Summary
This study tested the hypothesis that different doses of nandrolone decanoate (ND) will cause changes in the estrous cycle and ovarian tissue of adult rats; and investigated the duration of the recovery period that is sufficient to restore the damage in the animals treated with different doses. Wistar rats were treated with ND at doses of 1.87, 3.75, 7.5 and 15 mg/kg body weight, or received mineral oil (control group) for 15 days, subcutaneously. All animals were divided into three groups according to the treatment periods: (i) ND treatment for 15 days; (ii) ND treatment followed by a 30‐day recovery; and (iii) ND treatment followed by a 60‐day recovery. Estrous cycle was monitored daily, and at the end of each period, the animals were euthanized for histopathological analysis. During ND treatment and after 30‐day recovery, all animals exhibited persistent diestrus. After a 60‐day recovery, persistent diestrus was only maintained in the group that had received the highest dose. Ovarian weight was decreased significantly after the 30‐day recovery, regardless of ND doses, compared with the control group. There was a reduction (P < 0.05) in the number of corpora lutea and antral and growing follicles, in contrast to an increase (P < 0.05) in atretic follicles in a dose‐ and time‐dependent manner. Remarkable histopathological changes occurred in the ovaries of all ND‐treated groups. In conclusion, the different doses of ND caused changes in the estrous cycle and ovarian tissue of rats, and recovery periods (30 and 60 days) were insufficient to completely restore the damage in the animals treated with the highest dose.
Keywords: estrous cycle, nandrolone decanoate, ovarian histopathology, rats, recovery period
Anabolic androgenic steroids (AAS) are substances synthesized from testosterone or one of their derivatives, with anabolic or androgenic effects, depending on the target tissue (Lise et al. 1999; Bahrke & Yesalis 2004). The prescription and commercialization of these drugs are strictly controlled in many countries. However, AAS have been used indiscriminately by athletes and non‐athletes to increase muscle mass and resistance in a short time (Iriart et al. 2009).
Anabolic androgenic steroids are clinically indicated for the treatment of chronic diseases associated with the catabolic state of the patient, in conditions of AIDS, chronic obstructive pulmonary disease, hepatic or renal failure, cancer, and in cases of burns and postsurgical recovery (Karbalay‐Doust & Noorafshan 2006; Kicman 2008). They are also recommended for androgen replacement therapy after menopause (Arlt 2006), and during age‐related sarcopaenia (Evans 2004).
Among the AAS derivatives, nandrolone decanoate (ND) is the most commonly used injectable steroid (Boff 2010). Usually, AAS are administered at supraphysiological doses (Fermo et al. 2008), which are fivefold to 29‐fold higher than the dose recommended for the hormonal replacement (Perry et al. 2005) or reach up to 100 times the therapeutic dose recommended for the treatment of various diseases (Clark & Fast 1996).
Over the past decade, the abusive use of AAS by women has increased significantly (Bahrke & Yesalis 2004; Thiblin & Petersson 2004). In women, the side effects may include deepening of the voice, growth of body hair, atrophy of the breasts, aggressiveness, menstrual irregularity and clitoris hypertrophy (Maravelias et al. 2005; Hoffman & Ratamess 2006; Kicman 2008), with these alterations being dose‐ and time dependent (Silva et al. 2002; Cunha et al. 2004; Kam & Yarrow 2005; Bonetti et al. 2008;). Moreover, changes in gonadal function such as delayed puberty, luteal‐phase deficiency, oligo‐amenorrhea or anovulation may occur in addicted women (Cannavò et al. 2001). Unfortunately, some of these changes are irreversible after cessation of AAS administration (Kam & Yarrow 2005).
Although deleterious effects promoted by AAS are well understood, few studies have focused attention to their effects on female reproductive morphological parameters. The estrous cycle is a periodic physiologic process, with an average duration of 4.5 days, ranging from 3.5 to 5.5 days in Wistar rats (Paccola et al. 2013), and can be influenced by endogenous or exogenous factors that commonly express a change in normal morphology of the reproductive tract or a disturbance in the duration of particular phases of the cycle (Westwood 2008). We have recently reported that ND causes changes in the estrous cycle (Gerez et al. 2005; Camargo et al. 2009a; Chuffa et al. 2011b), histopathological alterations in the ovaries and uterus (Gerez et al. 2005; Camargo et al. 2009a, 2014; Chuffa et al. 2011b) and damage to the fertility (Camargo et al. 2009b; Belardin et al. 2014). However, no study has evaluated the dose‐dependent effect of the ND on ovarian tissue and whether this effect can be reversed after discontinuing steroid treatment.
Therefore, we aimed to investigate the effects of different doses of ND on estrous cycle and ovarian tissue of rats, in the post‐treatment (15 days) and postrecovery periods (30 and 60 days). This study brings novel information regarding the reversibility of some effects promoted by ND in the reproductive cycle and ovaries.
Materials and methods
Animals
Ninety adult female Wistar rats (Rattus norvegicus albinus), 12 weeks old, weighing approximately 250 g, were obtained from the Univ. Estadual Paulista (UNESP‐ Botucatu, Brazil) and kept in appropriate cages at the Central Biotherium of the Faculty of Sciences and Letters (UNESP‐ Assis, Brazil). The females were housed in polypropylene cages (43 cm × 30 cm × 15 cm) with laboratory‐grade pine shavings as bedding and also maintained under controlled room temperature (23 ± 1°C) and lighting conditions (12‐h L, 12‐h D photoperiod, lights switched on at 7 a.m.). Water and commercial diet (Nuvital™) were offered ad libitum. The experimental protocol followed the ethical principles in animal research adopted by the Brazilian College of Animal Experimentation and was approved by the Ethical Committee for Animals Use (Permit number: 005/2011, August 31).
Drug
Nandrolone decanoate (17β‐hydroxy‐19‐nor‐4‐androstene‐3‐one) was purchased from Schering‐Plough Laboratory (São Paulo, Brazil), under the name of Deca Durabolin™. It is an injectable solution, containing 50 mg of the androgen, available as oily solution (Marqueti et al. 2010).
Experimental design
Females with regular estrous cycles were weighed and randomly divided into six groups (n = 15/group): ND‐treated groups at doses of 1.87 mg/kg body weight (b.w.), 3.75 mg/kg b.w., 7.5 mg/kg b.w. and 15 mg/kg b.w.; CE group: control group euthanized in estrus phase; and CD group: control group euthanized in diestrus phase. Both CE and CD groups received subcutaneous (s.c.) injections of 0.1 ml of mineral oil (NP‐35, Anidrol, São Paulo, Brazil) as vehicle. The ND‐treated animals received a daily s.c. injection of ND diluted in vehicle for 15 consecutive days. The first injection was administered at the estrus phase of the cycle in all animals. The CD group was used in this study based on previous reports that AAS treatment promotes persistent diestrus (Camargo et al. 2009a,b; Bento‐Silva et al. 2010; Chuffa et al. 2011b). In this regard, the evaluation of ovarian tissue was carried out in a specific stage of the estrous cycle that androgenized females persisted or maintained for a long time during the experimental period. Then, all data obtained from animals displaying estrus‐phase prolongation were compared with CE group, and data obtained from animals displaying persistent diestrus were compared with CD group.
Doses of 7.5 and 15 mg ND/kg were selected based on previous studies in animals (Blasberg et al. 1997; Mobini Far et al. 2007; Camargo et al. 2011) and mimic the conditions generally employed by non‐therapeutic users of AAS (ranging from 200 to 3200 mg/week; Evans 1997). Doses of 1.87 and 3.75 mg ND/kg were used to evaluate the effects of doses employed in various treatments (ranging from 50 to 200 mg/week; Evans 1997) or lowest doses than physiologic replacement doses (ranging from 25 to 600 mg/week; Bhasin et al. 2005).
