Abstract
Aims
Intrathecal baclofen (ITB) has proven to be an effective and safe treatment for severe spasticity. However, although ITB is used extensively, clinical decisions are based on very scarce pharmacokinetic–pharmacodynamic (PKPD) data. The aim of this study was to measure baclofen CSF concentrations and clinical effects after administration of various ITB boluses in patients with spasticity and to create a PKPD model for ITB.
Methods
Twelve patients with severe spasticity received four different bolus doses of ITB (0, 25, 50, 75 μg and an optional dose of 100 μg), administered via a catheter with the tip at thoracic level (Th) 10. After each bolus, 10 CSF samples were taken at fixed time intervals, using a catheter with the tip located at Th12. Clinical effect was assessed by measuring spasticity with the Modified Ashworth Scale (MAS). These data were used to develop a PKPD model.
Results
All patients achieved an adequate spasmolytic effect with ITB doses varying from 50 to 100 μg. No serious side effects were observed. CSF baclofen concentrations, as well as the clinical effects, correlated significantly with ITB doses. The PK model predicted a steep spinal concentration gradient of ITB along the spinal axis. The clinical effect could be predicted using a delayed‐effect model.
Conclusions
ITB is an effective and safe therapy with, however, a steep concentration gradient along the spinal axis. This means that the administered baclofen is staying mainly around the catheter tip, which stresses the importance to position the ITB catheter tip closely to the targeted spinal level.
Keywords: baclofen; injections, spinal; pharmacokinetics; pharmacology
What is Already Known About this Subject
Intrathecal baclofen (ITB) is an effective and safe treatment for severe spasticity.
Animal studies have shown that there is a steep gradient of baclofen along the spinal axis.
Pharmacokinetic and pharmacodynamic (PKPD) data are hardly available to justify clinical decisions and treatment strategies.
What this Study Adds
A novel PKPD model for ITB was created, using clinical data gathered in patients with severe spasticity.
The model confirms a steep spinal concentration gradient of baclofen along the spinal axis.
This finding stresses the importance to position the ITB catheter tip closely to the targeted spinal level.
Introduction
Baclofen is the most widely used spasmolytic agent. It is a muscle relaxant with its prime site of action in the spinal cord, where it binds to the inhibitory GABA‐B receptor 1. The uptake of baclofen across the blood–brain barrier is limited. Therefore high oral doses (60–100 mg day–1) are needed to achieve a therapeutic effect, often causing side effects such as drowsiness and sleepiness 2. In 1984, Penn & Kroin bypassed the blood–brain barrier by infusing baclofen directly into the cerebrospinal fluid (CSF), using a subcutaneous programmable pump, which was connected to an intrathecal lumbar drain 3. In intrathecal baclofen (ITB) therapy, low doses of baclofen (25–1000 μg day–1) produce high spinal concentrations, resulting in a good spasmolytic effect and few side effects.
The long term effects and safety of ITB therapy have been investigated extensively in the last two decades. However, very little pharmacokinetic and pharmacodynamic (PKPD) data are available to justify clinical decisions. Many clinical questions remain unanswered, like what is the preferable catheter tip location in a specific patient?, which infusion regimen (continuous or with intermittent boluses) should be used? and what are the predictive factors in the development of tolerance and how can it be treated? Many of these questions are related to the distribution of baclofen after administration into the CSF. What is the concentration gradient of ITB, related to the administered dose and the way the dose is administered? Animal studies have shown that there is a steep gradient of baclofen along the spinal axis, meaning that most ITB seems to remain around the catheter tip after infusion 4. However, ITB distribution along the spinal canal has never been studied properly in humans.
The aim of this study was to measure baclofen CSF concentrations and clinical effects at various intervals after the administration of four different bolus doses of ITB in patients with spasticity, using two different intrathecal catheters with the tips at different spinal levels (one catheter being used for ITB infusion and one for sampling), in order to create a PKPD model for ITB infusion in humans.
Methods
This study was approved by the Ethical Review Board of the University Medical Center Groningen (Protocol Number: METc 2008.198). Informed consent was obtained from each subject.
Subjects
Inclusion criteria were the same as those for a regular ITB test infusion: patients suffering from severe spasticity, which could not be treated sufficiently with oral antispastic therapy, due to a lack of efficacy or adverse effects. Exclusion criteria were pregnancy, an age below 18 years, and the presence of contra‐indications for intrathecal catheter placement (elevated risk of bleeding, elevated intracranial pressure). Twelve patients with spasticity of various aetiology were included in this study (Table 1) from November 2009–July 2011.
Table 1.
Patient characteristics
| Patient | Age (years) | Gender | Aetiology | Height (cm) | Weight (kg) | Oral baclofen (mg day–1) |
|---|---|---|---|---|---|---|
| 1 | 59 | M | SCI C4–C5 | 167 | 77 | 90 |
| 2 | 58 | F | MS | 165 | 57 | 60 |
| 3 | 59 | M | SCI C4–C5 | 178 | 94 | 90 |
| 4 | 50 | M | MS | 180 | 63 | 80 |
| 5 | 35 | M | MS | 198 | 100 | 90 |
| 6 | 38 | M | CP | 175 | 65 | 125 |
| 7 | 35 | F | CP + SCI C5–C6 | 165 | 65 | 0 |
| 8 | 53 | M | Stroke | 193 | 90 | 0 |
| 9 | 63 | M | SAH | 176 | 85 | 75 |
| 10 | 41 | M | MS | 170 | 80 | 80 |
| 11 | 47 | F | MS/Myelitis Th6 | 175 | 64 | 50 |
| 12 | 38 | F | CP | 160 | 75 | 30 |
CP, cerebral palsy; MS, multiple sclerosis; SAH, subarachnoid haemorrhage; SCI, spinal cord injury.
