Abstract
Aims
The purpose of the present study was to describe the scope, pattern and patient characteristics associated with incident opioid use among older adults with chronic obstructive pulmonary disease (COPD).
Methods
This was a retrospective population‐based cohort study using Ontario, Canada, healthcare administrative data. Study participants were individuals aged 66 years and older with physician‐diagnosed COPD, identified using a validated algorithm, who were not receiving palliative care. We examined the incidence of oral opioid receipt between 1 April 2003 and 31 March 2012, as well as several patterns of incident opioid drug use.
Results
Among 107 109 community‐dwelling and 16 207 long‐term care resident older adults with COPD, 72 962 (68.1%) and 8811 (54.4%), respectively, received an incident opioid drug during the observation period. Among long‐term care residents, multiple opioid dispensings (8.8%), dispensings for >30 days' duration (up to 19.8%), second dispensings (35–43%) and early refills (24.2%) were observed. Incident opioid dispensing was also observed to occur during COPD exacerbations (6.9% among all long‐term care residents; 18.1% among long‐term care residents with frequent exacerbations). These same patterns of incident opioid use occurred among community‐dwelling individuals, but with relatively lower frequencies.
Conclusions
New opioid use was high among older adults with COPD. Potential safety concerns are raised by the degree and pattern of new opioid use, but further studies are needed to evaluate if adverse events are associated with opioid drug use in this older and respiratory‐vulnerable population.
Keywords: COPD, elderly, narcotics, opioids, pharmacoepidemiology
What is Already Known About this Subject
The scope and pattern of opioid drug use among older adults with COPD are not well‐known.
Understanding opioid use among older adults with COPD is important because several potential safety concerns are raised by the use of these drugs in this population, including possible respiratory concerns.
What this Study Adds
New opioid use was frequent among older Ontario adults with COPD.
New opioids were used by older adults with COPD in ways that are potentially indicative of excessive use.
Potential safety concerns are raised by the degree and pattern of new opioid use in this older and respiratory‐vulnerable population.
Introduction
The scope and pattern of opioid drug use among older adults with chronic obstructive pulmonary disease (COPD) are not well known. Estimates of opioid drug use among general community‐dwelling older adults range from 1.5–17% 1, 2, 3, 4, 5. Older adults with COPD might be more likely than older adults without COPD to receive opioids, for several reasons. Opioids can be used in COPD to treat chronic musculoskeletal pain, which is reported in an estimated 70% of COPD patients 7, 8, 9. Because of their sedating properties, opioids can also be used by some individuals with COPD to treat insomnia symptoms, which occur in 50% or more of individuals with COPD 10, 11. Finally, opioids can be used to manage refractory dyspnoea and cough 12, 13, 14, which are an issue in an estimated 50% of individuals with advanced COPD 15.
Understanding the scope and pattern of opioid use among older adults with COPD is important because several potential safety concerns are raised by the use of these drugs in this population. Opioids are known to be linked with an increased risk for various adverse psychomotor and gastrointestinal events among older adults (including falls and fractures, dizziness, delirium, somnolence, constipation, and nausea and vomiting) 16, 17, 18 and COPD largely afflicts older adults 19. Results from animal and physiological studies raise the possibility that opioid drugs could also predispose to negative respiratory outcomes among respiratory‐vulnerable individuals with COPD through several possible mechanisms 20, 21, 22, 23, 24, 25. However, several clinical studies have found that systemic formulations of opioids can actually help to reduce dyspnoea in individuals with advanced COPD 26, 27, 28, 29, 30, 31. However, these clinical studies were generally limited by small numbers of subjects 26, 27, 28, 29, 30, 31, low or single opioid dosing 26, 27, 28, short follow‐up durations 26, 27, 28, 29 and study subjects who experienced significant adverse events or died were not always included in final analyses 29, 30, 31. A large population‐based prospective cohort study that was designed to evaluate for possible adverse drug‐related events found that, among individuals with COPD on supplemental oxygen, prevalent opioid users receiving an estimated >30 mg oral morphine equivalents per day had a 21% increased adjusted risk of all‐cause mortality compared to non‐users 32. However, there was no increased mortality risk among opioid recipients with ≦30 mg oral morphine equivalents per day 32. In another large population‐based prospective cohort study, users of inhaled and orally consumed recreational and medicinal opium were found to have a fivefold increased adjusted risk of COPD‐related mortality relative to non‐users 33. Advanced dyspnoea management guidelines 12, 13, 14 and the 2014 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines 34 support the use of opioids in advanced COPD. However, the GOLD guidelines also go on to state that opioids can ‘have serious side‐effects and the benefits might be limited to a few sensitive subjects’ 34.
The purpose of the present study was to describe the scope, pattern and patient characteristics associated with incident opioid use among older adults with COPD, using population‐level healthcare administrative data.
Methods
Study design
This was a retrospective population‐based cohort study using data from 1 April 1998 to 31 March 2013. Ontario provincial healthcare administrative databases were used to examine incident opioid use among an established cohort of individuals with COPD. Ontario has a multicultural population of over 13.5 million and constitutes close to 40% of Canada's population. Ethics approval was obtained from the Research Ethics Board at Sunnybrook Health Sciences Centre.