Following the treatment period, each experimental group was divided according to the three periods of the study (n = 5 rats/group/period, Figure 1a–c): ND‐treated animals receiving daily s.c. injections for 15 consecutive days (a), and treatment with ND for 15 days followed by recovery for 30 days (b), and for 60 days (c). At the end of each period, the females who had regular estrous cyclicity were euthanized in estrus phase, while the acyclic females were euthanized at the cycle phase wherein persisted. For both cases, the ovaries were collected and processed for histopathological analysis.
Figure 1.
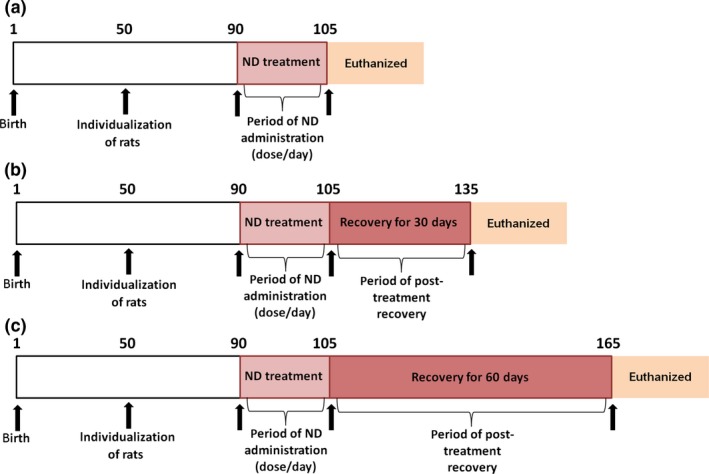
Chronological schedule for the experimental design in days. (a) At 90 days of age, five (5) rats per dose level (1.87, 3.75, 7.5, and 15 mg/kg) were assigned to the end of the dosing period (15 days) and euthanized. (b) Five (5) rats per dose level (1.87, 3.75, 7.5 and 15 mg/kg) received the respective treatment for 15 days and were maintained without further treatment into the 30‐day recovery period followed by euthanasia. (c) Five (5) rats per dose level (1.87, 3.75, 7.5, and 15 mg/kg) received the respective treatment for 15 days and were maintained without further treatment into the 60‐day recovery period followed by euthanasia.
Assessment of estrous cycle
During ND‐treatment and recovery periods, the estrous cycle was monitored daily by cytological examination (vaginal swabs, Marcondes et al. 2002). The time of collection was fixed at 9 a.m. (Chuffa et al. 2011a,b, 2013). Each slide containing cells from the vaginal epithelium was analysed under a light microscope (Olympus CX31 RBSFA, Tokyo, Japan) at 10× and 25× magnifications. The phases of the estrous cycle were identified as follows: (i) proestrus, consisting of clusters of round, nucleated epithelial cells; (ii) estrus, with predominance of cornified cells; (iii) metaestrus, consisting of a combination of leucocytes and cornified epithelial cells; (iv) diestrus, with predominance of leucocytes (Goldman et al. 2007).
Histopathological analysis
After each period (ND‐treatment, 30‐day recovery and 60‐day recovery), the females displaying regular or irregular estrous cycles were weighed and euthanized with an overdose of the anaesthetic sodium thiopental (Thiopentax™, Cristalia, São Paulo, Brazil), via intraperitoneal injection. The ovaries were collected and weighed, and the relative weights were obtained (organ weight/final body weight × 100, expressed in g/100 g b.w.).
The tissues were fixed in Bouin's solution, dehydrated in ethanol solutions and clarified in xylene for paraffin embedding (Paraplast Labware‐Oxford, St. Louis, MO, USA). The blocks were sliced into 5‐μm‐thick sections in a RM2125 LEICA microtome (Germany) and then stained with haematoxylin and eosin (HE), Mallory's Trichromic, Van Gieson and Orcein, for distinguishing cellular from extracellular matrix components in ovarian tissue during histopathological examination. All of the histological samples were handled in a blinded fashion. Finally, the slides were analysed and images captured with a digital photomicroscope (Scope A1‐Axio coupled with video camera AxioCam ICc3 and digitalized by the software axio vision, version 4.7.2; Carl Zeiss, Jena, Germany).
Follicular score and morphometric analysis
The identification of follicles subtypes was based on the classification proposed by Pedersen and Peters (1968), as presented by Plowchalck et al. (1993). In each ovary (10 sections/rat/group), the growing, antral and atretic follicles, and corpora lutea were counted. The criterion for the identification of atretic follicles was based on the presence of three characteristics: pyknotic nuclei in the antrum, involution of oocyte and thin granulosa layer. Measurements of the area of antral and atretic follicles, and corpora lutea were obtained using a Zeiss Scope A1‐Axio microscope (Carl Zeiss, Jena, Germany) connected to an AxioCam ICc3 camera, and the digitalized images were obtained by the image analyser axio vision version 4.7.2.
Immunohistochemistry
Ovarian sections (n = 5/group/period) were deparaffinized in xylene based on the areas previously identified during the morphological characterization. Tissue sections were microwaved (700–800 W) while immersed in 0.01 M sodium citrate buffer, pH 6.0, for antigen retrieval. After blocking endogenous peroxidase activity, the tissue was incubated with 3% bovine serum albumin (BSA, 3%) for 1 h to avoid non‐specific binding. Subsequently, the ovarian sections were incubated in a humid chamber overnight at 4°C with anti‐AR and anti‐inhibin A primary rabbit polyclonal antibody (dilution 1:100, ab7970; Abcam, Cambridge, MA, USA). After immunoreactions, the slides were washed in TBS‐T buffer and incubated with secondary antibody (Polymer Anti‐Mouse IgG or Anti‐Rabbit – DAKO® CYT) at room temperature for 1 h. Then, the slides were reacted with chromogen diaminobenzidine (DAB; Sigma, St. Louis, MO, USA) for 5 min. Then, sections were counterstained with haematoxylin. Negative controls were made by omitting the primary antibody. The results were analysed under a Zeiss Axiophot II microscope (Carl Zeiss, Oberkochen, Germany) based on the levels of staining intensity as absent, weak, moderate and strong reaction.
Immunofluorescence assays
Ovarian tissues were washed with phosphate‐buffered saline (PBS; sodium chloride, potassium chloride, dihydrogen phosphate and disodium hydrogen phosphate), fixed in 4% paraformaldehyde for 10 min, and permeabilized with PBS at room temperature (RT). Non‐specific binding sites were blocked with 1% BSA for 60 min. The samples were incubated with the anti‐inhibin A primary rabbit polyclonal antibody (dilution 1:100, ab7970; Abcam) overnight at 4°C, followed by a secondary polyclonal anti‐rabbit IgG conjugated to FITC (1:200, sc‐2012; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h at RT. Nuclei were stained with 6‐diamidino‐2‐phenylindole (DAPI, 5 min) at RT. Primary and secondary antibodies were diluted in blocking buffer (1% BSA). For negative immunolabelling, no primary antibody was added. The immunopositive cells were analysed using a fluorescence microscope (Zeiss Axiophot II, Oberkochen, Germany) at 40× magnification (immunofluorescence: excitation 590 nm, emission filter 650 nm) and for DAPI staining (excitation 365 nm, emission filter 435–485 nm). The fluorescence in the merged images was performed using image j software (National Institute of Health, Bethesda, Maryland, USA).
