S. & Lin L.M. (2011) Aberrant expression in multiple components of the transforming growth factor‐β1‐induced Smad signaling pathway during 7,12‐dimethylbenz[a]anthracene‐induced hamster buccal‐pouch squamous‐cell carcinogenesis. Oral Oncol. 47, 262–267. [DOI] [PubMed] [Google Scholar]
- Cotrim P., de Andrade C.R., Line S., de Almeida O.P. & Coletta R.D. (2002) Expression and activity of matrix metalloproteinase‐2 (MMP‐2) in the development of rat first molar tooth germ. Braz Dent J 13, 97–102. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Guyot C. & Gabbiani G. (2004) The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int. J. Dev. Biol. 48, 509–517. [DOI] [PubMed] [Google Scholar]
- Egeblad M. & Werb Z. (2002) New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2, 161–174. [DOI] [PubMed] [Google Scholar]
- Eyden B., Banerjee S.S., Shenjere P. & Fisher C. (2009) The myofibroblast and its tumours. J. Clin. Pathol. 62, 236–249. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Moles M.A., Ruiz‐Avila I., Gil‐Montoya J.A., Esteban F. & Bravo M. (2010) Analysis of Ki‐67 expression in oral squamous cell carcinoma: why Ki‐67 is not a prognostic indicator. Oral Oncol. 46, 525–530. [DOI] [PubMed] [Google Scholar]
- Hoque M.O., Azuma M. & Sato M. (1998) Significant correlation between matrix metalloproteinase activity and tumor necrosis factor‐alpha in salivary extravasation mucoceles. J. Oral Pathol. Med. 27, 30–33. [DOI] [PubMed] [Google Scholar]
- Ii M., Yamamoto H., Adachi Y., Maruyama Y. & Shinomura Y. (2006) Role of matrix metalloproteinase‐7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 231, 20–27. [DOI] [PubMed] [Google Scholar]
- Kawashiri S., Tanaka A., Noguchi N. et al (2009) Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck 31, 1346–1353. [DOI] [PubMed] [Google Scholar]
- Kellermann M.G., Sobral L.M., da Silva S.D. et al (2007) Myofibroblasts in the stroma of oral squamous cell carcinoma are associated with poor prognosis. Histopathology 51, 849–853. [DOI] [PubMed] [Google Scholar]
- Kellermann M.G., Sobral L.M., da Silva S.D. et al (2008) Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 44, 509–517. [DOI] [PubMed] [Google Scholar]
- Krunic A.L., Garrod D.R., Madani S., Buchanan M.D. & Clark R.E. (1998) Immunohistochemical staining for desmogleins 1 and 2 in keratinocytic neoplasms with squamous phenotype: actinic keratosis, keratoacanthoma and squamous cell carcinoma of the skin. Br. J. Cancer 77, 1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa H., Matsumoto S., Murata T. et al (2003) Immunohistochemical study of syndecan‐1 down‐regulation and the expression of p53 protein or Ki‐67 antigen in oral leukoplakia with or without epithelial dysplasia. J. Oral Pathol. Med. 32, 513–521. [DOI] [PubMed] [Google Scholar]
- Lin L.M., Huang G.T. & Rosenberg P.A. (2007) Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healing. J Endod 33, 908–916. [DOI] [PubMed] [Google Scholar]
- Luna‐Ortiz K., Güemes‐Meza A., Villavicencio‐Valencia V. & Mosqueda‐Taylor A. (2004) Lip cancer experience in Mexico. An 11‐year retrospective study. Oral Oncol. 40, 992–999. [DOI] [PubMed] [Google Scholar]
- Ma W., Wlaschek M., Tantcheva‐Poór I. et al (2008) Chronological ageing and photoageing of the fibroblasts and the dermal connective tissue. Clin. Exp. Dermatol. 26, 592–599. [DOI] [PubMed] [Google Scholar]
- Mohtasham N., Babakoohi S., Shiva A. et al (2013) Immunohistochemical study of p53, Ki‐67, MMP‐2 and MMP‐9 expression at invasive front of squamous cell and verrucous carcinoma in oral cavity. Pathol. Res. Pract. 209, 110–114. [DOI] [PubMed] [Google Scholar]
- Pesce C., Clapasson A., Valente S. & Passarello S. (2003) Tissue repair and remodelling in extravasation mucocele. Histopathology 42, 516–517. [DOI] [PubMed] [Google Scholar]