Data sources
Multiple Ontario provincial healthcare administrative databases were linked at an individual patient level using unique encoded identifiers and analysed at the Institute for Clinical Evaluative Sciences (ICES). An established validated database of Ontario adults with physician‐diagnosed COPD was used. COPD diagnosis was based on a highly specific algorithm using three or more ambulatory claims for COPD within any 2‐year period or one or more hospitalization(s) for COPD {specificity 95.4% [95% confidence interval (CI) 92.6%, 97.4%]; sensitivity 57.5% [95% CI 47.9%, 66.8%]} 35. The COPD health administrative codes had been previously validated against an expert respirology panel review of 442 randomly selected patient medical charts (with primary care practice diagnoses of COPD, asthma, other respiratory condition and no respiratory condition) from a random sample of primary care practices in the province of Ontario 35. The expert respirology panel was allowed to consider all available information in a patient's medical charts when deciding about COPD diagnosis, including full respiratory history, smoking status, physical examination, pulmonary function tests, chest imaging, etc. 35. Medication records were obtained from the Ontario Drug Benefit (ODB) database, which contains information on all publicly funded outpatient medications dispensed to patients in Ontario aged 65 years and older. The coding accuracy and completeness of drug claims in the ODB are excellent, with an error rate of 0.7% (95% CI 0.5%, 0.9%) 36. The Ontario Health Insurance Plan (OHIP) database (containing information on patient contact by physicians in both ambulatory and hospital settings), the Canadian Institute for Health Information Discharge Abstract Database (CIHI‐DAD) (containing information on all hospital admissions) and the National Ambulatory Care Reporting System (NACRS) database (containing information on all emergency room (ER) visits) were used to identify COPD exacerbations and comorbidities. Other healthcare administrative databases used in the present study are described in the Supplementary Appendix.
Study population
To be included in the study, individuals had to be Ontario residents, have validated physician‐diagnosed COPD and be 66 years of age or older by 1 April 2003 (i.e. this was a non‐rolling cohort). Individuals with COPD younger than 66 years of age were excluded as information on incident medication receipt was not available for such persons in the ODB database. Although individuals with COPD younger than 66 years of age were not included, COPD is a disease of older adults, with the vast majority of affected individuals being older than 65 years 19. Individuals receiving palliative care (based on physician service codes in OHIP and CIHI‐DAD databases) in the year prior to incident opioid receipt (defined below) were intentionally excluded as the appropriateness and safety concerns of opioid use will understandably differ in such a setting.
Exposure to opioids and index date definition
Oral and transdermal formulations of opioid drugs contained in the ODB database were considered (Table 1). Both tablet/capsule and liquid oral formulations were considered, and tablet/capsule opioids were distinguished by half‐life activity category. Injectable and rectally administered opioids were not included as these were felt to be unlikely to be used in the outpatient COPD setting. Partial agonist–partial antagonist opioid agents (i.e. suboxone) and combination opioid and glutamate receptor agonists (i.e. methadone) were not considered, as these are different classes of medication and have different indications. Opioid users were defined by incident dispensing of any opioid listed in Table 1 between 1 April 2003 and 31 March 2012. Incident dispensing was defined as receipt of any opioid drugs in Table 1 with no receipt of any of the opioids in Table 1 in the previous year. This definition of incident drug use has been used previously 37, 38. We elected to study incident drug use, rather than prevalent use, as the former is a more relevant usage pattern to describe from a drug safety perspective. ‘Healthy user’ bias is potentially introduced when considering prevalent use because individuals who have previously suffered an adverse opioid drug‐related effect and then discontinued the medication, or died, might not be included among prevalent users. ‘Healthy user’ bias is less of an issue among incident users, who have no prior personal experience with the drug to potentially influence drug receipt. For the purposes of describing patterns of new opioid use among users and patient characteristics associated with new use, the index date was the date that the incident opioid was dispensed. Incident use was counted only once per individual, even if an individual met the criteria for incident use more than once during the observation window, and only the first dispensing was considered. The follow‐up window lasted until 31 March 2013, until death or until 1 year after incident use, whichever came first. We described trends in incident opioid use over time among older adults with COPD by estimating cumulative incidence function curves for new use over the period 1 April 2003 to 31 March 2012. For this specific analysis, the index date was 1 April 2003 and only the first incident dispensing was considered per individual.
Table 1.
Opioid drugs covered under the Ontario Drug Benefit program
| Oral tablet/capsule agents | Oral liquid agents | Transdermal agents | ||
|---|---|---|---|---|
| Opioid‐only agonists | Opioid/non‐opioid combination agents | |||
| Shorter‐acting agents | Longer‐acting agents | |||
| Anileridine | Codeine sulphate | Acetaminophen–caffeine–codeine | Acetaminophen–codeine phosphate | Fentanyl transdermal system |
| Codeine phosphate | Hydromorphone HCL | Acetaminophen–codeine | Codeine phosphate | |
| Hydromorphone HCL | Levorphanol | Acetylsalicylic acid–codeine–caffeine | Morphine HCL | |
| Morphine HCL | Morphine sulphate | Acetylsalicylic acid–codeine | Morphine sulphate | |
| Meperidine | Oxycodone HCL ER/SR | Oxycodone HCL–acetaminophen | ||
| Oxycodone HCL | Propoxyphene HCL; dextropropoxyphene HCL | Oxycodone HCL–acetylsalicylic acid | ||
Rectal and intravenous opioid formulations were not considered. ER, extended release; HCL, hydrochloride; IR, immediate release; SR, slow release.