Statistical analysis
The data were analysed by parametric analysis of variance (anova) followed by Tukey's test, or by nonparametric Kruskal–Wallis test followed by Student–Newman–Keuls test, according to the characteristics of each variable. The results were expressed as the mean ± standard deviation (SD) or median ± interquartile deviation. Statistical significance was set at P < 0.05. Statistical analysis was performed using graphpad prism software, version 5.00 (GraphPad Software Inc., San Diego, California, USA).
Results
Estrous cycle
Nandrolone decanoate‐treated females at different dose levels presented an increase (P < 0.05) in the number of days in diestrus during the treatment period (Figure 2a), compared with control group. All ND‐treated groups remained at diestrus during the 30‐day recovery period (Figure 2b). Females treated with lower doses (1.87 and 3.75 mg ND/kg) exhibited diestrus for 52 and 50 days, respectively, during the 60‐day recovery period (Figure 2c). At the end of this period, the rats restored the cycle, but irregularly. The group treated with 7.5 mg ND/kg remained in diestrus until the 58th day and thereafter restored estrus phase. Due to estrous cycle restoration, these groups (1.87, 3.75 and 7.5 mg ND/kg) were euthanized in the estrus phase at the end of 60‐day recovery period. Persistent diestrus was only maintained through the 60‐day recovery period at the 15 mg ND/kg dose level (Figure 2c).
Figure 2.
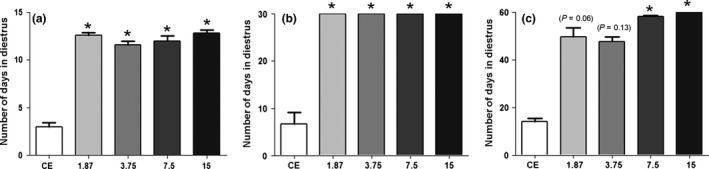
Number of days in diestrus in the nandrolone decanoate (ND)‐treated groups (1.87, 3.75, 7.5 and 15 mg/kg) after the treatment period (a), 30‐day recovery period (b) and 60‐day recovery period (c). Control group with regular cycles was euthanized in the estrus phase (CE). Histograms with an asterisk show significant differences (P < 0.05) among the ND‐treated group and CE group. Nonparametric Kruskal–Wallis test followed by Student–Newman–Keuls test. Data are expressed as the median and interquartile deviation.
Ovarian histopathology
A summary of the most common ovarian histopathological features in ND‐treated groups is shown in Table 1. During the treatment period, the ovarian histopathology showed that ND‐treated groups (Figure 3d,g,j,m) exhibited several atretic follicles, evidenced by a large antrum, a thin granulosa layer and cellular debris within the antrum. This histopathological change still remained after recovery for 60 days in the highest dosages (Table 1). The control group (Figure 3a) presented follicles in various stages of development and mature corpora lutea. Notably, the group treated with 1.87 mg/kg had ovaries with anomalous follicles characterized by seminiferous tubule‐like structures, devoid of oocyte, containing Sertoli cells (Figure 5a). These cells presented polymorphonuclear aspect with prominent nucleolus, and abundant and pallid cytoplasm. Moreover, this group exhibited fibrous cortex during the post‐treatment recovery for 30 days (Table 1). In the group treated with 7.5 mg/kg, a marked vasodilation and vascular congestion were common in the medulla, along with abundant interstitial tissue in the cortex (Figure 3j). The only exception was the post‐treatment recovery for 60 days (Table 1).
Table 1.
Summary of the relevant ovarian histopathological features in the nandrolone decanoate (ND)‐treated groups in each period (treatment period and post‐treatment recovery for 30 and 60 days)
| Histopathological features | Experimental groups (n = 5/group/period) | |||
|---|---|---|---|---|
| Treatment period | ||||
| 1.87 mg/kg | 3.75 mg/kg | 7.5 mg/kg | 15 mg/kg | |
| Atretic follicles | X | X | X | X |
| Vasodilation | — | — | X | — |
| Abundant interstitial tissue | — | — | X | — |
| Ovarian atrophy | — | — | — | — |
| Fibrous stroma | — | — | — | — |
| Anomalous follicles | X | — | — | — |
| Epithelial cysts | — | — | — | — |
| Post‐treatment recovery for 30 days | ||||
| Atretic follicles | X | X | X | X |
| Vasodilation | — | — | X | — |
| Abundant interstitial tissue | X | X | X | X |
| Ovarian atrophy | X | X | X | X |
| Fibrous stroma | X | X | X | X |
| Anomalous follicles | X | X | X | X |
| Epithelial cysts | X | X | X | X |
| Post‐treatment recovery for 60 days | ||||
| Atretic follicles | — | — | X | X |
| Vasodilation | — | — | — | — |
| Abundant interstitial tissue | X | X | X | X |
| Ovarian atrophy | — | — | — | X |
| Fibrous stroma | — | — | — | X |
| Anomalous follicles | X | — | X | X |
| Epithelial cysts | — | — | X | X |
(X) Presence; (—) Absence.
Figure 3.
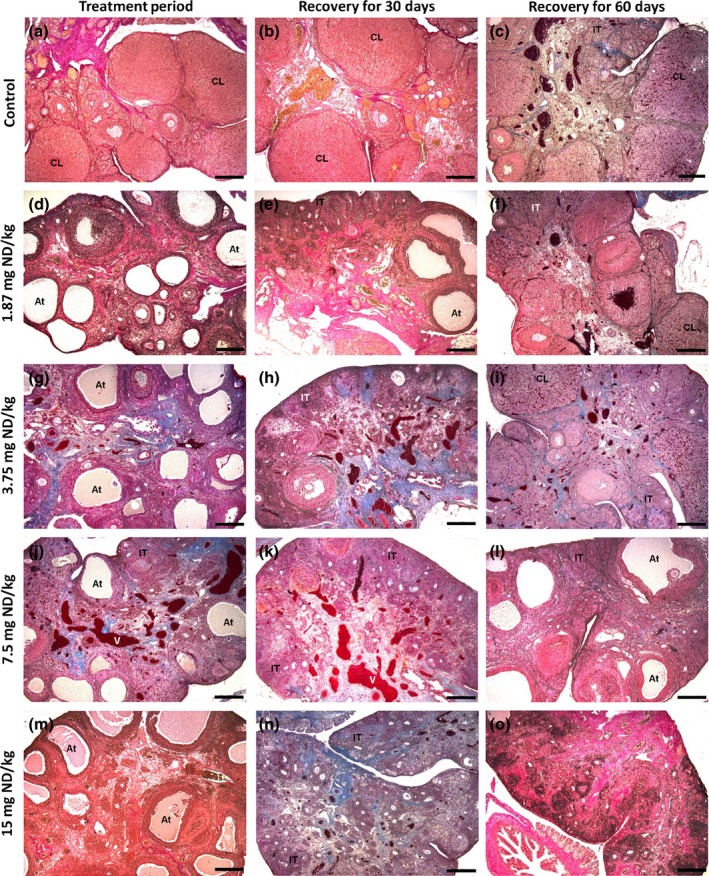
Photomicrographs of the ovaries in the different groups after the nandrolone decanoate (ND)‐treatment period and recovery for 30 and 60 days. Figures (a and b) represent the control group euthanized in the diestrus phase (CD); Figure (c) represents the control group euthanized in the estrus phase (CE); and d–o represent ND treatment at 1.87 mg/kg (d, e, f), 3.75 mg/kg (g, h, i), 7.5 mg/kg (j, k, l) and 15 mg/kg (m, n, o). Note the follicular atresia (At) in all ND‐treated groups. In the 7.5 mg/kg group (j, k), note the medullary vasodilatation (v). After 30 days of recovery, the ovaries exhibited atrophy and abundant interstitial tissue (IT). After 60 days of recovery, the groups treated with 1.87 and 3.75 mg/kg (f, i, respectively) contained corpora lutea (CL). The group treated with 15 mg/kg (o) showed ovarian atrophy and loss of follicular units. Stained with Mallory's trichrome (c, f, g, h, i, j, k, l, n) and Van Gieson (a, b, d, e, m). Bar = 200 μm.