Intrathecal catheter placement
All patients received two intrathecal catheters. One catheter was used for the administration of ITB, the other for CSF sampling. The catheters were identical to those used during ITB pump‐implantation (Intrathecal Catheter Spinal Segment Revision Kit 8598‐A; Medtronic, Minneapolis, USA). Both catheters were inserted at different lumbar levels using a 15 gauge Tuohy needle. The catheter tips were located inside the spinal canal, using the radiopaque tip and X‐ray guidance. The tip of the ITB administration catheter was placed at spinal level Th10 (at the upper endplate of the vertebra), which is the standard position of the ITB catheter tip in our centre. The spinal segments of the lower limbs are located at this level of the spinal cord. The tip of the sampling catheter was placed at the upper level of Th12. The two segment distance between administration and sampling was based on a pig model, which showed that an appropriate ITB concentration–time curve could be created, using a sampling catheter at least 5 cm below the level of administration 4. Based on the human anatomy, our catheter tips were separated 58 ± 6 mm from each other 5. No previous human data are available using this two catheter approach.
Drug administration
Baclofen (3RS)‐4‐amino‐3‐(4‐chlorophenyl)butanoic acid (Excella GmbH, Feucht, Germany; European Pharmacopoeia quality) was used to prepare the baclofen intrathecal ampoules (50 μg ml–1, ampoule 5 ml, made isotonic with 0.9% NaCl). The ampoules were manufactured by the hospital pharmacy of the University Medical Center Groningen, which has a manufacturing license for drugs in clinical trials. ITB was infused by a Crono Five infusion pump (CANE medical technologies, Turin, Italy). All patients received at random four different doses (0, 25, 50 and 75 μg) of ITB, on 4 different days. The sequence of doses was randomized by the hospital pharmacy in a double blind setting for patients and physicians. The ITB doses were administered by a physician who was not involved in the assessment of the clinical effect. Only patients who did not show an adequate response on the 75 μg dose, received an extra 100 μg dose of ITB.
Sampling protocol
All CSF samples were collected from the sampling catheter at Th12, which was used for sampling only. Each sample was taken after having cleaned the catheter with 0.5 ml of CSF. The sample itself consisted of 0.5 ml CSF. Samples were taken according a fixed time schedule: baseline, 10 min, 20 min, 40 min, 1 h, 1.5 h, 2 h, 4 h, 8 h, 12 h and 24 h. All samples were stored at −18°C, before being analyzed.
CSF baclofen concentration analysis
Baclofen was analyzed at the Laboratory for Therapeutic Drug Monitoring and Clinical Toxicology, Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, The Netherlands. Analysis was performed on a triple Quadrupole LC‐MS/MS system, with a C18 column held at 20°C. The HPLC column was a Thermo Electron HyPurity Aquastar (50 × 2,1 mm 5 μm), using 4‐chloro‐DL‐phenylalanine in 10% trichloroacetic acid as an internal standard. A HPLC Surveyor MS pump (Thermo Scientific), a Surveyor plus® autosampler with an integrated column oven (Thermo Scientific) as well as the TSQ Quantum detector were used. The mobile phase consisted of an aqueous buffer, water and acetonitrile. The aqueous buffer consisted of ammonium acetate, acetic acid and trifluoroacetic acid anhydride. Chromatographic separation was performed by means of a gradient with a flow of 0.3 ml min–1 and a runtime of 3.6 min. Xcalibur software was used for quantification of the results. The analytical method validation was based on the FDA guidelines for bioanalytical method validation 6. The lower limit of quantification (LLOQ) of the baclofen analysis was 2.0 μg l–1 with an overall bias of 3.3% and overall coefficient of variation of 16.1% during validation. The highest limit of quantification (HLOQ) of the baclofen analysis was validated at 1500 μg l–1 with an overall bias of −7.6% and overall coefficient of variation of 3.4% during validation. Baclofen proved to be stable in CSF at 22°C for 73 h and as processed sample in the autosampler at 22°C for 78 h.
Clinical assessments
The clinical effect was assessed by measuring spasticity with the Modified Ashworth Scale (MAS). The MAS is a six point ordinal scale ranging from no increase in muscle tone (MAS 0) to severe spasticity (MAS 5), with the affected parts rigid in flexion or extension. The MAS has shown an adequate inter‐rater reliability 7. Clinical effects were assessed at baseline and after 1, 2, 4 and 8 h after each ITB dose. MAS scores were measured for hip adduction and abduction, hip, knee and ankle flexion and extension. All scores for both legs were summed together resulting in the total MAS score. Although each MAS is scored on an ordinal scale, the total MAS score was considered as a continuous variable in the PD analysis.