Patterns of opioid use
We described five drug receipt patterns: receipt of long‐acting agents; simultaneous receipt of multiple opioids; duration of dispensing greater than 30 days; a second opioid dispensing (assuming the second dispensing occurred within 120% and 200% of the days supplied by the index prescription); and early refills (defined as any opioid dispensed within ≤80% of the days supplied by the index prescription). The first three of these patterns of medication use have been previously characterized as ‘questionable’ for other sedating medications by Tamblyn et al. 39. We also identified the receipt of meperidine as the American Geriatrics Society Updated Beers Criteria strongly recommend that this specific opioid be avoided in older adults because of limited effectiveness and an increased risk of side effects 40. Receipt of opioids during COPD exacerbations requiring presentation to hospital was also identified, as exacerbations are times of respiratory status instability, and opioids are not indicated in COPD guidelines for the management of acute respiratory exacerbations 34, 41. Opioid use was characterized by the timing of drug receipt relative to the exacerbation (i.e. 7 days before an ER presentation or hospital admission, on the day of an ER presentation or discharge or hospital admission or discharge, and 7 days after an ER or hospital discharge for a COPD exacerbation). Finally, prescribing physician specialty was described.
Patient characteristics
We distinguished individuals by COPD exacerbation frequency status, as COPD exacerbation frequency is one important marker of disease severity. COPD exacerbation frequency is known to be associated with the severity of underlying airflow obstruction 42, the risk of future exacerbations 43 and mortality 44. Canadian 41 and newer global 34 COPD guidelines use COPD exacerbation frequency to distinguish COPD severity. We defined ‘frequent COPD exacerbators’ as individuals with a recent history of clinically significant COPD exacerbations – i.e. one or more ER visits or hospitalizations for COPD or pneumonia within the year prior to incident opioid receipt. We defined ‘infrequent COPD exacerbators’ as individuals with zero ER visits or hospitalizations for COPD or pneumonia within the year prior to incident opioid receipt. A cut‐off of one or more exacerbations in the preceding year was used, as this cut‐off is contained in Canadian COPD guidelines 41. Other patient characteristics that were examined are detailed in the Supplementary Appendix.
Statistical analysis
We estimated cumulative incidence function curves for incident opioid use that accounted for the competing risk of death. Descriptive statistics were used to describe patterns of opioid use among new users. Patterns of opioid use were also examined stratified by COPD exacerbation frequency status. All analyses were performed separately for those living in the community vs. in a long‐term care facility at index. Long‐term care residents tend to be frailer than the general population, and they also have different access to provider care, which are factors that might influence exposure to opioids. All analyses were performed using SAS statistical software (SAS version 9.3, SAS Institute, Cary, NC, USA).
Results
We identified a cohort of 107 109 community‐dwelling and 16 207 long‐term care resident older adults with COPD. Of these, 72 962 (68.1%) and 8811 (54.4%), respectively, received an incident opioid drug during the observation period. In the community‐dwelling group, the cumulative incidence of opioid receipt increased progressively over about 6 years following the start of observation, and then plateaued. The cumulative incidence functions were near‐identical for frequent and infrequent COPD exacerbators (Figure 1A). In the long‐term care resident group, the cumulative incidence of opioid receipt increased progressively over about 8 years following the start of observation and then started to plateau. The cumulative incidence of drug receipt was persistently greater among frequent COPD exacerbators vs. infrequent COPD exacerbators (Figure 1B).
Figure 1.
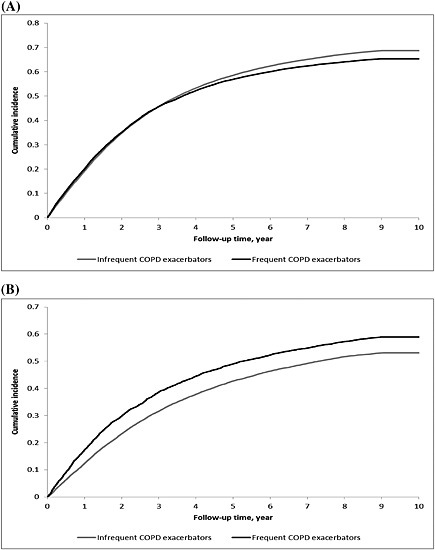
Cumulative incidence function curves for new opioid use among older adults with chronic obstructive pulmonary disease (COPD) from 2003 to 2012, distinguishing by COPD exacerbation frequency status, for: (A) community‐dwelling individuals; (B) long‐term care residents
Patient characteristics associated with new opioid use for the community‐dwelling group and for the long‐term care resident group are presented and described in the Supplementary Appendix. Among community‐dwelling individuals (even before covariate adjustment) and long‐term care residents (only after covariate adjustment), frequent COPD exacerbators had significantly reduced odds of new opioid receipt compared to infrequent COPD exacerbators.
Patterns of new opioid use for the community‐dwelling group and the long‐term care resident group are presented in Table 2. A detailed description of the patterns of new opioid use for both groups is presented in the Supplementary Appendix. In both groups, combination opioid/non‐opioid pills were the most frequently dispensed drug type. Multiple opioid dispensings, dispensings for >30 days' duration, second dispensings of opioids, early refills and opioids dispensed during COPD exacerbations were observed in both groups. These patterns of opioid use occurred with higher frequency among new users in the long‐term resident group compared to the community‐dwelling group.
