Abstract
Olaparib is used to treat BReast CAncer susceptibility protein (BRCA)‐associated, platinum‐sensitive ovarian cancer. Olaparib inhibits poly(ADP‐ribose) polymerase, thereby blocking the repair of single‐strand DNA breaks. This results in synthetic lethality in BRCA‐associated cancer cells, which have a dysfunction of another DNA repair pathway – homologous recombination.
In this series, we draw attention to medicines that have entered the European market with an entirely new mechanism of action. Publication is not to be confused with endorsement of use in clinical practice. Copyright to the images belongs to Leiden University, but use of the images (also available at http://coo.lumc.nl/trc and the app stores) is free.
Introduction
Ovarian cancer is the fifth most common cancer in women in Europe 1. Early diagnosis is rare and although chemotherapy can be effective in the short term, the 5‐year survival rate is only 20%. Impaired function of the homologous recombination pathway, a DNA repair mechanisms involving BReast CAncer type 1/2 suspectibility protein (BRCA), is associated with higher sensitivity to platinum‐based chemotherapy 5. Germline BRCA mutation is present in about 20% of the cases of high‐grade serous ovarian cancer and a BRCA‐like phenotype is observed in roughly 50% of all cases 2.
Mechanism
Under normal conditions, single‐strand breaks (SSBs) in DNA are repaired by an error‐free, poly(ADP‐ribose) polymerase (PARP)‐mediated mechanism 3. When SSBs are present in the DNA during replication, double‐strand breaks (DSBs) can occur (Figure 1A). These are preferentially repaired by the BRCA 1/2‐mediated homologous recombination that is typically regarded as an error‐free repair mechanism, although recent reports suggest that it can be mutagenic 4.
Figure 1.
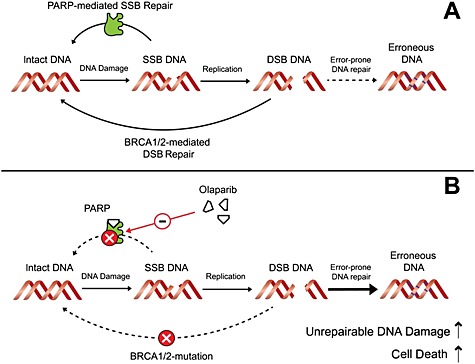
(A) DNA repair under normal conditions. Single‐strand breaks (SSBs) and double‐strand breaks (DSBs) are preferentially repaired by error‐free mechanisms that include poly(ADP‐ribose) polymerase (PARP) and BReast CAncer susceptibility protein (BRCA)1/2, respectively. (B) Synthetic lethality of olaparib in BRCA‐associated cancer cells. With both error‐free repair mechanisms blocked, DNA can only be repaired with more error‐prone repair mechanisms. The resulting build‐up of DSBs and erroneous DNA ultimately leads to cell death
Olaparib inhibits the PARP‐mediated error‐free repair of SSB, resulting in synthetic lethality in BRCA‐associated cancer cells as DNA is then repaired with more error‐prone repair mechanisms – single‐strand annealing and non‐homologous end joining 3. These alternative repair mechanisms are overwhelmed in the presence of large amounts of DNA damage – e.g. after treatment with genotoxic agents (Figure 1B). This triggers a build‐up of DSBs, erroneous DNA and, ultimately, cell death.
Indication
Olaparib is indicated in adult patients with high‐grade serous epithelial ovarian, fallopian tube and primary peritoneal cancer. The European Medicines Agency has approved olaparib for maintenance treatment of tumours that are both BRCA mutated and platinum sensitive (currently in response to last platinum therapy and ≥6 month duration of progression‐free survival after penultimate platinum therapy 6).
Clinical application
Maintenance treatment with olaparib improved median progression‐free survival in a Phase II trial with patients with platinum‐sensitive BRCA‐mutated serous epithelial ovarian, fallopian tube or primary peritoneal cancer (11.2 months vs. 4.3 months for placebo) 7. However, no significant difference in overall survival was observed between the treatments. Maintenance treatment with olaparib should begin no later than 8 weeks after the final dose of platinum therapy. Patients are directed to take 400 mg orally twice daily, at least 1 hour after food. It is recommended that treatment with olaparib is stopped upon disease progression 6.
Adverse effects
Olaparib monotherapy is generally well tolerated, with adverse effects generally of mild‐to‐moderate severity. Treatment discontinuation was not frequent in clinical trials. The most commonly observed side effects (>10%) are fatigue, nausea, vomiting, diarrhoea, dyspepsia, headache, altered taste, decreased appetite and dizziness. An increase in serum creatinine was also observed in >10% of patients 6. Haematological toxicity (anaemia, neutropenia, thrombocytopenia and lymphopenia) has been reported (>10%) in patients treated with olaparib, therefore, patients should not start treatment with olaparib until they have recovered from any previous chemotherapy‐related haematological abnormalities. Regular blood counts are advised as a risk management strategy 1. Uncommon serious adverse effects included myelodysplastic syndrome, acute myeloid leukaemia and pneumonitis.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Goulooze, S. C. , Cohen, A. F. , and Rissmann, R. (2016) Olaparib. Br J Clin Pharmacol, 81: 171–173. doi: 10.1111/bcp.12761.
References
- 1. European Medicines Agency . Summary of the risk management plan (RMP) for Lynparza (olaparib) [online]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Risk‐management‐plan_summary/human/003726/WC500176260.pdf (last accessed 21 February 2015).
- 2. Clamp A, Jayson G. PARP inhibitors in BRCA mutation‐associated ovarian cancer. Lancet Oncol 2015; 16: 10–2. [DOI] [PubMed] [Google Scholar]
- 3. Javle M, Curtin NJ. The role of PARP in DNA repair and its therapeutic exploitation. Br J Cancer 2011; 105: 1114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodgers K, McVey M. Error‐prone repair of DNA double‐strand breaks. J Cell Physiol 2016; 231: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fong PC, Yap TA, Boss DS, Carden CP, Mergui‐Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S, Messiou C, A'Hern R, Tutt A, Ashworth A, Stone J, Carmichael J, Schellens JH, de Bono JS, Kaye SB. Poly(ADP)‐ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum‐free interval. J Clin Oncol 2010; 28: 2512–9. [DOI] [PubMed] [Google Scholar]
- 6. European Medicines Agency . Lynparza: EPAR – product information [online]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/003726/WC500180151.pdf (last accessed 20 February 2015).
- 7. Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott CL, Meier W, Shapira‐Frommer R, Safra T, Matei D, Fielding A, Spencer S, Dougherty B, Orr M, Hodgson D, Barrett JC, Matulonis U. Olaparib maintenance therapy in patients with platinum‐sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014; 15: 852–61. [DOI] [PubMed] [Google Scholar]


