Summary
Cadmium is a well‐known testicular toxicant, and parts of the world population are exposed chronically by inhalation or by food and water intake. Grape products have been highlighted as important sources of bioactive compounds, having anti‐inflammatory, anti‐oxidant and metal chelating properties. Since maintenance of tissue morphology is essential for testicular sperm development and hence male fertility, we analysed the protective effect of grape juice concentrate (GJC) (G8000®) consumption on testicular morphology in rats exposed to cadmium. Thus, four groups of male Wistar rats (n = 6 per group), 50 days old, ingested either water or G8000® (2 g/kg/day) until they had completed one spermatogenic cycle in adult life (136 days old). Cadmium (1.2 mg / kg) was injected intraperitoneally when the animals were 80 days old into one of the water and one of the G8000 groups; intraperitoneal saline was used as a control in the other two groups. Animals anaesthetised and exsanguinated at 136 days and then perfused with Karnovsky's fixative and then the testes were collected for morphological analysis. We describe evident disruption of testicular morphology by cadmium, with alteration in tissue component proportions, reduced Leydig cells volume and initial signs of an inflammatory process. Ultrastructural analysis showed greater damage, suggesting spermatogenesis disruption. G8000® ingestion allowed tissue architecture to be re‐established, as was corroborated by our stereological and morphometric findings. Animals from the group where G8000® had been administered together with cadmium revealed a significant reduction in macrophages and blood vessel volume, suggesting diminished inflammation, when compared to animals that received only cadmium. Moreover, smaller number of ultrastructural alterations was noted, revealing fewer areas of degeneration and disorganized interstitium. In conclusion, our results demonstrate that GJC consumption prevented the spermatogenic disruption promoted by cadmium, and thus could be a promising form of therapy against male infertility.
Keywords: heavy metal, male intoxication, male reproduction, morphometry, polyphenols
Cadmium is a very toxic metal that causes significant dysfunction in animals and humans. Nowadays exposure to this metal is very frequent, since it is present in pesticides, cigarettes and some foods, and is considered by the World Health Organization to be one of the worst food pollutants (Who 1992; El‐Shahat et al. 2009).
Considering reproductive parameters, cadmium has been reported to cause irreversible damage to seminiferous epithelium and testis interstitial compartments mainly due to oxidative stress induction (Liu et al. 1996). The action of this metal includes primary changes in the vascular system, triggering an increase in inflammatory processes, with testicular oedema, haemorrhage and necrosis, which can affect both epithelial and interstitial compartments (Blanco et al. 2007; Siu et al. 2009). Moreover, irreversible damage has been reported in Sertoli cells, blood–testicular barrier destabilization, lipid peroxidation, DNA fragmentation, loss of germ cells, reduction in testicular weight, destabilization of the body's anti‐oxidant barrier, lower sperm motility and an increase in abnormal sperm morphology (Liu et al. 1996; Kusakabe et al. 2008; Oliveira et al. 2009; Siu et al. 2009; Wang et al. 2011). Furthermore, in Leydig cells, this metal can reduce the synthesis and secretion of testosterone, without altering the cell viability (Messaoudi et al. 2010).
To reverse or diminish this alteration, many anti‐oxidants have been studied, including phenolic compounds, present in grapes (Mustali & Dohadwala Joseph 2009). These compounds are divided into two categories: flavonoids (such as anthocyanin and catechin) and non‐flavonoids (for example, resveratrol), which include simple compounds such as phenolic acids, and highly polymerized compounds (Abe et al. 2007; Ho et al. 2010).
The benefits of these substances have been described widely in the literature, as well as their anti‐oxidant properties (scavenging oxygen reactive species), their capacity to modulate oxidative stress markers, to reduce blood pressure, and to show anti‐inflammatory properties (Mustali & Dohadwala Joseph 2009; Ho et al. 2010). Considering reproductive parameters, Jiang et al. (2008) demonstrated the increase in testicular weight and improvement of seminiferous tubule morphology of rats exposed to 2,5‐hexanedione and resveratrol.
Based on the fact that daily ingestion of these substances in certain quantities can favourably modify metabolism, preventing changes that can lead to more severe damage (Anjo 2004), the aim of this research was to evaluate the preventive effect of one of the phenolic compounds derived from grapes, grape juice concentrate (GJC), on testis morphology and ultrastructure in rats intoxicated with cadmium.
Material and methods
Grape juice concentrate
To develop this study, the GJC G8000® from Golden Sucos, Farroupilha‐RS, Brazil, was used. The animals received the concentrate daily in the dose of 2 mg/kg body weight (BW), by oral administration. In order to be consistent with the American Dietetic Association recommendation of 200–500 ml of grape juice that is required in order to develop positive physiological effects in humans (American Dietetic Association 2004), the present dosage was determined to be the equivalent to eight glasses (400 ml) of natural grape juice and was further adjusted to rat metabolism, which is faster than in humans (Zorzano & Herrera 1990; Scollon et al. 2009). The complete characterization of the GJC used, as well as the nature of its bioactive compounds, was previously described in Aguiar et al. (2011).
Animals
The study was carried out with 50‐day‐old male Wistar rats obtained from the Central Animal Raising Unit of UNICAMP (State University of Campinas, Campinas, SP, Brazil). All the procedures were developed according to the Guide for Care and Use of Laboratory Animals and were approved by the Ethics Committee for Animal Experimentation of UNICAMP (2900‐1). The animals were housed three per cage, with a 12‐h light–dark cycle. Food and water were provided ad libitum.
Experimental design
Twenty‐four Wistar rats were randomly divided into four groups, as described above:
GC: water and an intraperitoneal injection of saline (0.9%);
GCd: water and an intraperitoneal injection of CdCl2;
GCdJ: grape juice concentrate and an intraperitoneal injection of CdCl2;
GJ: grape juice concentrate and an intraperitoneal injection of saline (0.9%).
The water or GJC (2 g/kg BW) was administered daily by gavage using rats from 50 to 80 days old, which is the period of sexual maturation, according to Zanato et al. (1994). Juice or water administration continued until the end of one spermatogenic cycle in adult life (136 days old) (Russell et al. 1990). The cadmium chloride (CdCl2 P.A. Nuclear® cod. 318430) dose of 1.2 mg/kg was diluted in 0.5 ml of distilled water (Predes et al. 2010) and injected when rats became adults (80 days old) (Zanato et al. 1994). This dose was chosen based on the previous work that tested low doses of cadmium that can cause testicular injury (Predes et al. 2010). Saline solution (0.9%) was intraperitoneally injected when the animals were 80 days old, volume 0.5 ml, to maintain the same experimental conditions.