Table 2.
Patterns of opioid dispensing among community‐dwelling and long‐term care resident older adult new users with COPD, distinguishing by COPD exacerbation frequency status
| Community‐dwelling group | Long‐term care resident group | |||||
|---|---|---|---|---|---|---|
| Total COPD | Infrequent COPD exacerbator | Frequent COPD exacerbator | Total COPD | Infrequent COPD exacerbator | Frequent COPD exacerbator | |
| n = 107 109 | n = 88 911 | n = 18 198 | n = 16 207 | n = 12 714 | n = 3493 | |
| Incident opioid dispensing (2003–2012) N (%) | 72 962 (68.1%) | 61 067 (68.7%) | 11 895 (65.4%) | 8811 (54.4%) | 6752 (53.1%) | 2059 (58.9%) |
| Type of incident narcotic dispensed N (%) | ||||||
| Long‐acting tablet/capsule | 1586 (2.2%) | 1233 (2.0%) | 353 (3.0%) | 374 (4.2%) | 277 (4.1%) | 97 (4.7%) |
| Short‐acting tablet/capsule (including meperidine/anileridine) | 4690 (6.4%) | 3616 (5.9%) | 1074 (9.0%) | 1240 (14.1%) | 919 (13.6%) | 321 (15.6%) |
| Meperidine/anileridine specifically | 481 (0.7%) | 382 (0.6%) | 99 (0.8%) | 34 (0.4%) | 26 (0.4%) | 8 (0.4%) |
| Opioid/non‐opioid combination tablet/capsule agent | 64 260 (88.1%) | 54 267 (88.9%) | 9993 (84.0%) | 6442 (73.1%) | 4981 (73.8%) | 1461 (71.0%) |
| Liquid formulation | 2146 (2.9%) | 1746 (2.9%) | 400 (3.4%) | 330 (3.7%) | 248 (3.7%) | 82 (4.0%) |
| Liquid formulation followed by a tablet/capsule dispensing | 245 (0.3%) | 188 (0.3%) | 57 (0.5%) | 56 (0.6%) | 43 (0.6%) | 13 (0.6%) |
| Transdermal formulation | 764 (1.0%) | 582 (1.0%) | 182 (1.5%) | 623 (7.1%) | 476 (7.0%) | 147 (7.1%) |
| Multiple opioid dispensing N (%) | 3, 080 (4.2%) | 2391 (3.9%) | 689 (5.8%) | 772 (8.8%) | 595 (8.8%) | 177 (8.6%) |
| Duration of incident dispensing | ||||||
| Mean (SD) | 10.3 ± 9.9 | 10.2 ± 9.8 | 10.6 ± 10.1 | 12.1 ± 9.0 | 11.9 ± 8.9 | 12.5 ± 9.1 |
| Median (IQR) | 7 (4–12) | 7 (4–12) | 7 (4–14) | 10 (7–15) | 10 (6–15) | 10 (7–15) |
| Proportion with script ≥30 days | 7, 688 (10.5%) | 6, 310(10.3%) | 1, 358 (11.4%) | 1, 359 (15.4%) | 1, 025 (15.2%) | 334 (16.2%) |
| Second dispensing N (%) | ||||||
| Within 120% of days of incident script supply | 7, 345 (10.1%) | 5, 954 (9.7%) | 1, 391 (11.7%) | 3, 076 (34.9%) | 2, 385 (35.3%) | 619 (33.6%) |
| Within 200% of days of incident script supply | 10 961 (15.0%) | 8883 (14.5%) | 2078 (17.5%) | 3765 (42.7%) | 2910 (43.1%) | 855 (41.5%) |
| Duration of incident + repeat dispensing * (assuming 120% of days of incident script supply) | ||||||
| Mean (SD) | 14.4 ± 28.8 | 14.2 ± 28.3 | 15.6 ± 30.9 | 36.5 ± 70.6 | 37.2 ± 72.2 | 34.3 ± 65.3 |
| Median (IQR) | 7 (4–15) | 7 (4–15) | 7 (5–15) | 10 (7–30) | 10 (7–30) | 10 (7–30) |
| Proportion with at least one script ≥30 days | 8730 (12.0%) | 7157 (11.7%) | 1573 (13.2%) | 1701 (19.3%) | 1279 (18.9%) | 422 (20.5%) |
| Duration of incident + repeat dispensing * (assuming 200% of days of incident script supply) | ||||||
| Mean (SD) | 15.5 ± 29.8 | 15.2 ± 29.4 | 16.8 ± 31.8 | 37.8 ± 70.6 | 38.4 ± 72.2 | 35.6 ± 65.2 |
| Median (IQR) | 7 (4–15) | 7 (4–15) | 8 (5–17) | 14 (7–30) | 14 (7–30) | 14 (7–30) |
| Proportion with at least one script ≥30 days | 9208 (12.6%) | 7535 (12.3%) | 1673 (14.1%) | 1746 (19.8%) | 1312 (19.4%) | 434 (21.1%) |
| Early refill N (%) | 4503 (6.2%) | 3679 (6.0%) | 824 (6.9%) | 2136 (24.2%) | 1656 (24.5%) | 480 (23.3%) |
| Prescriber N (%) | ||||||
| Family physician | 38 818 (53.2%) | 32 161 (52.7%) | 6657 (56.0%) | 7568 (85.9%) | 5771 (85.5%) | 1797 (87.3%) |
| Respirologist | 281 (0.4%) | 183 (0.3%) | 98 (0.8%) | ≤5† | ≤5† | 0 |
| Other specialist | 14 763 (20.2%) | 12 470 (20.4%) | 2293 (19.3%) | 519 (5.9%) | 420 (6.2%) | 99 (4.8%) |
| Unknown specialty | 19 629 (26.9%) | 16 700 (27.3%) | 2929 (24.6%) | 786 (8.9%) | 613 (9.1%) | 173 (8.4%) |
| Dispensing within the context of a COPD exacerbation: | ||||||
| Within 7 days before an ER visit or hospital admission for a COPD exacerbation N (%) | 1121 (1.5%) | n/a | 510 (4.3%) | 175 (2.0%) | n/a | 74 (3.6%) |
| On the date of an ER presentation or discharge for a COPD exacerbation N (%) | 360 (0.5%) | n/a | 149 (1.3%) | 15 (0.2%) | n/a | 9 (0.4%) |
| On the date of a hospital admission or discharge for a COPD exacerbation N (%) | 949 (1.3%) | n/a | 378 (3.2%) | 233 (2.6%) | n/a | 102 (5.0%) |