- Pietras K. & Ostman A. (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp. Cell Res. 316, 1324–1331. [DOI] [PubMed] [Google Scholar]
- Pillai S., Oresajo C. & Hayward J. (2005) Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation‐induced matrix degradation ‐ a review. Int. J. Cosmet. Sci. 27, 17–34. [DOI] [PubMed] [Google Scholar]
- Poulopoulos A.K., Andreadis D. & Markopoulos A.K. (2013) Expression of matrix metalloproteinases 9 and 12 in actinic cheilitis. World J. Exp. Med. 3, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadori G., Dos Santos J.N., Martins M.A. et al (2014) Ki‐67, TGF‐β1, and elastin content are significantly altered in lip carcinogenesis. Tumour Biol. 35, 7635–7644. [DOI] [PubMed] [Google Scholar]
- dos Santos J.N., de Sousa S.O., Nunes F.D., Sotto M.N. & de Araújo V.C. (2003) Altered cytokeratin expression in actinic cheilitis. J. Cutan. Pathol. 30, 237–241. [DOI] [PubMed] [Google Scholar]
- Sena M.F., Costa A.P.S., Nobrega A.G.S., Ferreira A.A.F. & Costa A.L.L. (2010) Avaliação dos fatores prognósticos relacionados ao câncer de lábio: revisão sistemática. Revista Brasileira de Cancerologia 56, 93–102. [Google Scholar]
- Shimizu Y., Kondo S., Shirai A., Furukawa M. & Yoshizaki T. (2008) A single nucleotide polymorphism in the matrix metalloproteinase‐1 and interleukin‐8 gene promoter predicts poor prognosis in tongue cancer. Auris Nasus Larynx 35, 381–389. [DOI] [PubMed] [Google Scholar]
- Souza Freitas V., de Andrade Santos P.P., de Almeida Freitas R., Pereira Pinto L. & de Souza L.B. (2011) Mast cells and matrix metalloproteinase 9 expression in actinic cheilitis and lip squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 112, 342–348. [DOI] [PubMed] [Google Scholar]
- Suwabe H., Serizawa A., Kajiwara H., Ohkido M. & Tsutsumi Y. (1999) Degenerative processes of elastic fibers in sun‐protected and sun‐exposed skin: immunoelectron microscopic observation of elastin, fibrillin‐1, amyloid P component, lysozyme and alpha1‐antitrypsin. Pathol. Int. 49, 391–402. [DOI] [PubMed] [Google Scholar]
- Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C. & Brown R.A. (2002) Myofibroblasts and mechano‐regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363. [DOI] [PubMed] [Google Scholar]
- Tumuluri V., Thomas G.A. & Fraser I.S. (2002) Analysis of the Ki‐67 antigen at the invasive tumour front of human oral squamous cell carcinoma. J. Oral Pathol. Med. 31, 598–604. [DOI] [PubMed] [Google Scholar]
- Vered M., Allon I., Buchner A. & Dayan D. (2009) Stromal myofibroblasts accompany modifications in the epithelial phenotype of tongue dysplastic and malignant lesions. Cancer Microenviron 2, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vicente J.C., Fresno M.F., Villalain L., Vega J.A. & Hernández Vallejo G. (2005) Expression and clinical significance of matrix metalloproteinase‐2 and matrix metalloproteinase‐9 in oral squamous cell carcinoma. Oral Oncol. 41, 283–293. [DOI] [PubMed] [Google Scholar]
- Vieira R.A., Minicucci E.M., Marques M.E. & Marques S.A. (2012) Actinic cheilitis and squamous cell carcinoma of the lip: clinical, histopathological and immunogenetic aspects. An. Bras. Dermatol. 87, 105–114. [DOI] [PubMed] [Google Scholar]
- Vilen S.T., Salo T., Sorsa T. & Nyberg P. (2013) Fluctuating roles of matrix metalloproteinase‐9 in oral squamous cell carcinoma. ScientificWorldJournal 2013, 920595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visse R. & Nagase H. (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92, 827–839. [DOI] [PubMed] [Google Scholar]
- Ziober B.L., Turner M.A., Palefsky J.M., Banda M.J. & Kramer R.H. (2000) Type I collagen degradation by invasive oral squamous cell carcinoma. Oral Oncol. 36, 365–372. [DOI] [PubMed] [Google Scholar]