Interestingly, deleterious effects promoted by ND in ovaries were intensified after 30 days of recovery (Table 1, Figure 3). There was absence of developing follicles and corpora lutea, ovarian atrophy and abundant interstitial tissue in both cortex and medulla (Figure 3e,h,k,n). Epithelial cysts, characterized by an irregular shape with columnar to cuboidal epithelium (Figure 4a), were present in the fibrous stroma of all animals. Atypical areas of fibrous or elastic tissue (Figure 4b,d) showed degenerative aspects in the surrounding stroma, with vacuolated (Figure 4b) and undifferentiated cells (Figure 4c). Furthermore, there was an increase in vascularization of these areas (Figure 4c). Seminiferous tubule‐like structures were observed in all ND‐treated groups.
Figure 4.
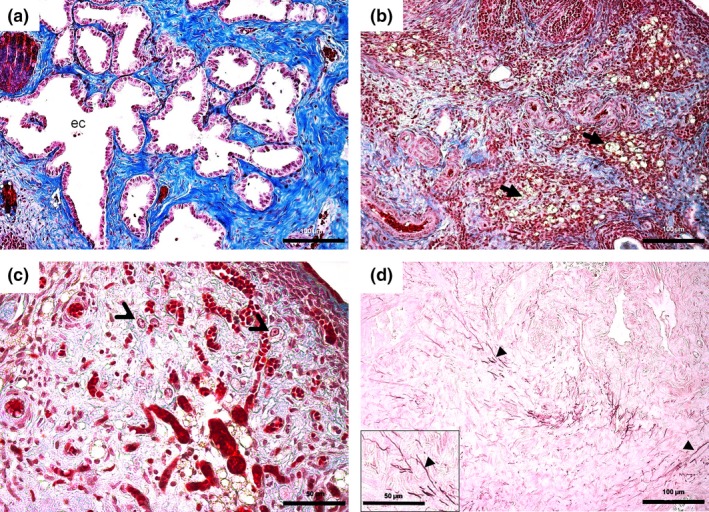
Details of the ovarian histopathological changes in the different nandrolone decanoate (ND)‐treated groups. Figure (a) shows epithelial cysts (ec) with irregular outlines and cuboid or columnar epithelium, surrounded by fibrous tissue (present in all groups after 30 days of recovery). Figure (b) shows areas of stromal degeneration containing vacuolated cells (arrows), surrounded by fibrous tissue. Figure (c) shows undifferentiated cells (thin arrow head) located in a notably vascular stroma. Figure (d) shows highlighted elastic fibres (arrow head, in detail) in the midst of a fibrous stroma in animals treated with 15 mg/kg after 60 days of recovery. Bar = 50 μm (c, d‐detail), 100 μm (a, b, d). Stained with Mallory's trichrome (a–c) and Orcein (d).
After 60 days of recovery, the animals treated with lower doses of ND (1.87 and 3.75 mg/kg) exhibited corpora lutea and follicles at various stages of development or atresia (Figure 3f,i), similar to the controls (Figure 3c). Conversely, the animals treated with higher doses of ND (7.5 and 15 mg/kg) had absence of corpora lutea and lack of healthy follicles (Figures 3l,o). Ovarian atrophy, fibrosis and abundant interstitial tissue were predominant in the stroma of animals treated with 15 mg/kg (Table 1, Figure 3o). Anomalous follicles, characterized by seminiferous tubule‐like structures, were scarce in the animals receiving doses of 1.87, 7.5 and 15 mg/kg, and none was reported in the animals treated with 3.75 mg/kg.
After ND treatment and 30 days of recovery period, the androgen receptor (AR) was strongly marked in the nucleus of Sertoli cells of the anomalous follicles (Figure 5b), whereas AR immunoreaction was weak to moderate after 60 days of recovery period (Figure 5b, detail), regardless of ND‐dose level. The immunoreaction for inhibin‐A (Figure 5c) was strong in Sertoli cells in all of the ND‐treated groups, after ND‐treatment and recovery periods. Immunofluorescence assay confirmed a positive reactivity for inhibin‐A in the cytoplasm of Sertoli cells (Figure 5d).
Figure 5.
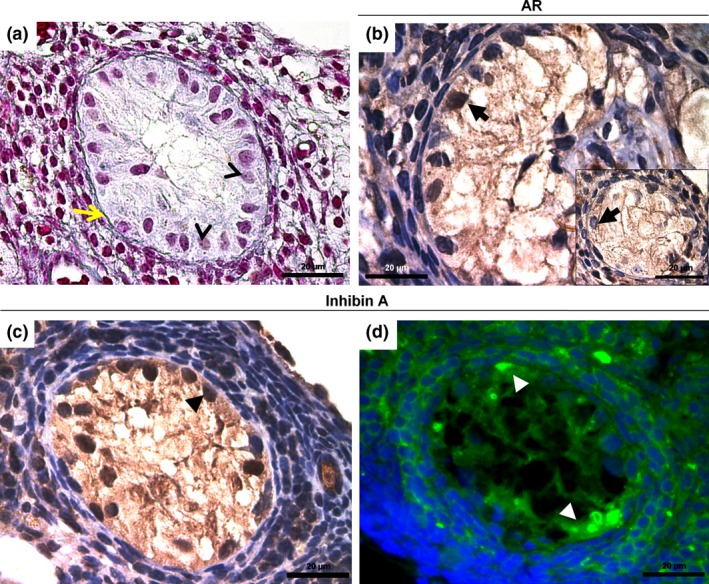
Histological sections of seminiferous tubule‐like structures containing Sertoli cells in the androgenized groups. Figure (a) shows polymorphonuclear cells with prominent nucleolus (thin arrow head) and abundant cytoplasm within the anomalous follicle devoid of oocyte and delineated by a continuous basal membrane (yellow arrow). Figure (b) shows an intense AR immunoreaction in the Sertoli cells (arrow), after treatment period. In detail, observe the moderate nuclear AR immunostaining (arrow) after 60 days of recovery. Figure (c) indicates the nuclear staining for inhibin‐A (arrow head). This result occurred after the treatment and recovery periods. Figure (d) shows the immunopositive cells for inhibin‐A (FITC, arrow head). Immunoreaction of inhibin‐A appears in the inner and surrounding cells of anomalous follicle. Bars = 20 μm. Stained with Mallory's trichrome (a) and haematoxylin (b, c).
Ovarian analysis
Ovarian weight, follicular score and morphometrical data are shown in Table 2. The mean ovarian weights were similar (P > 0.05) in all ND‐treated groups during the treatment period (Table 2). However, there was a reduction in the organ weight (P < 0.05) after 30 days of recovery, when compared to CD group. After 60 days of recovery, the mean ovarian weight of the groups treated with lowest doses (1.87 and 3.75 mg/kg) was similar (P > 0.05) to that of CE group, whereas in the groups treated with highest doses (7.5 and 15 mg/kg), the weight remained reduced.
Table 2.