| Within 7 days after an ER or hospital discharge for a COPD exacerbation N (%) | 884 (1.2%) | n/a | 884 (7.4%) | 206 (2.3%) | n/a | 206 (10.0%) |
| Total during a COPD exacerbation (i.e. total of all the above) N (%) | 3108 (4.3%) | n/a | 1750 (14.7%) | 606 (6.9%) | n/a | 373 (18.1%) |
Repeat dispensings were counted for up to a 1 year period from the incident dispensing;
Percentages are not presented, according to Institute for Clinical Evaluative Sciences reporting rules, because of small cell size. COPD, chronic obstructive pulmonary disease; ER, emergency room; IQR, interquartile range; n/a, not available (we do not present data for opioid receipt during exacerbation for the infrequent exacerbation group as, by definition, they are experiencing zero exacerbations in the preceding year); SD, standard deviation.
Discussion
New use of opioids was found to be high among older Ontario adults with COPD between 2003 and 2012, occurring in over half of long‐term care residents and over two‐thirds of community‐dwelling individuals. Several patterns potentially indicative of excessive opioid use were seen among older adults with COPD and especially among frailer long‐term care residents.
Our estimate of new opioid use among community‐dwelling older adults with COPD is higher than previously reported prevalence estimates of opioid use in the general older adult community‐dwelling population (Canadian estimates range from 1.5% to 17% 1, 2, 3 and non‐Canadian estimates range from 3% to 17% 4, 5, 6). Opioids might be more frequently prescribed among older adults with COPD than in the general older adult population as chronic musculoskeletal pain 7, 8, 9 and insomnia 10, 11 are common problems in COPD, and as opioids can be prescribed to treat dyspnoea and cough that are refractory to traditional COPD pharmacotherapy 12, 13, 14. Our estimate of opioid use among older Ontario adults with COPD is also higher than a previously reported estimate among individuals in Sweden between 2005 and 2009 with advanced COPD receiving supplemental oxygen (23%) 32. The finding of higher opioid use in the present study vs. the Swedish study is noteworthy for two reasons. First, our data reflect incident use, whereas the Swedish study reflected prevalent use. Second, the Swedish study examined use among individuals with more severe COPD (in whom problems of pain, insomnia and refractory respiratory symptoms are even more common 8, 10), whereas the present study included individuals from the broad COPD population with varying disease severity. Compared to previous studies, the higher opioid use found in the present study might be related to the fact that we evaluated drug receipt over a longer follow‐up period and used more recent data (as there is evidence indicating that opioid use is rising progressively 6, 45, 46. Our cumulative incidence function curve estimates suggest that incident opioid use is increasing over time in the older adult COPD population as the curves plateaued relatively late during our observation period.
Frequent COPD exacerbators in both the community‐dwelling and long‐term care resident groups were less likely after adjustment to receive opioids, even though pain, insomnia and refractory respiratory symptoms occur more commonly among individuals with more advanced COPD 8, 10. Decreased opioid use among older adults with frequent COPD exacerbations might reflect concerns in the medical community regarding the use of these drugs from a respiratory safety perspective in this high‐risk population 47, 48, 49. We previously found greater use of benzodiazepines among older adults with frequent vs. infrequent COPD exacerbations 50. The findings of lower opioid use, but higher benzodiazepine use, among older adults with frequent exacerbations vs. infrequent exacerbations are interesting as opioids might have a role in mitigating refractory dyspnoea in advanced COPD 26, 27, 28, 29, 30, 31, whereas benzodiazepines do not 51. Our finding of relatively lower incident opioid use among long‐term care residents compared to the community‐dwelling individuals is somewhat surprising as others have previously reported the opposite 5. This might be explained by the fact that prescribers are concerned about adverse opioid‐related effects among more vulnerable long‐term care residents 47, 48, 49.