Tissue preparation for light and electron transmission microscopy analyses
The animals (136 days old) were anaesthetized with a mixture of ketamine and xylazine (10 and 80 mg/kg, respectively), and the chest cavity opened to collect blood in heparinized tubes by puncture of the left ventricle. The animals were first perfused with saline (0.9%) to clear the testis vascular bed, then with Karnovsky [5% glutaraldehyde, 2.5% paraformaldehyde 0.2 M in sodium phosphate buffer (pH 7.2)] for at least 20 min. The right testicle was removed and weighed with an analytical scale (Ohaus®, Barueri, São Paulo, Brazil). The organs were postfixed overnight in the same solution. The following day, small blocks of the right testicle was embedded in glycol methacrylate. They were sectioned at 3 μm thickness and stained with toluidine blue/1% sodium borate, for light microscopy analyses.
For ultrastructural analysis, samples were postfixed in osmium tetroxide (1%), dehydrated in acetone and embedded in epoxy resin. Ultrathin sections were obtained and stained with uranyl acetate (2%) and lead citrate (0.2%), before observation with a transmission electron microscope (Zeiss, Leo 906‐Oberkochen, Germany).
Biometric and morphometric analysis
The BW gain was obtained by subtracting the animal weights at the end of the experiment from their initial weights. The relative testis weight, known as the gonadosomatic index (GSI), was calculated relating total testis weight and total BW (Gomes et al. 2011). To obtain a more precise parenchyma weight, the albuginea weight was subtracted.
For morphometry, the Image Pro Plus software associated with an Olympus BX‐40 microscope was used. The tubular diameter and epithelium height were measured for thirty tubules, randomly chosen at 100× magnification (Gomes et al. 2011). The seminiferous tubule total length was calculated according to the following formula: TL = TVS/πR 2, considering TVS = total volume of seminiferous tubules and πR 2 = area of the seminiferous tubule cross sections.
Stereological analysis
Using the Image Pro Plus software associated with an Olympus BX‐40 microscope, ten images were randomly obtained with 400× magnification, and the tubule epithelium, tubule lumen and the interstitium were measured. Using a grid with 432 intersections over the images, the proportion of each component was obtained based on the total of intersections (modified from Predes et al. 2010). Moreover, considering the density of this organ as 1 (1.03–1.04), the testicular weight was considered the same as its volume (França & Russel 1998). These data were used to calculate the volume of these components.
To analyse the interstitium elements (lymphatic space, blood vessels, macrophages and Leydig cells), 2000 intersections were counted on images randomly obtained with 400× magnification, using a grid with 432 intersections. The proportion of each component was obtained based on the total of intersections (modified from Predes et al. 2010). Moreover, to calculate their volume, the total volume of the testis was used. This was obtained considering the testicular volume to be the same as its weight, as explained above (França & Russel 1998). To quantify the number of blood vessels, they were counted in 25 micrographs for each animal obtained with 400× magnification. For the blood vessels incompletely shown in the image, only those on the lower and right‐hand borders of the image were considered. The results were expressed in number of blood vessels per μm2.
To verify the individual Leydig cell volume, the method found in the study of Leite et al. (2013) was used. Thus, 1000 intersections were counted on random images with 1000× magnification, using a grid with 432 intersections. The diameters of 10 nuclei were measured. The nuclear and cytoplasmic proportions were calculated based on 1000 intersections. Using these data and the following formulas, the nuclear and cytoplasmic volumes were calculated: nuclear volume = 4/3πR³, considering R = nuclear radius and the cytoplasmic volume = (% cytoplasm × nuclear volume)/% nucleus. Adding these volumes, the individual Leydig cell volume could be calculated. The number of Leydig cells per testis was obtained dividing the total Leydig cell volume per individual Leydig cell volume. To calculate the number of Leydig cells per gram of testis, the number of Leydig cells per testis was divided by the respective parenchyma weight.
Statistical analysis
The comparison of control values and other groups was made using the anova test, followed by Tukey's post‐test. The results were considered significant for P < 0.05. Moreover, for all values, the means ± standard deviation was calculated.
Results
Biometric and morphometric data
Body weight gain was reduced in all groups when compared to GC. However, final BW was reduced only in groups GCdJ and GJ relative to GC. There was no treatment effect on testis absolute weight or GSI (Table 1). Moreover, the GSI and the testis absolute weight were not altered (Table 1).
Table 1.
Biometry and morphometric data (mean values ± standard deviation)
| Parameters | GC | GCd | GCdJ | GJ |
|---|---|---|---|---|
| Body weight gain (g) | 296.7 ± 41.19 | 251.3 ± 18.35* | 243.0 ± 30.98** | 253.3 ± 30.30** |
| Final body weight (g) | 536.33 ± 52.64 | 482.67 ± 19.65 | 453.5 ± 40.60** | 460.17 ± 38.45** |
| Testis absolute weight (g) | 1.99 ± 0.20 | 1.77 ± 0.23 | 1.85 ± 0.16 | 1.92 ± 0.27 |
| GSI (%) | 0.373 ± 0.04 | 0.368 ± 0.05 | 0.409 ± 0.03 | 0.417 ± 0.05 |
| Tubular diameter (μm) | 338.86 ± 20.54 | 308.11 ± 10.35* | 297.48 ± 19.79** | 302.93 ± 14.17** |
| Epithelium high (μm) | 68.65 ± 2.19 | 44.66 ± 3.78*** | 52.90 ± 2.62* | 56.85 ± 2.22* , a |
| Tubules total length (m) | 19.98 ± 3.42 | 15.68 ± 2.07* | 21.17 ± 3.20b | 21.59 ± 2.58b |
GSI, gonadosomatic index; GC, control group; GCd, cadmium group; GCdJ, cadmium and grape juice concentrate group; GJ, grape juice concentrate group. (n = 6) A significant difference was found for the following: *P < 0.05 with GC, **P ≤ 0.01 with GC, ***P ≤ 0.001 with GC. a P ≤ 0.05 with GCd, b P ≤ 0.01 with GCd.
Tubular diameter was reduced in all groups when compared to GC, and the same pattern was seen with respect to epithelium height. Moreover, seminiferous tubule total length was lower in GCd in relation to other groups. These data are demonstrated in Table 1.
Stereological evaluations
The volume of seminiferous tubules was reduced in GCd when compared to GC. A significant improvement in GCdJ compared to GCd was seen when comparing the percentage area occupied by seminiferous tubules. The tubular lumen percentage and volume were reduced for GCd compared to GC. Higher values of tubular lumen percentage and volume in GCdJ were also noted when compared to GCd. Moreover, the interstitium volume and percentage were improved in GCdJ relative to GCd. There were no changes noted in the tubular epithelium percentage and volume. All these results are summarised in Table 2.
Table 2.