Ovarian weight (g/100 g b.w.) and number of corpora lutea and antral, growing and atretic follicles in the different nandrolone decanoate (ND)‐treated groups and periods (treatment period and post‐treatment recovery for 30 and 60 days)
| Parameters | Experimental groups (n = 5/group/period) | ||||
|---|---|---|---|---|---|
| Treatment period | |||||
| Diestrus control† | 1.87 mg/kg | 3.75 mg/kg | 7.5 mg/kg | 15 mg/kg | |
| Ovarian weight* | 0.035 ± 0.003 a | 0.027 ± 0.003 a | 0.028 ± 0.005 a | 0.027 ± 0.004 a | 0.035 ± 0.010 a |
| Corpora lutea | 10 ± 3.7 a | 1 ± 1.7 b | 6 ± 2 c | 5 ± 3 c | 10 ± 4.7 a |
| Antral follicles | 8 ± 3 a | 5 ± 4.7 b | 11 ± 4 c | 4.5 ± 3.7 b | 6.5 ± 4.7 ab |
| Growing follicles | 6 ± 3 a | 0 ± 1 b | 4 ± 2.75 a | 1 ± 1.75 b | 2.5 ± 2 c |
| Atretic follicles | 5 ± 7 a | 20 ± 11 b | 16.5 ± 5.7 bd | 11.5 ± 14.2 cd | 13 ± 11.5 cd |
| Post‐treatment recovery for 30 days | |||||
| Ovarian weight* | 0.030 ± 0.003 a | 0.017 ± 0.001 b | 0.018 ± 0.002 b | 0.019 ± 0.003 b | 0.020 ± 0.003 b |
| Corpora lutea | 16.50 ± 4.5 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Antral follicles | 6 ± 4 a | 1 ± 2 b | 4 ± 3 a | 2.5 ± 3 c | 4 ± 3 a |
| Growing follicles | 4 ± 2.7 a | 0 ± 1 b | 2 ± 2 c | 1 ± 2 de | 0.5 ± 1 e |
| Atretic follicles | 6.5 ± 6 a | 5.5 ± 7.5 a | 5.5 ± 3.7 a | 5 ± 7 a | 4 ± 9 a |
| Post‐treatment recovery for 60 days | |||||
| Estrus control‡ | |||||
| Ovarian weight* | 0.035 ± 0.007 a | 0.030 ± 0.007 ab | 0.036 ± 0.004 a | 0.021 ± 0.005 bc | 0.014 ± 0.003 c |
| Corpora lutea | 11.5 ± 6.5 ab | 8 ± 8 a | 13 ± 2.7 b | 0 ± 0 c | 0 ± 0 c |
| Antral follicles | 4 ± 5.7 a | 4 ± 3 a | 4 ± 2 a | 1 ± 1 b | 0 ± 1 b |
| Growing follicles | 2 ± 2 ac | 1 ± 1 ab | 3 ± 4.7 c | 1 ± 2 b | 0 ± 0 b |
| Atretic follicles | 5 ± 6 ac | 5 ± 6.7 ab | 2 ± 2 b | 4 ± 6 c | 1 ± 2 b |
Data are expressed as the mean ± SD. *Data are expressed as the median ± interquartile deviation. In the same line, values followed by different letters indicate statistical differences among the groups (P < 0.05). †Control group in the diestrus phase. ‡Control group in the estrus phase.
During the ND treatment, the number and area of corpora lutea were decreased (P < 0.05) in the groups that received 1.87, 3.75 and 7.5 mg/kg. These structures were absent after 30 days of recovery, and in longer recovery period, the groups treated with 1.87 and 3.75 mg/kg restored the number and area of corpora lutea, while in the highest doses (7.5 and 15 mg/kg), the corpora lutea remained absent (Table 2).
There was a decrease in the number of antral follicles during ND treatment and after the recovery periods in a dose‐dependent manner (Table 2). The area of these follicles remained reduced in the groups treated with 1.87, 3.75 and 7.5 mg/kg after 60 days of recovery (Table 3). The number of growing follicles was reduced (P < 0.05) in the ND‐treated groups, except the 3.75 mg/kg group (Table 2). At 30‐day recovery, all groups still had few growing follicles, and a 60‐day recovery was sufficient to increase growing follicles in the groups that received the lowest doses (1.87 and 3.75 mg/kg). Despite no difference found in the atretic follicle area, there was an increased number of atretic follicles in ND‐treated groups (Tables 1 and 2). At 30‐day recovery, the number of atretic follicles in androgenized groups was similar (P > 0.05) to the control group (Table 2). The follicle area was lower after a 30‐day recovery, except in the group treated with 7.5 mg/kg (Table 3). After 60‐day recovery, the number and area of the atretic follicles varied among the groups in a dose‐dependent manner.
Table 3.
Measurements of area (mm2) of corpora lutea and antral and atretic follicles in the different nandrolone decanoate (ND)‐treated groups and periods (treatment period and post‐treatment recovery for 30 and 60 days)
| Parameters | Experimental groups (n = 5/group/period) | ||||
|---|---|---|---|---|---|
| Treatment period | |||||
| Diestrus control* | 1.87 mg/kg | 3.75 mg/kg | 7.5 mg/kg | 15 mg/kg | |
| Corpora lutea | 0.60 ± 0.56 a | 0.11 ± 0.10 b | 0.15 ± 0.08 b | 0.17 ± 0.10 b | 0.32 ± 0.41 a |
| Antral follicles | 0.04 ± 0.08 ac | 0.10 ± 0.09 a | 0.03 ± 0.04 cb | 0.02 ± 0.05 b | 0.04 ± 0.08 bc |
| Atretic follicles | 0.10 ± 0.05 a | 0.12 ± 0.06 a | 0.13 ± 0.08 a | 0.09 ± 0.16 a | 0.09 ± 0.14 a |
| Post‐treatment recovery for 30 days | |||||
| Corpora lutea | 0.46 ± 0.5 a | Absent | Absent | Absent | Absent |
| Antral follicles | 0.06 ± 0.12 ac | 0.02 ± 0.03 b | 0.10 ± 0.22 a | 0.05 ± 0.04 c | 0.01 ± 0.10 b |
| Atretic follicles | 0.09 ± 0.05 a | 0.05 ± 0.11 bc | 0.06 ± 0.03 b | 0.08 ± 0.09 ac | 0.07 ± 0.12 bc |
| Post‐treatment recovery for 60 days | |||||
| Estrus control† | |||||
| Corpora lutea | 0.36 ± 0.55 a | 0.37 ± 0.50 a | 0.50 ± 0.33 a | Absent | Absent |
| Antral follicles | 0.08 ± 0.07 a | 0.02 ± 0.02 b | 0.03 ± 0.03 b | 0.05 ± 0.10 b | Absent |
| Atretic follicles | 0.15 ± 0.25 ab | 0.11 ± 0.14 a | 0.25 ± 0.45 b | 0.25 ± 0.17 b | Absent |
Values expressed as the median ± interquartile deviation. In the same line, values followed by different letters indicate statistical differences among the groups (P < 0.05). *Control group in the diestrus phase. †Control group in the estrus phase.
Discussion
In this study, the estrous cycle and ovarian structures were affected by treatment with ND in all of the doses, and recovery periods did not restore the adverse effects, mainly when the steroid was administered at the highest dose level. The absence of changes in the ovarian weight after ND treatment probably occurred due to the presence of corpora lutea in the ovaries, even with a decrease in the population of follicles. After a 30‐day recovery, the lack of corpora lutea and the marked impairment in folliculogenesis contributed to a decrease in ovarian weights. At 60 days after treatment, only the groups treated with 1.87 and 3.75 mg/kg were able to restore ovarian weight similar to the CE group, as they showed histological signs of recovery. In the groups treated with highest doses, the ovarian weight remained unchanged. It is well documented that the AAS promotes reduction in reproductive organ weights (Camargo et al. 2009a,b, 2014; Bento‐Silva et al. 2010; Karbalay‐Doust & Noorafshan 2012).