We found patterns of new opioid use among older adults with COPD that have the potential to augment possible negative drug‐related effects 16, 17, 18, 32, 33, including multiple opioid dispensings, dispensings for >30 days' duration, second dispensings, early refills and drug receipt during COPD exacerbations. These patterns of opioid use occurred with even higher frequency among community‐dwelling older adults with frequent COPD exacerbations and among long‐term care residents, which are two more vulnerable subgroups. Similar to previous reports of benzodiazepine use in the general 52 and COPD‐specific 50 older adult populations, the majority of new opioid use during exacerbations occurred either on the day of hospital presentation or discharge, or in the days following hospital discharge. Therefore, drug reconciliation during hospitalization 53, 54 may be a key process for optimizing opioid drug use in this population. The majority of opioid prescriptions in the present study population originated from family physicians, suggesting that they may play an important role in optimizing opioid drug use as well. The fact that opioid/non‐opioid combinations were the most frequently dispensed formulation, and that long‐acting opioid dispensing occurred with relatively low frequency, might in part be related to prescriber perceptions of the relative safety of different opioid formulation types. Alternatively, the high use of opioid/non‐opioid combinations (which contain acetaminophen or aspirin, in addition to the opioid) among older adults with COPD might reflect physician intention to use multi‐modal analgesic therapy to treat chronic musculoskeletal pain, which is a common problem in COPD 26, 27, 28. However, several recent Cochrane reviews have concluded that there is minimal good‐quality evidence supporting opioids as an effective treatment for chronic musculoskelatal pain 55, 56, 57.
There were several limitations to our study. First, our COPD definition, although highly specific, had a sensitivity of 58% 35. Although the high specificity of this definition allowed us to be confident that patients included in the present study truly had COPD, the lower sensitivity could render our findings less generalizable to the entire COPD population. Second, the health administrative databases lack patient‐level clinical data, such as lung function and respiratory symptoms, so measures of COPD severity beyond frequency of significant exacerbations were not available. Third, our results on opioid use in COPD are likely underestimated as we examined only incident, and not prevalent, use. Fourth, information on indication for opioid receipt was not captured in the drug database. Fifth, we were unable to assess opioid dose accurately as standing vs. pro re nata (p.r.n.) medication prescriptions are not delineated in the drug database. Individuals are also often given some flexibility regarding the frequency and dose of opioid taken, making it difficult, again, to quantify dose accurately. Finally, our present focus was on describing patterns of opioid use in the older adult COPD population. Examining for potential respiratory‐related health outcomes of opioid use among older adults with COPD will be undertaken next.
New opioid use was high among older adults with COPD. Potential safety concerns are raised by the degree and pattern of new opioid use observed because opioids can be associated with several adverse health outcomes 16, 17, 18, 32, 33. Further studies though are needed to clarify the clinical impact and safety of new opioid use in this older and respiratory‐vulnerable population.
Competing Interest
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: NTV had support from The Lung Association‐Canadian Thoracic Society National Grant Review/Grant‐In‐Aid for the submitted work; XW, HDF, ASG, CMB, SSG, PCA, ALS and PAR had no support from any organization for the submitted work; DO received grants and personal fees from Boehringer Ingelheim, grants and personal fees from Astra Zeneca, grants from GlaxoSmithKline, personal fees from Novartis, in the previous 3 years; NTV, XW, HDF, ASG, CMB, SSG, PCA, ALS and PAR had no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; NTV, XW, HDF, ASG, CMB, SSG, DO, PCA, ALS and PAR had no other relationships or activities that could appear to have influenced the submitted work.
This research was funded by a grant from The Lung Association‐Canadian Thoracic Society National Grant Review/Grant‐In‐Aid. This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI. Dr. Austin was supported in part by a Career Investigator Award from the Heart and Stroke Foundation (Ontario office).
Contributors
NTV, HDF, ASG, CMB, SSG, DEO, PCA, ALS and PAR were involved in the conception and design; NTV, XW, HDF, ASG, CMB, SSG, DEO, PCA, ALS and PAR in the analysis and interpretation; and NTV, XW, HDF, ASG, CMB, SSG, DEO, PCA, ALS and PAR in drafting the manuscript.
Supporting information
Appendix S1
Supporting info item
Vozoris, N. T. , Wang, X. , Fischer, H. D. , Gershon, A. S. , Bell, C. M. , Gill, S. S. , O'Donnell, D. E. , Austin, P. C. , Stephenson, A. L. , and Rochon, P. A. (2016) Incident opioid drug use among older adults with chronic obstructive pulmonary disease: a population‐based cohort study. Br J Clin Pharmacol, 81: 161–170. doi: 10.1111/bcp.12762.