Testicular stereological data (mean value ± standard deviation)
| Parameters | GC | GCd | GCdJ | GJ |
|---|---|---|---|---|
| Seminiferous tubules | ||||
| ml | 1.59 ± 0.16 | 1.28 ± 0.20* | 1.51 ± 1.16 | 1.55 ± 0.25 |
| % | 83.44 ± 2.46 | 77.32 ± 4.68 | 86.57 ± 2.31c | 85.25 ± 1.92b |
| Epithelium | ||||
| ml | 1.15 ± 0.14 | 1.07 ± 0.18 | 1.14 ± 0.14 | 1.14 ± 0.18 |
| % | 60.71 ± 2.52 | 64.26 ± 5.23 | 65.01 ± 2.46 | 62.52 ± 1.13 |
| Lumen | ||||
| ml | 0.43 ± 0.07 | 0.21 ± 0.03*** | 0.38 ± 0.03a | 0.41 ± 0.07c |
| % | 20.49 ± 2.80 | 13.06 ± 1.22*** | 21.56 ± 0.50a | 22.73 ± 1.65c |
| Interstitium | ||||
| ml | 0.32 ± 0.07 | 0.37 ± 0.08 | 0.23 ± 0.03b | 0.26 ± 0.04a |
| % | 16.72 ± 1.98 | 22.67 ± 4.68 | 13.43 ± 2.31c | 14.75 ± 1.93b |
GC, control group; GCd, cadmium group; GCdJ, cadmium and grape juice concentrate group; GJ, grape juice concentrate group. (n = 6) A significant difference was found for the following: *P < 0.05 with GC, ***P ≤ 0.001 with GC. a P ≤ 0.05 with GCd, b P ≤ 0.01 with GCd, c P ≤ 0.001 with GCd.
With respect to interstitial components, cadmium decreased Leydig cell volume and percentage; increased blood vessel volume, percentage and number; reduced the lymphatic space percentage; and increased macrophage volume and percentage in GCd relative to GC. An improvement in Leydig cell percentage, blood vessel volume and macrophage volume was noted in GCdJ compared with GCd. Nevertheless, lower values were verified in GCdJ relative to GC with respect to Leydig cell volume and lymphatic space volume. These data are summarised in Table 3.
Table 3.
Stereological analysis of interstitium components (mean value ± standard deviation)
| Parameters | GC | GCd | GCdJ | GJ |
|---|---|---|---|---|
| Leydig cell | ||||
| ml | 0.09 ± 0.02 | 0.06 ± 0.02* | 0.06 ± 0.01* | 0.07 ± 0.01 |
| % | 27.74 ± 6.34 | 16.18 ± 3.62** | 25.14 ± 4.21a | 26.27 ± 4.22b |
| Blood vessels | ||||
| ml | 0.06 ± 0.03 | 0.14 ± 0.06** | 0.06 ± 0.01b | 0.06 ± 0.01b |
| % | 17.55 ± 8.03 | 37.55 ± 11.50** | 24.72 ± 5.76 | 22.64 ± 3.97a |
| Lymphatic space | ||||
| ml | 0.16 ± 0.03 | 0.11 ± 0.05 | 0.11 ± 0.02** | 0.13 ± 0.02 |
| % | 51.72 ± 6.21 | 31.92 ± 15.42** | 46.94 ± 7.82 | 48.84 ± 3.68a |
| Macrophages | ||||
| ml | 0.09 ± 0.03 | 0.19 ± 0.04* | 0.08 ± 0.03a | 0.06 ± 0.01c |
| % | 2.99 ± 0.73 | 5.19 ± 1.78* | 3.5 ± 1.12 | 2.24 ± 0.32c , + |
| N° blood vessels × 10−5 | ||||
| μm2 | 4.19 ± 0.09 | 5.7 ± 0.05** | 5.22 ± 1.27 | 4.94 ± 0.09 |
GC, control group; GCd, cadmium group; GCdJ, cadmium and grape juice concentrate group; GJ, grape juice concentrate group. (n = 6) A significant difference was found for the following: *P < 0.05 with GC, **P ≤ 0.01 with GC. a P ≤ 0.05 with GCd, b P ≤ 0.01 with GCd, c P ≤ 0.001 with GCd, + P ≤ 0.05 with GCdJ.
Analysis of Leydig cells in GCd showed lower values relative to GC, considering the nuclear diameter, volume, the cytoplasm volume and percentage, and total Leydig cell volume. The intoxicated animals that received GJC showed an improvement in nucleus and cytoplasm percentage, and total volume of a Leydig cell when compared to GCd. Nevertheless, GCdJ showed a decrease in nuclear diameter, nucleus and cytoplasm volume, and total volume of a Leydig cell when compared to GC. No differences were noted among the experimental groups with respect to the number of Leydig cells per testis or per gram of testis. These results are illustrated in Table 4.
Table 4.
Leydig cell stereology (mean value ± standard deviation)
| Parameters | GC | GCd | GCdJ | GJ |
|---|---|---|---|---|
| Nuclear diameter | ||||
| μm | 6.90 ± 0.51 | 6.09 ± 0.31** | 6.08 ± 0.20* | 7.02 ± 0.49b , ++ |
| % | 34.03 ± 1.35 | 43.15 ± 2.80** | 34.70 ± 4.19b | 34.47 ± 2.92b |
| Nucleus | ||||
| μm3 | 174.61 ± 35.45 | 119.11 ± 19.60** | 117.82 ± 12.08* | 183.73 ± 40.95b , ++ |
| % | 65.97 ± 1.35 | 56.85 ± 2.80** | 65.3 ± 4.19b | 65.53 ± 2.92b |
| Cytoplasm | ||||
| μm3 | 338.51 ± 70.03 | 158.06 ± 32.16*** | 226.75 ± 50.17** | 348.15 ± 62.50c , + |
| Total volume of a Leydig cell | ||||
| μm3 | 513.12 ± 104.37 | 277.17 ± 50.12*** | 344.58 ± 59.82* , c | 531.89 ± 99.03a |
| Number Leydig/testis | ||||
| ×107 | 17.30 ± 4.40 | 22.60 ± 9.90 | 17.32 ± 4.20 | 13.27 ± 2.53b |
| Number Leydig/g testis | ||||
| ×107 | 9.14 ± 2.37 | 13.29 ± 3.85 | 9.34 ± 2.21 | 7.50 ± 2.13b |
GC, control group; GCd, cadmium group; GCdJ, cadmium and grape juice concentrate group; GJ, grape juice concentrate group. (n = 6) A significant difference was found: *P < 0.05 with GC, **P ≤ 0.01 with GC, ***P ≤ 0.001 with GC. a P < 0.05 with GCd, b P ≤ 0.01 with GCd, c P ≤ 0.001 with GCd, + P < 0.05 with GCdJ, ++ P ≤ 0.01 with GCdJ.