The suppression of estrous cyclicity caused by AAS treatment was previously reported in various studies (Gerez et al. 2005; Mobini Far et al. 2007; Bento‐Silva et al. 2010; Camargo et al. 2011, 2014; Chuffa et al. 2011b) and severely affects the sexual cycle of women (Maravelias et al. 2005). According to Karbalay‐Doust and Noorafshan (2012), administration of exogenous steroids may increase circulating androgen levels, thus altering the control of the hypothalamus–pituitary–gonad axis which results in altered levels of hormones, such as follicle‐stimulating hormone (FSH), luteinizing hormone (LH), oestrogen and progesterone (Bronson et al. 1996a; Bronson 1996b; Blasberg et al. 1997). Moreover, these hormonal disturbances may affect the regulation of the estrous cycle as observed. In this study, the persistence in diestrus in ND‐treated females followed by 30 days of recovery indicates the physiological inability for resynchronization of the estrous cycle. Thus, it can be considered that these females showed a physiological anestrus. There was a pronounced effect on the estrous cycle in all of the dose levels at 60 days after recovery. The animals treated with the lower doses exhibited an irregular cycle at the end of the period, with extension of diestrus. Only females treated with the highest dose (15 mg ND/kg) had persistent diestrus after 60 days of recovery.
This study described for the first time the histopathological and morphometrical changes in the ovarian tissue arising from different doses of ND and varying according to the recovery periods. In general, both folliculogenesis and luteogenesis have been severely damaged by ND, indicating that neither doses nor period of treatment are safe when administered in therapeutic or supraphysiological levels. The lowest dose of ND (1.87 mg/kg), which is equivalent to the therapeutic dose used for treating anaemia (allometric scaling, Reagan‐Shaw et al. 2007), was able to promote changes in the ovarian tissue similar to the highest doses used in the study (7.5 and 15 mg/kg). The increase in follicular atresia and the decrease in the number of corpora lutea have already been reported in females who received a single dose of ND (Gerez et al. 2005; Camargo et al. 2009a, 2011) or a combination of two synthetic steroids (Camargo et al. 2014). These effects may be caused indirectly via the neuroendocrine axis or even directly via androgen receptors (AR) expressed in the granulosa and theca cells (Drummond 2006). Anabolic androgenic steroids concentration induces a dose‐dependent effect on the AR expression and/or oestrogen synthesis to regulate ovarian functions, so that high doses can result in advanced stage of atresia and follicular apoptosis, while lower doses stimulate folliculogenesis (Couse et al. 2006; Drummond 2006; Walters et al. 2008). Depending on AAS metabolism and oestrogen conversion rates, the inhibitory effect of androgens related to apoptotic DNA fragmentation in granulosa cells of early and pre‐antral follicles is expected even at lower doses (Goyeneche et al. 2002).
Androgens are extensively involved in supporting luteal progesterone production following their conversion into oestrogens (Goyeneche et al. 2002). An explanation for this is provided by the presence of oestrogen receptors in the rat corpora lutea (CL). However, oestrogen is not always required for the action of androgens in the CL. In animals receiving doses of 1.87, 3.75 and 7.5 mg ND/kg, there was few and small CL in ovaries after treatment period. Interestingly, this effect was not observed in the group treated with 15 mg ND/kg, where number and area of CL were similar to the control group in diestrus. This normal aspect is typical of a state of persistent diestrus (Li & Davis 2007). Indeed, there is a relationship between non‐aromatizable androgens and luteal progesterone secretion that is responsible for the inhibition of luteolysis (Goyeneche et al. 2002). In this context, high‐dose ND produced persistent CL throughout the experiment, while low‐dose‐treated animals underwent luteolysis. Additional studies are needed to clarify the mechanism(s) of androgen‐mediated formation and regression of CL.
These results suggest that the action of androstenedione on luteal progesterone production and secretion at the time of luteolysis occurs through an androgenic mechanism.
The interruption of ND treatment for 30 days was not sufficient to reverse the ovarian injuries. In addition, these ovaries showed severe histopathological alterations, characterized by marked atrophy and abundant interstitial tissue derived from changes in theca cells of the atretic follicles (Perez et al. 1999), decrease in the number of healthy follicles and the absence of corpora lutea. These effects were considered dose‐independent, as all of the treated groups exhibited the same response. The presence of stromal fibrosis in the different groups was due to tissue degeneration. Previous studies reported that the uterus showed fibrous stroma in the rats submitted to physical effort combined with nandrolone decanoate treatment (Chuffa et al. 2011b) or nandrolone decanoate alone (Gerez et al. 2005). Cortical fibrosis and injury to blood vessels are histopathological aspects of ovarian damage, observed in humans exposed to chemotherapy (Meirow et al. 2007). It is reported that pharmacological or supraphysiological doses of testosterone or AAS have resulted in vasodilatation (Sader et al. 2001; Littleton‐Kearney & Hurn 2004). This effect has been extensively reported in animal or human coronary arteries. In female rats, it is suggested that vasodilation is due to an increase in the aromatization of testosterone into estradiol (Shaw et al. 2001); however, this mechanism still needs further elucidation. According to Sader et al. (2001), the effects of AAS in promoting vasodilatation may be dependent on the dose, type of androgen and outcome measured. In the present study, vasodilation observed in ovarian medulla occurred only at the dose of 7.5 mg ND/kg, in both the post‐treatment and postrecovery for 30 days. Because other ND dosages promoted several histopathological changes at 30 days after treatment, we believe that this effect specifically to the animals treated with 7.5 mg ND/kg was dose dependent and 30 days of recovery was insufficient to reduce vasodilation.
The changes in the features of ovarian tissue, associated with a decrease in the population of follicular units, are capable of promoting a further significant reproductive loss for the animal, considering that both preservation and function of the follicles are damaged. Thus, a previous study conducted in our laboratory (Belardin et al. 2014) showed that 60 days after last ND dose, the females were receptive to males as sperm was observed in vaginal smears, except to those females receiving the highest dose of ND (15 mg/kg). Only two females receiving 1.87 mg ND/kg became pregnant, and the rats receiving doses of 3.75 and 7.5 mg ND/kg probably did not ovulate because no uterine implantation and/or resorption site was found. Based on these results, the current study suggested that the treatment of ND is able to affect ovulation.
In ND‐treated groups anomalous follicles were observed, characterized by seminiferous tubule‐like structures containing Sertoli cells. These structures have been often observed in ovaries of ageing rodents (Crumeyrolle‐Arias et al. 1976). Other studies reported the presence of this type of follicle in the eutherian (Britt et al. 2002) or irradiated ovary (Guigon et al. 2005). It is known that follicular cells can survive after oocyte loss and, subsequently, transdifferentiate into immature Sertoli cells, presumably under the control of the endocrine environment and more specifically of FSH (Guigon et al. 2005). According to Britt et al. (2002) and Davis et al. (2000), this transdifferentiation occurs due to an alteration in the apoptotic program of granulosa cells, in which oocyte/granulosa cell interaction is disrupted and the Sertoli cells develop within atretic follicles. The involvement of oestrogen replacement in prevention of this transdifferentiation has also been reported (Britt et al. 2002). In this study, the absence of viable oocytes and the intense follicular atresia induced by ND contributed to the formation of seminiferous tubule‐like structures, confirming the differentiation of granulosa cells into Sertoli cells. At 60 days of recovery, the incidence of anomalous follicles was decreased in the ND‐treated groups. Such structures were scarce in the most groups (varying from 1 to 2 anomalous follicles) and absent in the group treated with 3.75 mg ND/kg, suggesting that these follicles degenerated faster in this group. The mechanism which promoted the absence of anomalous follicles is unknown, but it is likely to be due to a dose‐independent effect, as the range between scarce and absent anomalous follicles is too small to assign a proper dose‐related response. The Sertoli cells displayed marked AR nuclear staining, similar to that observed in transdifferentiated oocyte‐depleted follicles (tODFs; Guigon et al. 2005). This positive reaction was reduced after a longer recovery period, probably due to a downregulation of AR. In this study, the inhibin‐A immunoreaction was intense in the Sertoli cells of androgenized females, agreeing with the results obtained by Guigon et al. (2005) in the irradiated ovaries.