PKPD modelling of ITB
So far, no validated human PKPD model is available for drug delivery in the intrathecal space. We used our PKPD data to develop such a model for ITB therapy.
Modelling the intrathecal space
Administration of ITB in pigs resulted in a steep concentration gradient of baclofen along the spinal axis, indicating that most of the infused baclofen stayed around the catheter tip 4. An intrathecal model should be able to describe this spinal gradient. Shafer et al. suggested a diffusion/distribution model, describing the intrathecal drug flow based on diffusion within the CSF and distribution from the CSF into the tissue of the spinal column 8. This model divides the intrathecal space in a number of compartments which are connected serially. The parameters for drug distribution are the same for each level, providing the model with a fixed number of parameters, independent of the number of compartments. Furthermore, the compartment for drug injection and the sampling compartment can be chosen, making it possible to calculate a concentration gradient with data from just one sampling location. Therefore this diffusion/distribution model was used as a basis for our ITB pharmacokinetic (PK) model 8.
ITB pharmacokinetic model
Our ITB PK model (Figure 1) divides the CSF in a series of compartments (V1). Each compartment corresponds with 1 cm of the intrathecal space. The mean human spine length (C3–L5) is known to be 57 cm 5. Therefore the model was configured as a series of 57 compartments (n = 57), number 1 being the most rostral, and number 57 being the most caudal compartment. Based on this model, baclofen administration was calculated to take place in compartment 30 (C3–Th10 = 29.7 cm), while CSF samples were taken from compartment 36 (C3–Th12 = 35.6 cm) 5.
Figure 1.
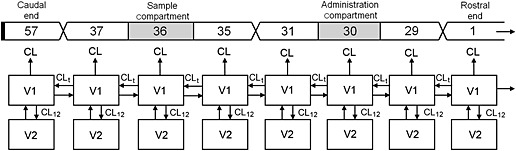
Pharmacokinetic model for intrathecal baclofen, based on the diffusion–distribution model from Shafer & Shafer 8. The intrathecal space (V1) is distributed in a series of 57 connected compartments. Each compartment communicates with the adjacent tissue (V2). The caudal end of the model is closed, the rostral end is open
ITB distributes between the CSF compartments with a distribution clearance CLt. To incorporate the diffusion of baclofen from the CSF into the spinal column, each CSF compartment (V1) is connected to a tissue compartment (V2). Distribution between these compartments is described by distribution clearance CL12. Since baclofen diffusion within the spinal cord is thought to be limited, there is no distribution constant between the neighbouring tissue compartments in our model.
Clearance of ITB is thought to take place through absorption of CSF and its constituents by the arachnoid villi, which are located in the spinal and cerebral intrathecal space 9. The spinal clearance is incorporated in the model by a one way flow (CL) out of each CSF compartment (V1), while cerebral clearance is represented by an additional one way flow (CLt) out of the most rostral (first) compartment. The model is closed at the caudal end, resembling the closed caudal dural sac.
The baclofen concentration as a result of the oral doses of baclofen was included in the model as a constant baseline baclofen concentration (C(0)).
PK modelling
The ITB PK model was programmed in MW\Pharm (version 3.80; MwPharm, Zuidhorn, the Netherlands), a computer‐aided therapeutic drug monitoring program, which can be used for pharmacokinetic modelling 10. The program was altered specifically for this study. A pharmacokinetic profile was programmed, based on our ITB PK model (Figure 1). Dosage regimen, sampling times and observed baclofen CSF concentrations were imported for each patient. A population pharmacokinetic analysis was performed using a Bayesian iterative two stage procedure, assuming a log‐normal distribution of inter‐individual variability. All parameters were considered Bayesian, except for C(0) (baseline baclofen concentration) since this parameter does not have typical population properties and may differ per patient due to the different oral doses of baclofen used as regular medication of the participating patients. The residual error was fixed at 10 μg l–1 + 0.1 × concentration (μg l–1). Both individual and population PK parameters were estimated.
PKPD modelling
In the PKPD analysis the observed concentration (C obs) was used as the driving force for the drug effect 11. The observed MAS scores and C obs values were used as the datafile for PKPD analysis by non‐linear mixed effects modelling using NONMEM 7.2.0 (Icon Development Solutions, Hanover, MD, USA) with first order conditional estimation (FOCE), interaction, and subroutine ADVAN6. NONMEM was running within PLTTools (PLTsoft, San Francisco, CA, USA).
Because the time profile of the MAS score lags behind the baclofen concentration in the sample compartment (see Figure 4), the drug effect was assumed to be related to a hypothetical effect compartment linked to the concentration in the sample compartment by
| (1) |
Figure 4.
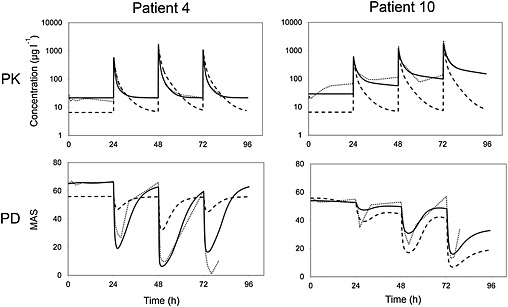
CSF baclofen concentration (upper) and MAS (lower) vs. time after administration of three doses of ITB in two patients. The dotted line represents the observed concentration (upper) and MAS (lower), the solid line the individual predicted fit and the dashed line the population predicted fit
where C e is the concentration in the effect compartment, C s the concentration in the sample compartment and k se the transfer rate constant.