Light and transmission electron microscopy (TEM) analyses
The GC and GJ groups showed normal tissue architecture. In the tubular compartment, Sertoli cells were distributed just inside the tunic, together with the spermatogonia. Normal development of spermatogenesis was observed. In the interstitium, normal blood vessels, lymphatic space, Leydig cells and macrophages were verified. In these groups, the Leydig cells were morphologically normal, organized in clusters of polymorphic cells. These clusters were, in most of the sections, associated with blood vessels and some macrophages. The overview of this architecture can be found in Figure 1(a,b). The ultrastructure of the GC and GJ groups can be seen in Figure 2.
Figure 1.
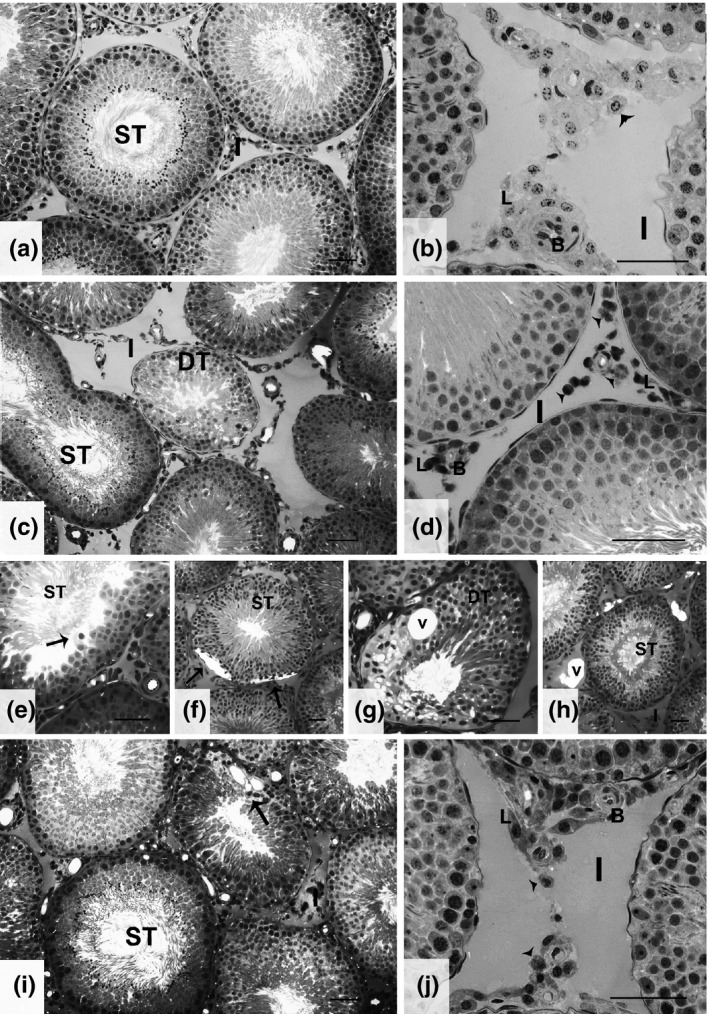
Testis tissue characteristics for the four experimental groups. (a,b) Representative areas for both GC and GJ groups showing normal seminiferous tubules (ST) and interstitial (I) regions with Leydig cell (L) clusters, blood vessels (B) and macrophages (arrowhead). (c,d) General characteristics of GCd showing some degenerated tubules (DT), disorganized Leydig cells (L) with compact nuclei and increased macrophages (arrowhead). (e–h) Other alterations found in GCd group, showing loss of germinal cells (E‐arrow), holes in epithelium (F‐arrow), tubular degeneration (DT) with vacuoles (G‐v) and interstitial vacuoles (H‐v). (i,j) GCdJ tissue structure, representing better preserved epithelium, with small degenerated areas (arrow). In this group, the interstitium presented a normal Leydig cell (L) distribution, as well as typical blood vessels (B) and macrophages (arrowhead). Bar = 50 μm.
Figure 2.
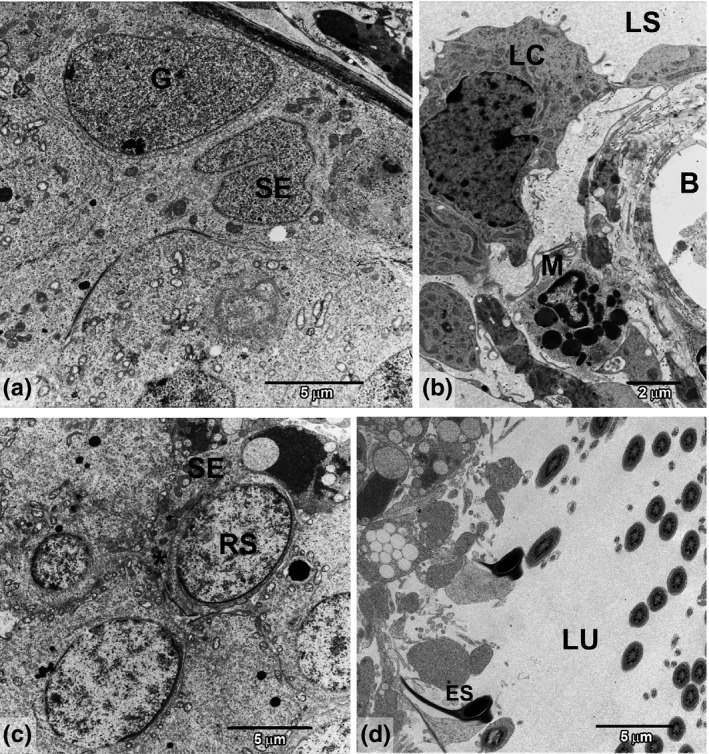
Representative areas of ultrastructure from GC and GJ groups. (a) Basal compartment showing spermatogonia (G) and Sertoli cells (SE). (b) Interstitial region showing lymphatic space (LS), Leydig cells (LC), blood vessel (B) and macrophage (M). (c) Representative of adluminal compartment showing juxtaposed round spermatids (RS) and Sertoli cell (SE) prolongation (*). (d) Lumen area (LU) showing elongated spermatids (ES) with regular shape and normal spermiation.
On the other hand, the GCd tissues showed regions with tubular degeneration and epithelial vacuolization. Testis ultrastructure showed many lipid droplets in the Sertoli cell cytoplasm. In the adluminal compartment, these droplets were denser and more numerous than in other groups. Large spaces between germ cells, apparently left by the degeneration of Sertoli cell prolongations, were observed. Moreover, it was possible to note a disorganization of the blood–testis barrier, and regions of degenerated cytoplasm and dilated tunic. After sperm release in the tubular lumen, a greater quantity of cytoplasm was seen to adhere to the flagella. This could be noted even for spermatozoa located more centrally in the lumen, at a certain distance from the epithelium, which is different from other groups. The mitochondria of the flagella were round, different from GC where they are elongated. During spermatid elongation, some vesicles appeared in the region that commonly is very compact and with little cytoplasm. The interstitium was considered disorganized with Leydig cells having compact nuclei and irregular cytoplasm. Ultrastructure of the interstitium showed Leydig cells with fewer lipid droplets, altered mitochondrial shape and some breaks in the blood vessels. These general alterations in GCd are illustrated in Figure 1(c–h) and its disorganized ultrastructure can be visualized in Figure 3.