Important features of the normal ovarian tissue were restored after the 60‐day recovery period only in the animals treated with the lowest doses of ND (1.87 and 3.75 mg/kg). In contrast, the groups treated with the highest doses of ND, especially 15 mg/kg, still presented signals of histopathological changes.
In summary, all the different doses of ND administered (1.87–15 mg/kg) were able to cause changes to the estrous cycle and ovarian tissue of rats. Furthermore, 30 and 60 days of recovery were insufficient to completely restore the damage, mainly in animals treated with the highest dose.
Conflict of interest
The authors declare no conflict of interest.
Funding source
The authors express their gratitude to FAPESP – São Paulo Research Foundation (Processes number 2012/03813‐0, 2012/01747‐0 and 2013/14510‐0) and FUNDUNESP – Foundation for the Development of UNESP (Process number 2178/002/14), for financial support.
References
- Arlt W. (2006) Androgen therapy in women. Eur. J. Endocrinol. 154, 1–11. [DOI] [PubMed] [Google Scholar]
- Bahrke M.S. & Yesalis C.E. (2004) Abuse of anabolic androgenic steroids and related substances in sport and exercise. Curr. Opin. Pharmacol. 4, 614–620. [DOI] [PubMed] [Google Scholar]
- Belardin L.B., Simão V.A., Leite G.A.A., Chuffa L.G.A. & Camargo I.C.C. (2014) Dose‐dependent effects and reversibility of the injuries caused by nandrolone decanoate in uterine tissue and fertility of rats. Birth Defects Res. B Dev. Reprod. Toxicol. 101, 168–177. [DOI] [PubMed] [Google Scholar]
- Bento‐Silva M.T., Martins M.C.C., Torres‐Leal F.L. et al (2010) Effects of administering testosterone undecanoate in rats subjected to physical exercise: effects on the estrous cycle, motor behavior and morphology of the liver and kidney. Braz. J. Pharm. Sci. 46, 79–89. [Google Scholar]
- Bhasin S., Woodhouse L., Casaburi R. et al (2005) Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J. Clin. Endocrinol. Metab. 90, 678–688. [DOI] [PubMed] [Google Scholar]
- Blasberg M.E., Langan C.J. & Clark A.S. (1997) The effects of 17 alpha‐methyltestosterone, methandrostenolone, and nandrolone decanoate on the rat estrous cycle. Physiol. Behav. 61, 265–272. [DOI] [PubMed] [Google Scholar]
- Boff S.R. (2010) Esteroides anabólicos e exercício: ação e efeitos colaterais. Rev. Bras. Cienc. Mov. 18, 81–88. [Google Scholar]
- Bonetti A., Tirelli F., Catapano A. et al (2008) Side effects of anabolic androgenic steroids abuse. Int. J. Sports Med. 29, 679–687. [DOI] [PubMed] [Google Scholar]
- Britt K.L., Kerr J., O'Donnel L. et al (2002) Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. FASEB J. 16, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Bronson F.H. (1996b) Effects of prolonged exposure to anabolic steroids on the behavior of male and female mice. Pharmacol. Biochem. Behav. 53, 329–334. [DOI] [PubMed] [Google Scholar]
- Bronson F.H., Nguyen K.Q. & De La Rosa J. (1996a) Effect of anabolic steroids on behavior and physiological characteristics of female mice. Physiol. Behav. 59, 49–55. [DOI] [PubMed] [Google Scholar]
- Camargo I.C.C., Souza R.B., Mesquita S.F.P., Chuffa L.G.A. & Frei F. (2009a) Ovarian histology and follicular score in female rats treated with nandrolone decanoate and submitted to physical effort. Acta Biol. Hung. 60, 253–261. [DOI] [PubMed] [Google Scholar]
- Camargo I.C.C., Gaspar A.L.C., Frei F. & Mesquita S.F.P. (2009b) Efeitos dos esteroides anabólicos androgênicos sobre o útero e parâmetros reprodutivos de ratas adultas. Rev. Bras. Ginecol. Obstet. 31, 453–460. [DOI] [PubMed] [Google Scholar]
- Camargo I.C.C., Gênova T.C., Machado M.C.P., Frei F. & Mesquita S.F.P. (2011) Administração experimental de esteróide anabólico androgênico e álcool causa alterações histológicas e morfométricas nos ovários e útero de ratas adultas. Biosci. J. 27, 656–665. [Google Scholar]
- Camargo I.C.C., Leite G.A.A., Pinto T. & Ribeiro‐Paes J.T. (2014) Histopathologycal findings in the ovaries and uterus of albino female rats promoted by co‐administration of synthetic steroids and nicotine. Exp. Toxicol. Pathol. 66, 195–202. [DOI] [PubMed] [Google Scholar]
- Cannavò S., Curtò L. & Trimarchi F. (2001) Exercise‐related female reproductive dysfunction. J. Endocrinol. Invest. 24, 823–832. [DOI] [PubMed] [Google Scholar]
- Chuffa L.G., Amorim J.P., Teixeira G.R. et al (2011a) Long‐term melatonin treatment reduces ovarian mass and enhances tissue antioxidant defenses during ovulation in the rat. Braz. J. Med. Biol. Res. 44, 217–223. [DOI] [PubMed] [Google Scholar]
- Chuffa L.G.A., Souza R.B., Frei F., Mesquita S.F.P. & Camargo I.C.C. (2011b) Nandrolone decanoate and physical effort: histological and morphometrical assessment in adult rat uterus. Anat. Rec. 294, 335–342. [DOI] [PubMed] [Google Scholar]
- Chuffa L.G., Seiva F.R., Fávaro W.J. et al (2013) Melatonin and ethanol intake exert opposite effects on circulating estradiol and progesterone and differentially regulate sex steroid receptors in the ovaries, oviducts, and uteri of adult rats. Reprod. Toxicol. 39, 40–49. [DOI] [PubMed] [Google Scholar]
- Clark A.S. & Fast A.S. (1996) Comparison of the effects of 17 alpha‐methyltestosterone, methandrostenolone, and nandrolone decanoate on the sexual behavior of castrated male rats. Behav. Neurosci. 110, 1478–1486. [DOI] [PubMed] [Google Scholar]
- Couse J.F., Hewitt S.C., Korach K.S. (2006) Chapter 15, Steroid Receptors in the Ovary and Uterus In: Knobil and Neill's Physiology of Reproduction, Third Edition, (eds Neill J.D.), Academic Press, New York, 593–608. [Google Scholar]
- Crumeyrolle‐Arias M., Scheib D. & Aschheim P. (1976) Light and electron microscopy of the ovarian interstitial tissue in the senile rat: normal aspect and response to HCG of “deficiency cell” and “epithelial cords”. Gerontology 22, 185–204. [DOI] [PubMed] [Google Scholar]
- Cunha T.S., Cunha N.S., Moura M.J.C.S. & Marcondes F.K. (2004) Esteroides anabólicos androgênicos e sua relação com a prática desportiva. Braz. J. Pharm. Sci. 40, 165–179. [Google Scholar]
- Davis B.J., McCurdy A.E., Miller B.D., Lucier G.W. & Tritscher A.M. (2000) Ovarian tumors in rats induced by chronic 2,3,7,8‐Tetrachlorodibenzo‐p‐Dioxin treatment. Cancer Res. 60, 5414–5419. [PubMed] [Google Scholar]