The time profile of C s was calculated by linear interpolation between the observed baclofen concentrations (C obs), assuming a constant concentration between the last observed concentration before the next dose and the time of the next dose. In all cases the last observed concentration was at least 23 h after dosing 11. C s was calculated for each patient at the time points of dosing, CSF concentration measurements and MAS measurements, as well as at intermediate time points every 1 min during the first 30 min after each dose, every 2 min during the next 30 min, every 5 min during the next 1 h every, 15 min during the next 2 h and every hour over the remaining period.
The drug effect E, i.e. MAS score, was modelled according to the inhibitory sigmoid Emax model:
| (2) |
where E0 is the baseline MAS score in the absence of baclofen, Emax is the MAS score at maximum drug effect, EC 50 is the baclofen concentration at 50% of the maximum effect, and γ is a slope parameter reflecting the steepness of the concentration–effect relationship.
Model building was performed starting with the simplest form of the model (i.e., γ = 1, Emax = 0 (i.e. MAS score = 0 at maximum drug effect), no inter‐individual variability (IIV), additive residual error model), and expanding the model with an estimated value for γ, a separate value for Emax, or IIV for each parameter separately, assuming a log‐normal distribution, until the decrease of the objective function value was not statistically significant using the chi‐square test with P = 0.05 for one additional model parameter, and P = 0.01 for one additional IIV. Also, a proportional error model was tested. The model building was continued by stepwise reducing the model by the constraint γ = 1 or Emax = 0, or by fixing IIV to zero, using the chi‐square test with P = 0.01 for model parameter, and P = 0.001 for IIV (backward elimination).
The final models were accepted as a valid result only if both minimization and covariance steps were successful. The goodness‐of‐fit for the final models was also assessed by visual inspection of the predicted vs. observed plots and the distribution of residual (weighted) errors.
To estimate the confidence intervals of the final model parameters, a bootstrap analysis was performed, based on 2000 sets of 11 patients each, randomly selected from the available 11 patients.
Results
In total 12 patients were included in the study. None of the patients experienced any serious complications or side effects. Most patients experienced a good clinical effect after the 75 μg dose. Two patients (#6 and #9) required an extra 100 μg dose, which resulted in adequate spasmolysis in both patients. These 100 μg data were included in the PKPD analysis. Two patients had some missing data, patient 1 (25 μg sampling) due to dysfunction of the sampling catheter and patient 3 (baseline sampling) because of an administrative error.
CSF baclofen concentration
The concentration–time curves show the measured baclofen CSF concentrations at spinal level Th12, approximately 6 cm below the level of ITB administration at Th10 (Figure 2). Each curve represents one patient. The baseline (0 μg) graph shows the CSF concentrations as a result of continued oral baclofen doses. Only two patients did not use oral baclofen. The oral doses of baclofen ranged between 30 and 125 mg day–1 (Table 1) resulting in CSF baclofen concentrations varying from 5 to 100 μg l–1. In eight out of 10 patients the CSF baclofen did not reach concentrations above 30 μg l–1.
Figure 2.
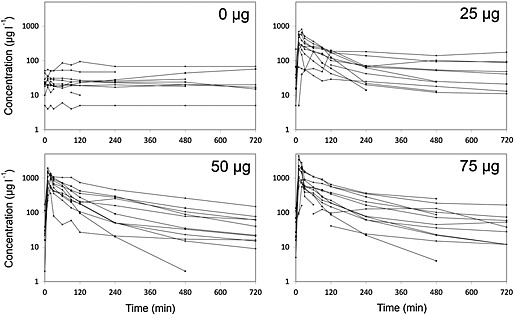
CSF baclofen concentration vs. time after the administration of different doses of ITB
A positive correlation was shown between ITB doses (25, 50 and 75 μg) and mean peak concentrations of baclofen (403, 1145 and 1700 μg l–1, respectively). The concentration mostly peaked after 10 min (62%) or 20 min (24%), whereas the curve fell off in a bi‐exponential manner (Figure 2). The measured CSF baclofen concentrations showed large inter‐individual differences for each of the three ITB doses.
MAS scores
Figure 3 shows the effect of ITB over time, as measured by the MAS. Each curve represents one patient. Patient 8 was excluded from the PD analysis because the MAS score was influenced by an unaffected left side, as a result of a right spastic hemiparesis after stroke. The baseline graph shows the MAS scores resulting from oral (or no) baclofen. The ITB doses (25, 50 and 75 μg) showed a positive correlation with the mean improvement of MAS scores after 4 h (21%, 50% and 57%, respectively), although in two patients maximal effect was already measured after the 50 μg dose. All doses resulted in an improvement of MAS scores within 1 h, with a maximal effect after 2–4 hours. Eight hours after the 25 μg dose, most patients had returned to their baseline MAS, while the effect lasted longer (up to 12 h) with higher doses. All patients were satisfied about the effect, resulting in ITB pump implantation in all cases.