Figure 3.
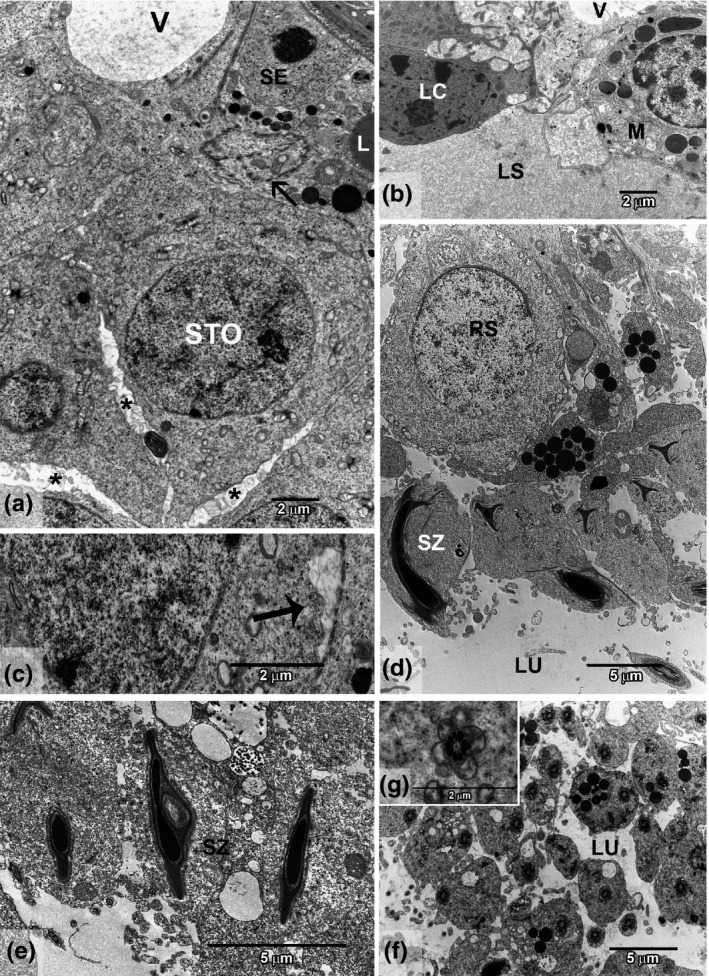
Ultrastructure of testis intoxicated with cadmium (GCd). (a) Basal compartment showing altered structure with an irregular blood–testis barrier (thin arrow), lipid droplets (L) in Sertoli cell (SE) and spermatocyte (STO), vacuolated region (V) and intercellular spaces (*). (b) Interstitial region showing vacuole (V) in lymphatic space (LS), Leydig cell (LC) and macrophage (M). (c) Cytoplasmic degeneration (thick arrow). (d) Region of spermiation showing an immature spermatid (RS) and compact vesicles. (e) Vacuole in sperm head (SZ) with retained cytoplasm. (f) Lumen area (LU) showing retained cytoplasm around flagella and abundant compact vesicles. (g) Detail of round mitochondria surrounding the axoneme.
The benefits of GJC were evident in these parameters. The daily administration of G8000® prior to and following a single injection of cadmium apparently reduced the amount and the size of vacuoles, and tubular degeneration was not observed in the light microscopy analyses. Moreover, in GCdJ there were fewer regions of disorganized interstitium and compact Leydig nuclei. Transmission electron microscopy analysis showed spaces between germ cells, some lipid droplets in Sertoli's cells cytoplasm and some regions with dilated tunic, but they were less evident than in GCd. Moreover, just a few flagella showed a large amount of cytoplasm and round mitochondria. In the interstitium, no alterations were noted in comparison with GC. Figure 1(i,j) shows an overview of the structure from GCdJ. In Figure 4, GCdJ ultrastructure is illustrated.
Figure 4.
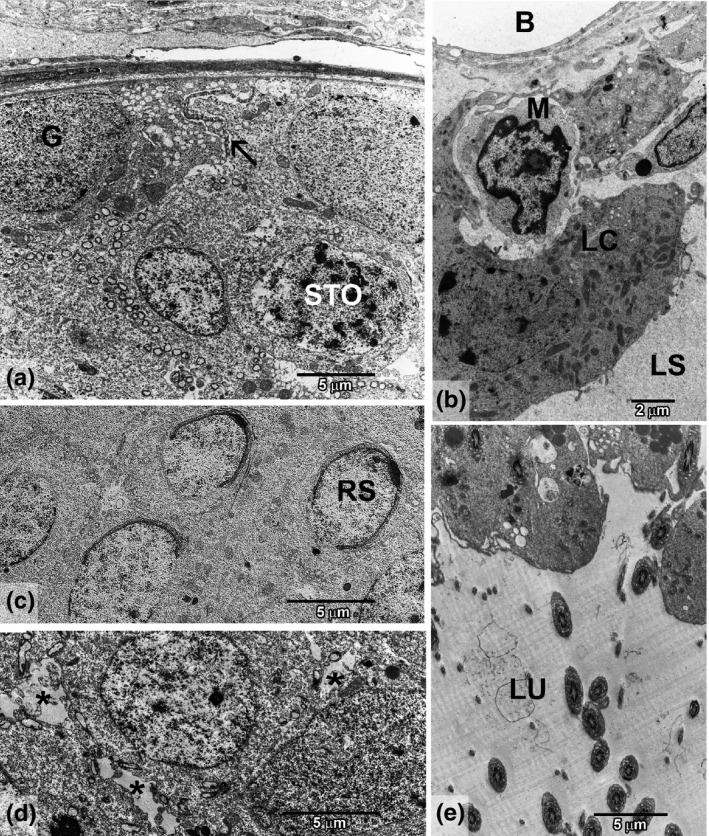
Testis ultrastructure of rats treated with cadmium and grape juice concentrate (GCdJ). (a) Basal compartment showing better preserved structure with spermatogonia (G), spermatocyte (STO), blood–testis barrier (arrow) and smaller spaces between cells. (b) Interstitial region showing a preserved lymphatic space (LS), Leydig cells (LC), blood vessel (B) and macrophage (M). (c) Adluminal region with normally structured round spermatids (RS), showing correct acrosomal development. (d) Intercellular spaces in the adluminal region (*). (e) Lumen region (LU) showing spermiation with the usual amount of cytoplasm around the axoneme.