- Drummond A.E. (2006) The role of steroids in follicular growth. Reprod. Biol. Endocrinol. 4, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans N.A. (1997) Gym & tonic: a profile of 100 male steroid users. Br. J. Sports Med. 31, 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans N.A. (2004) Current concepts in anabolic‐androgenic steroids. Am. J. Sports Med. 32, 534–542. [DOI] [PubMed] [Google Scholar]
- Fermo R.S., Rego J.N.I., Fraquini J.V.M. & Andrade T.U. (2008) Efeito da suplementação alimentar sobre ação anabólica do decanoato de nandrolona em ratos. Rev. Eletr. Farm. 5, 111–121. [Google Scholar]
- Gerez J.R., Frei F. & Camargo I.C.C. (2005) Histological assessment of ovaries and uterus of rats subjected to nandrolone decanoate treatment. Contraception 72, 77–80. [DOI] [PubMed] [Google Scholar]
- Goldman J.M., Murr A.S. & Cooper R.L. (2007) The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. B Dev. Reprod. Toxicol. 80, 84–97. [DOI] [PubMed] [Google Scholar]
- Goyeneche A.A., Calvo V., Gibori G. & Telleria C.M. (2002) Androstenedione interferes in luteal regression by inhibiting apoptosis and stimulating progesterone production. Biol. Reprod. 66, 1540–1547. [DOI] [PubMed] [Google Scholar]
- Guigon C.J., Coudouel N., Mazaud‐Guittot S., Forest M.G. & Magre S. (2005) Follicular cells acquire Sertoli cell characteristics after oocyte loss. Endocrinology 146, 2992–3004. [DOI] [PubMed] [Google Scholar]
- Hoffman J.R. & Ratamess N.A. (2006) Medical issues associated with anabolic steroid use: are they exaggerated? J. Sports Sci. Med. 5, 182–193. [PMC free article] [PubMed] [Google Scholar]
- Iriart J.A.B., Chaves J.C. & De Orleans R.G. (2009) Culto ao corpo e uso de anabolizantes entre praticantes de musculação. Cad. Saúde Pública. 25, 773–782. [DOI] [PubMed] [Google Scholar]
- Kam P.C.A. & Yarrow M. (2005) Anabolic steroid abuse: physiological and anaesthetic considerations. Anaesthesia 60, 685–692. [DOI] [PubMed] [Google Scholar]
- Karbalay‐Doust S. & Noorafshan A. (2006) Stereological study of the effects of nandrolone decanoate on the rat prostate. Micron 37, 617–623. [DOI] [PubMed] [Google Scholar]
- Karbalay‐Doust S. & Noorafshan A. (2012) Stereological estimation of ovarian oocyte volume, surface area and number: application on mice treated with nandrolone decanoate. Folia Histochem. Cytobiol. 50, 275–279. [DOI] [PubMed] [Google Scholar]
- Kicman A.T. (2008) Pharmacology of anabolic steroids. Br. J. Pharmacol. 154, 502–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. & Davis B. (2007) Evaluating rodent vaginal and uterine histology in toxicity studies. Birth Defects Res. B Dev. Reprod. Toxicol. 80, 246–252. [DOI] [PubMed] [Google Scholar]
- Lise M.L.Z., Gama e Silva T.S., Ferigolo M., Barros H.M.T. (1999) O abuso de esteroides anabólicos androgênicos em atletismo. Rev. Ass. Med. Bras. 45, 364–370. [DOI] [PubMed] [Google Scholar]
- Littleton‐Kearney M. & Hurn P.D. (2004) Testosterone as modulator of vascular behavior. Biol. Res. Nurs. 5, 276–285. [DOI] [PubMed] [Google Scholar]
- Maravelias C., Dona A., Stefanidou M. & Spiliopoulou C. (2005) Adverse effects of anabolic steroids in athletes – A constant threat. Toxicol. Lett. 158, 167–175. [DOI] [PubMed] [Google Scholar]
- Marcondes F.K., Bianchi F.J. & Tanno A.P. (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz. J. Biol. 62, 609–614. [DOI] [PubMed] [Google Scholar]
- Marqueti R.C., Prestes J., Wang C.C. et al (2010) Biomechanical responses of different rat tendons to nandrolone decanoate and load exercise. Scand. J. Med. Sci. Sports 21, e91–e99. [DOI] [PubMed] [Google Scholar]
- Meirow D., Dor J., Kaufman B. et al (2007) Cortical fibrosis and blood‐vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum. Reprod. 22, 1626–1633. [DOI] [PubMed] [Google Scholar]
- Mobini Far H.R., Agren G., Lindqvist A.S., Marmendal M., Fahlke C. & Thiblin I. (2007) Administration of the anabolic androgenic steroid nandrolone decanoate to female rats causes alterations in the morphology of their uterus and a reduction in reproductive capacity. Eur. J. Obstet. Gynecol. Reprod. Biol. 131, 189–197. [DOI] [PubMed] [Google Scholar]
- Paccola C.C., Resende C.G., Stumpp T., Miraglia S.M. & Cipriano I. (2013) The rat estrous cycle revisited: a quantitative and qualitative analysis. Anim. Reprod. 10, 677–683. [Google Scholar]
- Pedersen T., Peters H. (1968) Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil., 17, 208–212. [DOI] [PubMed] [Google Scholar]
- Perez J.F., Conley A.J., Dieter J.A., Sanz‐Ortega J. & Lasley B.L. (1999) Studies on the origin of ovarian interstitial tissue and the incidence of endometrial hyperplasia in domestic and feral cats. Gen. Comp. Endocrinol. 116, 10–20. [DOI] [PubMed] [Google Scholar]
- Perry P.J., Lund B.C., Deninger M.J., Kutscher E.C. & Schneider J. (2005) Anabolic steroid use in weightlifters and bodybuilders: an internet survey and drug utilization. Clin. J. Sport Med. 15, 326–330. [DOI] [PubMed] [Google Scholar]
- Plowchalck D.R., Smith B.J. & Mattison C.R. (1993) Assessment of toxicity to the ovary using follicle quantification and morphometrics. Methods Toxicol. 3, 57–68. [Google Scholar]
- Reagan‐Shaw S., Nihal M. & Ahmad N. (2007) Dose translation from animal to human studies revisited. FASEB J. 22, 659–661. [DOI] [PubMed] [Google Scholar]
- Sader M.A., Griffiths K.A., McCredie R.J., Handelsman D.J. & Celermajer D.S. (2001) Androgenic anabolic steroids and arterial structure and function in male bodybuilders. J. Am. Coll. Cardiol. 37, 224–230. [DOI] [PubMed] [Google Scholar]
- Shaw L., Taggert M. & Austin C. (2001) Effects of the oestrus cycle and gender on acute vasodilatory responses of isolated pressurized rat mesenteric arteries to 17β–oestradiol. Br. J. Pharmacol. 132, 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P.R.P., Danielski R. & Czepielewski M.A. (2002) Esteroides anabolizantes no esporte. Rev. Bras. Med. Esporte. 8, 235–243. [Google Scholar]
- Thiblin I. & Petersson A. (2004) Pharmacoepidemiology of anabolic androgenic steroids: a review. Fundam. Clin. Pharmacol. 19, 27–44. [DOI] [PubMed] [Google Scholar]
- Walters K.A., Allan C.M. & Handelsman D.J. (2008) Androgen actions and the ovary. Biol. Reprod. 78, 380–389. [DOI] [PubMed] [Google Scholar]
- Westwood F.R. (2008) The female rat reproductive cycle: a practical histological guide to staging. Toxicol. Pathol. 36, 375–384. [DOI] [PubMed] [Google Scholar]