Figure 3.
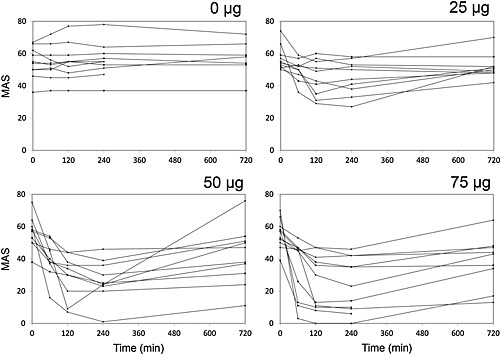
MAS vs. time after the administration of different doses of ITB
Pharmacokinetic modelling
During the model development process various variants of the model were tested, aiming to keep the Akaike information criterion (AIC) as low as possible, while keeping the model as simple as possible. A one compartment model (removing V2) increased the AIC and was therefore discarded. Decreasing (n = 29) and expanding (n = 114) the number of consecutive compartments resulted in a higher and comparable AIC, respectively. The addition of parameters for clearance from the tissue compartment (V2) and/or transfer clearance between adjacent V2 compartments did not result in a better fit, and were left out to keep the number of parameters minimal.
The population PK parameters of the final ITB PK model (Figure 1) are shown in Table 2. No bootstrap analysis was performed due to the very long runtime.
Table 2.
Parameters of the ITB PK model
| Mean | SD | ||
|---|---|---|---|
| C(0) | Baseline baclofen CSF concentration (μg l–1) | 6.6 | 26.0 |
| V1 | Volume of the intrathecal compartment (l) | 0.116 | 0.122 |
| V2 | Volume of the tissue compartment (l) | 0.083 | 0.093 |
| CL | Clearance from the intrathecal compartment (l h–1) | 0.055 | 0.060 |
| CLt | Clearance between adjacent intrathecal compartments (l h–1) | 4.5 | 6.3 |
| CL12 | Clearance between intrathecal and tissue compartments (l h–1) | 0.036 | 0.012 |
n = 57; dosing‐compartment #30; sample compartment #36; values for volumes and clearances refer to the total of intrathecal and tissue compartments (for each of the n compartments the actual value is multiplied by 1/n).
Figure 4 shows the observed concentrations with both the individual predicted fit and the population predicted fit of the final PK model in two patients (#4 and #10). Diagnostic plots of the goodness‐of‐fit can be found in the Supporting Information (Figures S1–S3).
The model‐predicted concentration–time profiles of each of the 57 compartments, i.e. for all spinal levels, were calculated during the total duration of the study for each patient, as shown for patients 4 and 10 in Figure 5 after administration of the 50 μg dose. Immediately after injection, the predicted peak concentration around the catheter tip of these patients lies around 25 000 μg l–1 (very high, due to the concentrated injection of baclofen) which drops to 3000–4000 μg l–1 within a few minutes. Compared with the latter, predicted peak concentration at 6 and 12 cm distance drop to 30–35% (1100 μg l–1) and 10–15% (450 μg l–1), respectively. The model predicts that baclofen concentrations decrease about three times every 6 cm, resulting in a steep baclofen CSF concentration gradient along the spinal axis. Baclofen diffuses from the catheter tip, causing the gradient to decrease over time. However, even after 2 h, the baclofen concentration around the catheter tip is still roughly 5–10 times higher as compared with the rostral end of the spinal axis. The asymmetrical shape of the curves results from the open rostral end of the model, causing baclofen to be cleared from the spinal CSF into the cranial CSF, thereby lowering the baclofen concentration at the rostral end much more as compared with the closed caudal end.
Figure 5.
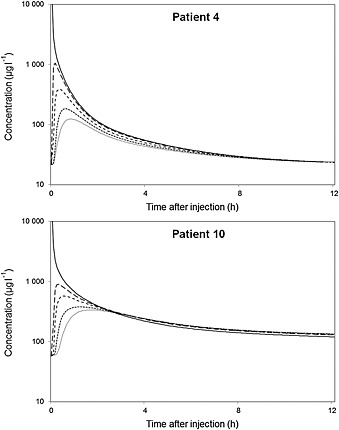
Predicted CSF baclofen concentration–time profile at different distances below the catheter tip after administration of 50 μg for patient 4 and 10. — 0 cm (administration), – – 6 cm (sample), ‐‐‐ 12 cm, ‐‐‐‐‐ 18 cm, ········· 24 cm
PKPD modelling
The parameter Emax was found to be close to 0, and fixed Emax to zero did not worsen the fit significantly. Therefore the final models have an Emax value of 0, implying that the predicted MAS score at high baclofen concentrations is 0, which is in accordance with the observed MAS scores in this study. The additive error model resulted in the lowest OFV and acceptable residuals plots, which was therefore used in the final model. The parameters of the PKPD analysis are shown in Table 3, including the results of the bootstrap analysis. Figure 4 shows the MAS vs. time with both the individual predicted fit and the population predicted fit of the final PKPD model in two representative patients (#4 and #10). Diagnostic plots of the goodness‐of‐fit can be found in the Supporting Information (Figures S4–S9).