References
- 1. Elby EM, Hogan DB, Fung TS. Potential adverse outcomes of psychotropic and narcotic drug use in Canadian seniors. J Clin Epidemiol 1997; 50: 857–63. [DOI] [PubMed] [Google Scholar]
- 2. Kelly KD, Pickett W, Yanniakoulias N, Rowe BH, Schopflocher DP, Svenson L, Voaklander DC. Medication use and falls in community‐dwelling older persons. Age Ageing 2003; 32: 503–9. [DOI] [PubMed] [Google Scholar]
- 3. Sadowski CA, Carrie AG, Grymonpre RE, Metge CJ, St JP. Access and intensity of use of prescription analgesics among older Manitobans. Can J Clin Pharmacol 2009; 16: e322–30. [PubMed] [Google Scholar]
- 4. Landi F, Onder G, Cesari M, Gambassi G, Steel K, Russo A, Lattanzio F, Bernabei R. Pain management in frail, community‐living elderly patients. Arch Intern Med 2001; 161: 2721–4. [DOI] [PubMed] [Google Scholar]
- 5. Jensen‐Dahm C, Gasse C, Astrup A, Mortensen PB, Waldemar G. Frequent use of opioids in patients with dementia and nursing home residents – a study of the entire elderly population of Denmark . Alzheimers Dement 2014; 11: 691–9. [DOI] [PubMed] [Google Scholar]
- 6. Steinman MA, Komaiko KD, Fung KZ, Ritchie CS. Use of opioids and other analgesics by older adults in the United States, 1999–2010. Pain Med 2015; 16: 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edmonds P, Karlsen S, Khan S, Addington‐Hall J. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliat Med 2001; 15: 287–95. [DOI] [PubMed] [Google Scholar]
- 8. Elkington H, White P, Addington‐Hall J, Higgs R, Edmonds P. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med 2005; 19: 485–91. [DOI] [PubMed] [Google Scholar]
- 9. Borge CR, Wahl AK, Moum T. Pain and quality of life with chronic obstructive pulmonary disease. Heart Lung 2011; 40: e90–101. [DOI] [PubMed] [Google Scholar]
- 10. Klink ME, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest 1987; 91: 540–6. [DOI] [PubMed] [Google Scholar]
- 11. Cormick W, Olson LG, Hensley MJ, Saunders NA. Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung disease. Thorax 1986; 41: 846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen‐Flaschen J, Heffner JE, Levy M, Mularski RA, Osborne ML, Prendergast TJ, Rocker G, Sibbald WJ, Wilfond B, Yankaskas JR; ATS End‐of‐Life Care Task Force . An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med 2008; 177: 912–27. [DOI] [PubMed] [Google Scholar]
- 13. Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri‐Kohlman V, Curtis JR, Manning HL, Mularski RA, Varkey B, Campbell M, Carter ER, Chiong JR, Ely EW, Hansen‐Flaschen J, O'Donnell DE, Waller A. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest 2010; 37: 674–91. [DOI] [PubMed] [Google Scholar]
- 14. Marciniuk D, Goodridge D, Hernandez P, Rocker G, Balter M, Bailey P, Ford G, Bourbeau J, O'Donnell DE, Maltais F, Mularski RA, Cave AJ, Mayers I, Kennedy V, Oliver TK, Brown C; Canadian Thoracic Society COPD Committee Dyspnea Expert Working Group . Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J 2011; 18: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elkington H, White P, Addington‐Hall J, Higgs R, Pettinari C. The last year of life of COPD: a qualitative study of symptoms and services. Respir Med 2004; 98: 439–45. [DOI] [PubMed] [Google Scholar]
- 16. Buckeridge D, Huang A, Hanley J, Kelome A, Reidel K, Verma A, Winslade N, Tamblyn R. Risk of injury associated with opioid use in older adults. J Am Geriatr Soc 2010; 58: 1664–70. [DOI] [PubMed] [Google Scholar]
- 17. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing 2011; 40: 23–9. [DOI] [PubMed] [Google Scholar]
- 18. Papaleontiou M, Henderson CR Jr, Turner BJ, Moore AA, Olkhovskaya Y, Amanfo L, Reid MC. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta‐analysis. J Am Geriatr Soc 2010; 58: 1353–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gershon AS, Wang C, Wilton AS, Raut R, To T. Trends in chronic obstructive pulmonary disease prevalence, incidence, and mortality in Ontario, Canada, 1996 to 2007: a population‐based study. Arch Intern Med 2010; 170: 560–5. [DOI] [PubMed] [Google Scholar]
- 20. Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 1999; 286: 1566–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin‐1 receptor‐expressing neurons mediate opioid‐induced respiratory depression. J Neurosci 2011; 31: 1292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lalley PM. Opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol 2003; 285: R1287–304. [DOI] [PubMed] [Google Scholar]
- 23. Weil JV, McCullough RE, Kline JS, Sodal IE. Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N Engl J Med 1975; 292: 1103–6. [DOI] [PubMed] [Google Scholar]
- 24. Adcock JJ, Schneider C, Smith TW. Effects of codeine, morphine and a novel opioid pentapeptide BW443C, on cough, nociception and ventilation in the unanaesthetized guinea‐pig. Br J Pharmacol 1988; 93: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vallejo R, de Leon‐Casasola O, Benyamin R. Opioid therapy and immunosuppression: a review. Am J Ther 2004; 11: 354–65. [DOI] [PubMed] [Google Scholar]
- 26. Woodcock AA, Gross ER, Gellert A, Shah S, Johnson M, Geddes DM. Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gases. N Engl J Med 1981; 305: 1611–6. [DOI] [PubMed] [Google Scholar]
- 27. Johnson MA, Woodcock AA, Geddes DM. Dihydrocodeine for breathlessness in “pink puffers”. Br Med J (Clin Res Ed) 1983; 286: 675–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax 2002; 57: 939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003; 327: 523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Currow DC, McDonald C, Oaten S, Kenny B, Allcroft P, Frith P, Briffa M, Johnson MJ, Abernethy AP. Once‐daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. J Pain Symptom Manage 2011; 42: 388–99. [DOI] [PubMed] [Google Scholar]
- 31. Rocker GM, Simpson AC, Young J, Horton R, Sinuff T, Demmons J, Donahue M, Hernandez P, Marciniuk D. Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: patients' experiences and outcomes. CMAJ Open 2013; 1: E27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ekström M, Bornefalk‐Hermansson A, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ 2014; 348: g445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khademi H, Malekzadeh R, Pourshams A, Jafari E, Salahi R, Semnani S, Abaie B, Islami F, Nasseri‐Moghaddam S, Etemadi A, Byrnes G, Abnet CC, Dawsey SM, Day NE, Pharoah PD, Boffetta P, Brennan P, Kamangar F. Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50,000 adults in Iran . BMJ 2012; 344: e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated2014). Available at http://www.goldcopd.com/uploads/users/files/GOLD_Report_2014_Oct30.pdf (last accessed 31 January 2015).