Discussion
Cadmium toxicity to the reproductive system has already been described in the literature, but a large variety of results have been found (Gunnarsson et al. 2007; Predes et al. 2010; Wang et al. 2012). Our contributions relate to a widely studied research area, showing diminished testicular injury due to this metal.
Metallothioneins (MT) are related to cadmium toxicity, in which they can bind to the metal, protecting the tissue (Xu et al. 2005; Predes et al. 2010). It has been established that toxicity is the consequence of lower MT levels compared with the free cadmium found in the tissue. Although it has been stated that testes are the male reproductive organ with the highest levels of MT, it is also known as the most sensitive organ, because of its unique vasculature (Xu et al. 2005; Prozialeck et al. 2008; Siu et al. 2009). In this way, Predes et al. (2010) considered that the variability of cadmium damage is related to the differing levels of defence occurring in the animals. Moreover, Liu et al. (2001) considered that the level of cadmium‐developed alterations is related to their genetic background, and reported that some resistant strains do not produce visible testicular damage even at lethal doses.
According to what has been stated above, the testicular response to cadmium found in the present work was different from that described in the study of Pires et al. (2013) and Predes et al. (2010). In our research some regions with degenerated epithelium were observed using the light microscope, although the modifications were less severe than in the studies cited above, which demonstrated that the 1.2 mg/kg BW dosage was able to completely destroy the seminiferous tubules, with necrosis and the presence of multinucleated germ cells. On the other hand, we found many alterations in the ultrastructure, and in morphometric and stereological parameters, which show that even without a destruction clearly observed using the light microscope, this metal is very harmful to testis morphology, culminating in altered sperm formation. This fact highlights the importance and sensitivity of morphometric and stereological analyses, which are able to detect non‐evident alterations that could trigger severe consequences.
The reduced tubule and lumen as well as the increase in the interstitium are in agreement with lower epithelium height, reduced tubular diameter and tubular length found in the group treated only with the metal. Sinha‐Hikim et al. (1989) proposed a positive relationship between seminiferous tubule diameter and the spermatogenic process. This statement corroborates with our findings. Moreover, it is known that cadmium can alter signalling pathways, triggering epithelium disruption and an inflammatory process (Siu et al. 2009). These alterations together with cadmium‐induced DNA damages (Wang et al. 2012) may alter stability and expression of adhesion proteins such as occludin, destabilizing tight junctions between germ and Sertoli cells (Siu et al. 2009). All these direct effects inflicted by cadmium could be related to the morphometric and stereological alterations described here. These data corroborate the ultrastructural alterations where a disruption in the sperm development was noted. Spaces between cells, regions of cytoplasmic degeneration, vacuoles in sperm head, liberation of sperm still surrounded by a generous amount of cytoplasm and round mitochondria in the flagella are among the defects described.
Prozialeck et al. (2006) reported that doses between 1 and 2 mg/kg BW can cause testicular damage without pathological alterations. Instead, in our research, the vacuolated epithelium, increased proportion of macrophages and blood vessel levels are evidence of an incipient inflammatory process. Leite et al. (2013) also described an increase in macrophages in the testis of rats treated with 1.15 mg/kg BW of cadmium. The increase in blood vessel number, not just in volume or volumetric proportion, prompted us to consider this as the beginning of an angiogenic process. The endothelial cells are considered a target of cadmium toxicity (Siu et al. 2009). In this study, some breaks in the endothelium were observed in images of testis ultrastructure of GCd. The low dose of cadmium administered induced testicular injury in our research and contributed to an initial damage to the vascular system which promoted revascularization in order to try to maintain organ irrigation, but also collaborating with the metal distribution.
When an intense inflammatory process is described, it is followed by reduced GSI and absolute testis weight, but the fact that it can cause a reduction in BW gain is controversial (Predes et al. 2011; Leite et al. 2013; Pires et al. 2013). Pires et al. (2013) attributed the reduction in testis weight to organ atrophy, with the presence of necrosis, inflammatory infiltrates and vascular congestion. Moreover, they described reduced BW gain as a cause of lower organ relative weight. In our research, in spite of a reduction in BW gain by cadmium, there was no alteration in IGS. We attributed this to the fact that we noticed some signs of an initial inflammatory process with less intense tubular atrophy.
Leydig cells are considered a target for cadmium (Yang et al. 2003). As in the present research, Leite et al. (2013) also found a reduction in the volume of these cells in the testis of rats intoxicated with this metal. Thus, considering that we verified a reduction in Leydig cell volume, but not a decrease in cell number, we related cadmium aggressiveness to Leydig cell atrophy rather than cell death. Corroborating these data, Yang et al. (2003) considered cadmium very toxic to Leydig cells, causing a reduction in cell viability and DNA damage in cell culture. Leydig cells are responsible for testosterone, MT‐1 and MT‐2 production (Suzuki et al. 1998). According to McKenna et al. (1996), even low doses of cadmium can alter this synthesis. Moreover, Pires et al. (2013), using the same cadmium dose as the present study, observed a reduction in testosterone levels. Considering testosterone essential to spermatogenesis and MT levels crucial to combat cadmium toxicity, alterations in Leydig cell volume have been attributed to cadmium aggressiveness.
The stimuli generated by a healthy food pattern to avoid alterations of the organism have gained more attention in recent years (Anjo 2004; El‐Shahat et al. 2009; Lenquiste et al. 2012). Thus, natural products such as grapes and their bioactive compounds have been widely studied (Flechtner‐Mors et al. 2004; Jiang et al. 2008; Pires et al. 2013). The abilities of grape bioactive compounds to have anti‐inflammatory, antiviral and anti‐tumour properties are known in the scientific community (Frèmont 2000). The polyphenols' ability to act as metal chelators could contribute to the elimination of heavy metals (Fraga et al. 2010). This is an important mechanism that could have contributed to the reduced morphological and inflammatory damage observed when intoxicated animals ingested the GJC. This chelation triggers a reduction in genotoxicity and in the inflammatory process, which are stimulated by heavy metals, allowing the maintenance of regular testicular architecture and physiology.
Moreover, these molecules can interact with transcription factors or genes related to inflammation (Makenzie et al. 2009; Fraga et al. 2010). The reduced level of macrophages and blood vessels observed in GCdJ, as well as its improved morphology, corroborates this concept. Moreover, G8000® permitted the proportion of tubules and interstitium to remain similar to that of the control. Although tubular diameter and epithelium height did not alter with GJC ingestion, we noted greater tubular length. Thus, there is an increase in spermatogenic tissue, which is a positive aspect.
Another characteristic of polyphenols that contributes to GJC positive action, as described in the present research, is their ability to interact with membranes altering their structure as well as physical and electric properties (Fraga et al. 2010). This fact permits functional alterations such as signalling pathways related to membrane receptors and improvement of the chelating property (Fraga et al. 2010).