Table 3.
Parameters of the pharmacodynamics analysis
| PKPD modelling | Bootstrap analysis | |||
|---|---|---|---|---|
| Parameter | Estimate | SE | IIV | 95% CI |
| EC50 (μg l–1) | 194 | 50 | 76% | 112, 350 |
| Slope | 2.26 | 0.35 | * | 1.27, 3.89 |
| E0 | 56.3 | 3.2 | 20% | 49.8, 62.7 |
| Emax | 0 | FIXED | ||
| ke0 (h−1) | 0.178 | 0.031 | * | 0.096, 0.231 |
| Residual variability | 6.87 | 0.56 | * | 5.56, 7.87 |
No IIV was identified for this parameter. IIV, inter‐individual variability; CI, confidence interval; SE, standard error.
The PKPD analysis was also performed using the predicted concentration (C pred) from the PK analysis. This resulted in the same structural model, the same significant inter‐individual variability, and the same residual error model. However, parameters were different (e.g. EC 50: 135 vs. 194 μg l–1).
Finally the population predicted values of the PK analysis were used in the PD population analysis, resulting in a final PKPD population prediction based on the ITB PKPD model. Figure 6 shows the population predicted PKPD curves after administration of four doses of ITB.
Figure 6.
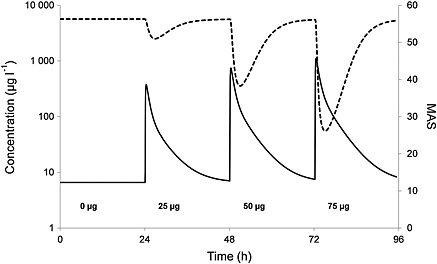
Population prediction for PK (baclofen concentration in sample compartment) and PD (MAS) after four doses of baclofen, based on the PKPD model for ITB. — population predicted PK, ‐‐‐ population predicted PD
Discussion
This is the first human study presenting detailed ITB concentration–time curves after different bolus doses in patients. It is also the first human study presenting a PKPD model for ITB.
CSF baclofen concentrations
The results described are based on bolus doses of baclofen, which cannot be extrapolated to continuous dosing 4. The concentration–time curves (Figure 2) showed a positive correlation between ITB bolus dose and CSF baclofen concentrations. Peak concentrations mostly appeared within the first 20 min after administration, irrespective of the dose. The measured concentrations showed large inter‐individual differences for each of the three ITB doses. This can be explained by the relatively small and heterogeneous patient population. Other studies report large inter‐individual differences in intrathecal concentrations as well, which is likely due to anatomical differences between patients 12, 13, 14. It is difficult to compare these data with other studies, because of large differences in dosing, administration and sampling 12, 13, 14. Despite these problems there is a good match with a concentration–time curve from one study, in which a peak concentration of 1100 μg l–1 was measured in the first sample after 60 min, also decreasing bi‐exponentially, dropping below 100 μg l–1 after 8–10 h 13. These results match the 75 μg curves (Figure 2) quite well, considering the slightly higher dose. The CSF baclofen concentration generated by oral baclofen intake remained below 30 μg l–1 in eight out of 10 patients (baseline curves in Figure 2). These results are comparable with previous data, measuring CSF baclofen concentrations after oral baclofen (30–90 mg day–1), showing baclofen CSF concentrations below 30 μg l–1 in nine out of 11 patients, while concentrations above 100 μg l–1 were never reached 15.
Clinical efficacy
A positive correlation between MAS improvement and ITB dose was shown. The MAS scores at baseline were fairly constant, indicating the absence of a clinically relevant placebo effect, in contrast to another study, measuring a clear placebo effect after administration of normal saline 16.
In all patients, the first effect was measured 1 h after the ITB bolus administration. However, the latency of onset could have been shorter. Maximal effect was measured after 2–4 h and in most patients spasticity started to return 8 h after administration, which may guide the timing of bolus regimens in clinical practice. After 24 h spasticity was back at baseline level in all patients. Other studies report similar results, with the first effects occurring within 1–2 h, maximum effect after 4–6 h, with a total effect lasting 6–16 h after ITB bolus administration 13, 16, 17. The variety in clinical effect may be explained by the heterogeneity of the population with respect to severity of spasticity and pathophysiology.
The MAS scores lagged behind the measured ITB concentrations (Figure 4), which likely reflects the time baclofen needs to diffuse from the CSF into the spinal cord.
PK model
The individual predicted PK fit followed the observed concentrations well, whereas the population prediction was less accurate, which is likely a result of large inter‐individual variations in the distribution of baclofen within the CSF, supported by the large SD of the model parameters. This large variation might be explained by multiple anatomical (height, weight, gender) and physiological variables (blood pressure, CSF density), although we could not find any significant covariates. Since this is the first ITB PK model reported in the literature, it is not possible to compare our data with other studies. However, it is interesting to compare some PK parameters with spinal anatomy. For example, V1 (0.116 l) corresponds fairly well to the total spinal CSF volume (0.081 l), while V2 (0.083 l) is 2–3 times larger than the total spinal cord volume (0.033 l; including the cauda equina), into which baclofen diffuses 18.