- 35. Gershon AS, Wang C, Guan J, Vasilevska‐Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. J COPD 2009; 6: 388–94. [DOI] [PubMed] [Google Scholar]
- 36. Levy R, O'Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol 2003; 10: 67–71. [PubMed] [Google Scholar]
- 37. Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long‐term analgesic use after low‐risk surgery: a retrospective cohort study. Arch Intern Med 2012; 172: 425–30. [DOI] [PubMed] [Google Scholar]
- 38. Vozoris NT, Fischer HD, Wang X, Stephenson AL, Gershon AS, Gruneir A, Austin PC, Anderson GM, Bell CM, Gill SS, Rochon PA. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2014; 44: 332–40. [DOI] [PubMed] [Google Scholar]
- 39. Tamblyn RM, McLeod PJ, Abrahamowicz M, Monette J, Gayton DC, Berkson L, Dauphinee WD, Grad RM, Huang AR, Isaac LM, Schnarch BS, Snell LS. Questionable prescribing for elderly patients in Quebec . CMAJ 1994; 150: 1801–9. [PMC free article] [PubMed] [Google Scholar]
- 40. The American Geriatrics Society 2012 Beers Criteria Expert Panel . American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60: 616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Donnell DE, Hernandez P, Kaplan A, Aaron S, Bourbeau J, Marciniuk D, Balter M, Ford G, Gervais A, Lacasse Y, Maltais F, Road J, Rocker G, Sin D, Sinuff T, Voduc N. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2008 update – highlights for primary care. Can Respir J 2008; 15S: 1A–8A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57: 847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal‐Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators . Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–38. [DOI] [PubMed] [Google Scholar]
- 44. Connors AF Jr, Dawson NV, Thomas C, Harrell FE Jr, Desbiens N, Fulkerson WJ, Kussin P, Bellamy P, Goldman L, Knaus WA. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996; 154: 959–67. [DOI] [PubMed] [Google Scholar]
- 45. Gomes T, Mamdani MM, Paterson JM, Dhalla IA, Juurlink DN. Trends in high‐dose opioid prescribing in Canada . Can Fam Physician 2014; 60: 826–32. [PMC free article] [PubMed] [Google Scholar]
- 46. Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in veterans health administration 2004–2012. J Gen Intern Med 2015; 30: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rocker G, Young J, Donahue M, Farquhar M, Simpson C. Perspectives of patients, family caregivers and physicians about the use of opioids for refractory dyspnea in advanced chronic obstructive pulmonary disease. CMAJ 2012; 184: E497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Young J, Donahue M, Farquhar M, Simpson C, Rocker G. Using opioids to treat dyspnea in advanced COPD: attitudes and experiences of family physicians and respiratory therapists. Can Fam Physician 2012; 58: e401–7. [PMC free article] [PubMed] [Google Scholar]
- 49. Janssen DJ, de Hosson SM, Bij de Vaate E, Mooren KJ, Baas AA. Attitudes toward opioids for refractory dyspnea in COPD among Dutch chest physicians. Chron Respir Dis 2015; 12: 85–92. [DOI] [PubMed] [Google Scholar]
- 50. Vozoris NT, Fischer HD, Wang X, Anderson GM, Bell CM, Gershon AS, Stephenson AL, Gill SS, Rochon PA. Benzodiazepine use among older adults with chronic obstructive pulmonary disease: a population‐based cohort study. Drugs Aging 2013; 30: 183–92. [DOI] [PubMed] [Google Scholar]
- 51. Simon ST, Higginson IJ, Booth S, Harding R, Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non‐malignant diseases in adults. Cochrane Database Syst Rev 2010; 1: CD007354. [DOI] [PubMed] [Google Scholar]
- 52. Bell CM, Fischer HD, Gill SS, Zagorski B, Sykora K, Wodchis WP, Herrmann N, Bronskill SE, Lee PE, Anderson GM, Rochon PA. Initiation of benzodiazepines in the elderly after hospitalization. J Gen Intern Med 2007; 22: 1024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gallagher PF, O'Connor MN, O'Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther 2011; 89: 845–54. [DOI] [PubMed] [Google Scholar]
- 54. Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L, Tulkens PM. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc 2007; 5: 658–65. [DOI] [PubMed] [Google Scholar]
- 55. Chaparro LE, Furlan AD, Deshpande A, Mailis‐Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low‐back pain. Cochrane Database Syst Rev 2013; 8: CD004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gaskell H, Moore RA, Derry S, Stannard C. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2014; 6: CD010692. [DOI] [PubMed] [Google Scholar]
- 57. Santos J, Alarcão J, Fareleira F, Vaz‐Carneiro A, Costa J. Tapentadol for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2015; 5: CD009923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Supporting info item