In our research, we could verify GJC positive effects on testis morphology against cadmium intoxication. Grape juice concentrate was able to improve Leydig cell percentage, as well as their individual volume. According to Juan et al. (2005), resveratrol, a bioactive compound from grapes, can stimulate FSH, LH and testosterone production. Moreover, according to these authors, this molecule can interact with oestrogen receptors inducing secretion of gonadotrophins and testosterone. An improvement in the testosterone level was demonstrated by Pires et al. (2013) using GJC against cadmium toxicity. Taking into account the relation between Leydig cell and testosterone, and the importance of this hormone to the spermatogenesis process, we consider that the improvement in Leydig cell parameters in GCdJ contributed to the better preservation of epithelial morphology observed.
All these data corroborate with our ultrastructural findings where we noted an improvement in the spermatogenic tissue in GCdJ relative to GCd. The areas with intracellular spaces, increased lipid droplets as well as tissue degeneration were much reduced. Moreover, we found no alterations in acrosome formation and sperm liberation.
Data about grapes or the bioactive compounds in them which might have effects on the reproductive system are very limited. Our results showed that GJC could reduce body mass gain. Flechtner‐Mors et al. (2004) demonstrated that grape juice consumption reduced the BW of obese patients. As a consequence of the high energy intake provided by the concentrate, which has a large amount of glycosides, these authors demonstrated reduced food ingestion. This tendency could also have contributed to the reduced BW gain in GCdJ in our study.
Considering the testicular parameters, Juan et al. (2005) demonstrated reduced tubular diameter and epithelium height with trans‐resveratrol supplementation. However, in this research an increase in tubular length and sperm production was also described (data not shown). The presence of narrow tubules in rats treated with resveratrol was verified by Jiang et al. (2008). In our findings, lower tubular diameter and epithelium height in GJ were found in spite of the lack of alterations in tubular length. Nevertheless, in a previous study by our group (data not shown), we observed normal sperm production and morphology in the group treated with G8000® for the same time period. Thus, our research is in agreement with data showing that GJC consumption had no toxic effect (Pires et al. 2013).
In conclusion, we demonstrated that GJC consumption protects the testicular morphology of cadmium‐intoxicated rats. The concentrate consumption in GCdJ allows better preserved testicular architecture with fewer areas of epithelium degeneration and disorganized interstitium. Thus, we considered that GJC ingestion prevented spermatogenic disruption observed in GCd, therefore offering a potential method of mitigation of cadmium‐induced male infertility.
Acknowledgements
We are grateful to Henrique Ceretta Oliveira for the statistical support. The authors declare no conflict of interest. This paper was supported by a grant to the author Lamas, C.A. from the Brazilian agency Capes (Coordination for the Improvement of Higher Education Personnel).
References
- Abe L.T., Mota R.V., Lajolo F.M. & Genovese M.I. (2007) Compostos fenólicos e capacidade antioxidante de cultivares de uvas Vitis labrusca L. e Vitis vinifera L. Ciênc. Tecnol. Aliment. 27, 394–400. [Google Scholar]
- Aguiar O., Gollücke A.P., De Moraes B.B. et al (2011) Grape juice concentrate prevents oxidative DNA damage in peripheral blood cells of rats subjected to a high‐cholesterol diet. Br. J. Nutr. 105, 694–702. [DOI] [PubMed] [Google Scholar]
- American Dietetic Association (2004) Position of the American Dietetic Association: functional foods. J. Am. Diet. Assoc. 104, 814–826. [DOI] [PubMed] [Google Scholar]
- Anjo D.F.C. (2004) Alimentos funcionais em angiologia e cirurgia vascular. J. Vasc. Bras. 3, 145–154. [Google Scholar]
- Blanco A., Moyano R., Vivo J. et al (2007) Quantitative changes in the testicular structure in mice exposed to low doses of cadmium. Environ. Toxicol. Pharmacol. 23, 96–101. [DOI] [PubMed] [Google Scholar]
- El‐Shahat A.E., Gabr A., Meki A.R. & Mehana E.S. (2009) Altered testicular morphology and oxidative stress induced by cadmium in experimental rats and protective effect of simultaneous green tea extract. Int. J. Morphol. 27, 757–764. [Google Scholar]
- Flechtner‐Mors M., Biesalki H.K., Jenkinson C.P., Adler G. & Ditschuneit H.H. (2004) Effect of moderate consumption of white wine on weight loss in overweight and obese subjects. Int. J. Obes. 28, 1420–1426. [DOI] [PubMed] [Google Scholar]
- Fraga C.G., Galleano M., Verstraeten S.V. & Oteiza P.I. (2010) Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects Med. 31, 435–445. [DOI] [PubMed] [Google Scholar]
- França L.R., Russel L.D. (1998) The testes of domestic animals In: Male Reproduction. A Multidisciplinary Overview, pp. 197–219 (eds Regadera J.& Martinez‐Garcia R.), London: Churchill Livingstone. [Google Scholar]
- Frèmont L. (2000) Biological effects of resveratrol. Life Sci. 66, 663–673. [DOI] [PubMed] [Google Scholar]
- Gomes M.L.M., Monteiro J.C., Freitas K.M., Sbervelheri M.M. & Dolder H. (2011) Association of the infusion of Heteropterys aphrodisiaca and endurance training brings spermatogenetic advantages. Biol. Res. 44, 235–241. [PubMed] [Google Scholar]
- Gunnarsson D., Nordberg G. & Selstam G. (2007) Differential effects of cadmium on the gene expression of seven‐transmembrane‐spanning receptors and GAPDH in the rat testis. Toxicol. Lett. 168, 51–57. [DOI] [PubMed] [Google Scholar]
- Ho C.T., Rafi M.M., Ghai G. (2010) Substâncias bioativas: nutracêuticas e tóxicas In: Química de alimentos de Fennema, pp. 585–608 (eds Damodaran S., Parkin K.L., Fennema O.R.), Porto Alegre: Artmed. [Google Scholar]