Spinal gradient
An interesting aspect of our PK model is its ability to visualize the spinal gradient of ITB. The calculated steep spinal gradient is important in ITB therapy, because this results in high local ITB concentrations in the direct vicinity of the catheter tip only, thereby reducing unwanted (central) side effects, such as drowsiness and sleepiness, as seen with oral baclofen 2. Figure 5 nicely shows the rapid drop in CSF baclofen concentration at a distance from the catheter tip. The spinal gradient has been measured previously in patients receiving continuous ITB therapy, where lumbar (L3) and cisternal (C1) CSF samples from five patients showed a baclofen steady‐state concentration ratio of 4.1 : 1 (range 1.8–8.7) 12. However, these were steady‐state concentrations after infusion, and can therefore not be compared with our data. Spinal ITB gradients have been measured also in a pig study 4. After the administration of a 2000 μg bolus in 5 min, the ITB peak concentration at 5 cm away from the catheter tip was reduced to 70–75%, which dropped further to 10% at a distance of 10 cm from the tip, with no measurable ITB at cerebral level 4. Our model predicted a peak concentration of 30% at 5 cm, 12% at 10 cm and 0.7% at cerebral level as compared with the tip concentration, 5 min after injection of a 50 μg bolus (based on the data of patient 4).
These data implicate that after a bolus injection, most baclofen remains around the catheter tip, which might be even more important during slow continuous infusion of ITB. The low injection speed reduces the initial distribution of baclofen along the spinal canal, resulting in an even higher spinal gradient. This was shown in a pig model. During the ITB infusion of 40 μg h–1, peak concentrations dropped to 2% at a distance of 5 cm from the tip 4.
The rapid drop in ITB concentration at a small distance from the catheter tip stresses the importance of careful catheter tip placement. For example, if the catheter tip is placed at Th10, most baclofen will be delivered in between Th8 and Th12, containing the lumbar enlargement of the spinal cord, including the spinal segments of the lower limbs. Therefore Th10 seems to be the best catheter tip location for patients suffering from lower extremity spasticity.
For patients with spasticity of the arms the targeted myelum levels should be within the cervical enlargement (C3‐Th2). To reach a sufficient ITB concentration at this cervical‐thoracic level with a catheter tip at a low thoracic level, higher infusion rates are required, associated with increased side effects and hypotonia of the lower extremities 19. If the catheter tip is positioned at a midthoracic level (Th6), this may provide better spasmolysis as compared with a low thoracic tip (Th11‐12), shifting the centre of the concentration gradient to a more rostral position 20. As an alternative, catheters with two tips at different locations would be an option. However these are not yet available.
PKPD model
Our PKPD model on ITB is the first one based on human data. The observed concentration data were used instead of the PK model‐predicted data, because of the high inter‐individual and intra‐individual differences in the model parameters, as well as to prevent PK modelling errors to be carried over to the PKPD model 21. The PKPD model is based on a delayed effect model and shows an adequate fit to the effect data as shown in Figure 4. The large variability in concentration and effect data results in high standard errors and inter‐individual variability for the model parameters (Table 3). Figure 6 shows the population predicted dose–effect relationship for three different bolus doses. The accuracy of the model may be improved with the inclusion of more patients.
The calculated EC 50 of 194 μg l–1 (95% CI 112, 350) provides a guideline for the targeted baclofen CSF concentration during the test infusion.
Limitations
This study and the PKPD model have several important limitations. Firstly, the number of included patients was relatively small, resulting in relatively large uncertainty in concentration and effect data. Secondly, our PK model, developed to describe the spinal ITB concentration gradient, is not yet validated, making it vulnerable to inaccuracies. The model and especially the concentration gradient are based on a single sample location. Multiple sample locations would have increased the validity of the model significantly, but this will be very difficult to realize in patients. Possibly a study with radiolabelled baclofen may solve this issue. The effect of ITB was measured using the MAS, which is an ordinal scale, making it less accurate to describe the concentration–effect relationship compared with a continuous scale. Furthermore, the validity of the MAS in measuring spasticity has been questioned as well 22. Unfortunately no real alternative is available at this moment.
Conclusion
In conclusion this study showed a steep concentration gradient of baclofen along the spinal axis, stressing the importance of catheter tip placement close to the targeted spinal level. We were able to develop a human ITB PKPD model, which hopefully will serve as an important tool to improve our insight in the PKPD relationship of ITB. More patient data are required to improve the accuracy of this model.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work.
We thank R.A. Koster, Laboratory for Clinical and Forensic Toxicology and Drugs Analysis, Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, The Netherlands, for the analytical method development, validation and bioanalysis of CSF baclofen.
Contributors
HWH collected, analyzed and interpreted the data and wrote the paper. JHP did the pharmacokinetic and pharmacodynamic analyses and wrote these sections of the paper. BHM coordinated the manufacturing and analysis of baclofen, coordinated dose randomization. All authors contributed to the interpretation, writing and editing of the paper.
Supporting information
Figure S1 Observed baclofen concentration vs. individual predicted baclofen concentration.
Figure S2 Residuals (observed minus individual predicted baclofen concentration) vs. time after administration.