- Jiang Y., Peng T., Luo Y., Ming‐chuan L. & Tum‐hua L. (2008) Resveratrol reestablishes spermatogenesis after testicular injury in rats caused by 2,5‐hexanedione. Chin. Med. J. 13, 1204–1209. [PubMed] [Google Scholar]
- Juan M.E., González‐Pons E., Munuera T., Ballester J., Rodríguez‐Gil J.E. & Planas J.M. (2005) Trans‐resveratrol, a natural antioxidant from grapes, increases sperm output in healthy rats. J. Nutr. 135, 757–760. [DOI] [PubMed] [Google Scholar]
- Kusakabe T., Nakajima K., Nakasato K. et al (2008) Changes of heavy metal, metallothionein and heat shock proteins in Sertoli cells induced by cadmium exposure. Toxicol. In Vitro 22, 1469–1475. [DOI] [PubMed] [Google Scholar]
- Leite R.P., Predes F.S., Monteiro J.C., Freitas K.M., Wada R.S. & Dolder H. (2013) Advantage of Guaraná (Paullinia cupana Mart.) supplementation on cadmium‐induced damages in testis of adult Wistar rats. Toxicol. Pathol. 41, 73–79. [DOI] [PubMed] [Google Scholar]
- Lenquiste S.A., Batista A.G., Marineli R.S., Dragano N.R.V. & Maróstica M.R. (2012) Freeze‐dried jaboticaba peel added to high‐fat diet increase HDL‐cholesterol and improves insulin resistance in obese rats. Food Res. Int. 49, 153–160. [Google Scholar]
- Liu J., Liu Y., Michalska A.E., Andy Choo K.H., Klaassen C.D. (1996) Distribution and retention of cadmium in metallothionein I and II null mice. Toxicol. Appl. Pharmacol. 136, 260–268. [DOI] [PubMed] [Google Scholar]
- Liu J., Corton C., Dix D.J., Liu Y., Waalkes M.P. & Klaassen C.D. (2001) Genetic background but not metallothionein phenotype dictates sensitivity to cadmium‐induced testicular injury in mice. Toxicol Appl. Pharmacol. 176, 1–9. [DOI] [PubMed] [Google Scholar]
- Makenzie G.G., Delfino J.M., Keen C.L., Fraga C.G. & Oteiza P.I. (2009) Dimeric procyanidins are inhibitors of NF‐қβ‐DNA binding. Biochem. Pharmacol. 78, 1252–1262. [DOI] [PubMed] [Google Scholar]
- McKenna I.M., Bare R.M. & Waalkes M.P. (1996) Metallothionein gene expression in testicular interstitial cells and liver of rats treated with cadmium. Toxicology 107, 121–130. [DOI] [PubMed] [Google Scholar]
- Messaoudi I., Hammouda F., El Heni J., Baati T. & Sai D.K. (2010) Reversal of cadmium‐induced oxidative stress in rat erythrocytes by selenium, zinc or their combination. Exp. Toxicol. Pathol. 62, 281–288. [DOI] [PubMed] [Google Scholar]
- Mustali M. & Dohadwala Joseph A. (2009) Vita grapes and cardiovascular disease. J. Nutr. 139, 1788–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira H., Spanò M., Santos C. & Pereira M.L. (2009) Adverse effects of cadmium exposure on mouse sperm. Reprod. Toxicol. 28, 550–555. [DOI] [PubMed] [Google Scholar]
- Pires V.C., Gollücke A.P., Ribeiro D.A., Lungato L., D'Almeida V. & Aguiar O. (2013) Grape juice concentrate protects reproductive parameters of male rats against cadmium‐induced damage: a chronic assay. Br. J. Nutr. 9, 1–10. [DOI] [PubMed] [Google Scholar]
- Predes F.S., Diamante M.A.S. & Dolder H. (2010) Testis response to low doses of cadmium in Wistar rats. Int. J. Exp. Pathol. 91, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predes F.S., Monteiro J.C., Matta S.L.P., Garcia M.C. & Dolder H. (2011) Testicular histomorphometry and ultrastructure of rats treated with cadmium and Ginkgo biloba . Biol. Trace Elem. Res. 140, 330–341. [DOI] [PubMed] [Google Scholar]
- Prozialeck W., Edwards J. & Woods J. (2006) The vascular endothelium as a target of cadmium toxicity. Life Sci. 79, 1493–1506. [DOI] [PubMed] [Google Scholar]
- Prozialeck W., Edwards J., Nebert D., Woods J., Barchowsky A. & Atchison W. (2008) The vascular system as a target of metal toxicity. Toxicol. Sci. 102, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L.D., Ettlin R.A., Hikim A.P.S., Clegg E.D. (1990) Histological and Histopathological Evaluation of the Testis. St. Louis: Cache River Press. [Google Scholar]
- Scollon E.J., Starr J.M., Godin S.J., DeVito M.J. & Hughes M.F. (2009) In vitro metabolism of pyrethroid pesticides by rat and human hepatic microsomes and cytochrome P450 isoforms. Drug Metab. Dispos. 37, 221–228. [DOI] [PubMed] [Google Scholar]
- Sinha‐Hikim A.P., Amador A.G., Klemcke H.G., Bartke A. & Russell L.D. (1989) Correlative morphology and endocrinology of Sertoli cells in hamster testes sin active and inactive states of spermatogenesis. Endocrinology 125, 1829–1843. [DOI] [PubMed] [Google Scholar]
- Siu E.R., Mruk D.D., Porto C.S. & Cheng C.Y. (2009) Cadmium‐induced testicular injury. Toxicol. Appl. Pharmacol. 238, 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J.S., Kodama N., Molotkov A., Aoki E. & Thyama C. (1998) Isolation and identification of metallothionein isoforms (MT‐1 and MT‐2) in rat testis. Biochem. J. 334, 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xu T., Lei W.‐W., Liu D.‐M. & Li Y.‐J. (2011) Cadmium‐induced oxidative stress and apoptotic changes in the testis of freshwater crab, Sinopotamon henanense. PLoS ONE 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Sun Y., Liu J. et al (2012) Protective effect of theaflavins on cadmium‐induced testicular toxicity in male rats. Food Chem. Toxicol. 50, 3243–3250. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1992) Cadmium. Geneva: Environmental Health Criteria, No. 134. [Google Scholar]
- Xu L.C., Sun H., Wang S.Y., Song L., Chang H.C. & Wang X.R. (2005) The roles of metallothionein on cadmium‐induced testes damages in Sprague–Dawley rats. Environ. Toxicol. Pharmacol. 20, 83–87. [DOI] [PubMed] [Google Scholar]
- Yang J., Arnush M., Chen Q., Wu X., Pang B. & Jiang X. (2003) Cadmium‐induced damage to primary cultures of rat Leydig cells. Reprod. Toxicol. 17, 553–560. [DOI] [PubMed] [Google Scholar]
- Zanato V.F., Martins M.P., Anselmo‐Franci J.A., Petenusci S.O. & Lamano‐Carvalho T.L. (1994) Sexual development of male Wistar rats. Braz. J. Med. Biol. Res. 27, 1273–1280. [PubMed] [Google Scholar]
- Zorzano A. & Herrera H. (1990) In vivo ethanol elimination in man, monkey and rat: a lack of relationship between the ethanol metabolism and the hepatic activities of alcohol and aldehyde dehydrogenases. Life Sci. 46, 223–230. [DOI] [PubMed] [Google Scholar]