Figure S3 Residuals (observed minus individual predicted baclofen concentration) vs. individual predicted baclofen concentration.
Figure S4 Observed MAS vs. population predicted MAS.
Figure S5 Observed MAS vs. individual predicted MAS.
Figure S6 Residuals (observed MAS minus population predicted MAS) vs. time after administration.
Figure S7 Residuals (observed MAS minus individual predicted MAS) vs. time after administration.
Figure S8 Residuals (observed MAS minus population predicted MAS) vs. population predicted MAS.
Figure S9 Residuals (observed MAS minus individual predicted MAS) vs. individual predicted MAS.
Supporting info item
Heetla, H. W. , Proost, J. H. , Molmans, B. H. , Staal, M. J. , and van Laar, T. (2016) A pharmacokinetic–pharmacodynamic model for intrathecal baclofen in patients with severe spasticity. Br J Clin Pharmacol, 81: 101–112. doi: 10.1111/bcp.12781.
References
- 1. Yang K, Wang D, Li YQ. Distribution and depression of the GABA(B) receptor in the spinal dorsal horn of adult rat. Brain Res Bull 2001; 55: 479–85. [DOI] [PubMed] [Google Scholar]
- 2. Meythaler JM, Clayton W, Davis LK, Guin‐Renfroe S, Brunner RC. Orally delivered baclofen to control spastic hypertonia in acquired brain injury. J Head Trauma Rehabil 2004; 19: 101–8. [DOI] [PubMed] [Google Scholar]
- 3. Penn RD, Kroin JS. Intrathecal baclofen alleviates spinal cord spasticity. Lancet 1984; 1: 1078. [DOI] [PubMed] [Google Scholar]
- 4. Bernards CM. Cerebrospinal fluid and spinal cord distribution of baclofen and bupivacaine during slow intrathecal infusion in pigs. Anesthesiology 2006; 105: 169–78. [DOI] [PubMed] [Google Scholar]
- 5. Busscher I, Ploegmakers JJ, Verkerke GJ, Veldhuizen AG. Comparative anatomical dimensions of the complete human and porcine spine. Eur Spine J 2010; 19: 1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guidance for Industry, bioanalytical method validation, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM) 2001.
- 7. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987; 67: 206–7. [DOI] [PubMed] [Google Scholar]
- 8. Shafer SL, Shafer A. Spinal pharmacokinetics In: Spinal drug delivery, 1st edn, ed Yaksh TL. Amsterdam: Elsevier, 1999; 271. [Google Scholar]
- 9. Edsbagge M, Tisell M, Jacobsson L, Wikkelso C. Spinal CSF absorption in healthy individuals. Am J Physiol Regul Integr Comp Physiol 2004; 287: R1450–5. [DOI] [PubMed] [Google Scholar]
- 10. Proost JH, Eleveld DJ. Performance of an iterative two‐stage Bayesian technique for population pharmacokinetic analysis of rich data sets. Pharm Res 2006; 23: 2748–59. [DOI] [PubMed] [Google Scholar]
- 11. Unadkat JD, Bartha F, Sheiner LB. Simultaneous modeling of pharmacokinetics and pharmacodynamics with nonparametric kinetic and dynamic models. Clin Pharmacol Ther 1986; 40: 86–93. [DOI] [PubMed] [Google Scholar]
- 12. Kroin JS, Penn RD. Cerebrospinal fluid pharmacokinetics of lumbar intrathecal baclofen In: Parenteral drug therapy in spasticity and Parkinson's disease, eds JPWF L, Delhaas EM, AWF R Casterton Hall, Carnforth: The Parthenon Publishing Group Limited, 1992; 67–78. [Google Scholar]
- 13. Sallerin‐Caute B, Lazorthes Y, Monsarrat B, Cros J, Bastide R. CSF baclofen levels after intrathecal administration in severe spasticity. Eur J Clin Pharmacol 1991; 40: 363–5. [DOI] [PubMed] [Google Scholar]
- 14. Muller H, Zierski J, Dralle D, Krauß D, Mutschler E. Pharmacokinetics of intrathecal baclofen In: Local spinal therapy of spasticity, eds Muller H, Zierski J, Penn RD. Berlin, Heidelberg, New York: Springer‐Verlag, 1988; 223–6. [Google Scholar]
- 15. Knutsson E, Lindblom U, Martensson A. Plasma and cerebrospinal fluid levels of baclofen (lioresal) at optimal therapeutic responses in spastic paresis. J Neurol Sci 1974; 23: 473–84. [DOI] [PubMed] [Google Scholar]
- 16. Van Schaeybroeck P, Nuttin B, Lagae L, Schrijvers E, Borghgraef C, Feys P. Intrathecal baclofen for intractable cerebral spasticity: a prospective placebo‐controlled, double‐blind study. Neurosurgery 2000; 46: 603–9; discussion 609‐12. [DOI] [PubMed] [Google Scholar]
- 17. Meythaler JM, Guin‐Renfroe S, Brunner RC, Hadley MN. Intrathecal baclofen for spastic hypertonia from stroke. Stroke 2001; 32: 2099–109. [DOI] [PubMed] [Google Scholar]
- 18. Edsbagge M, Starck G, Zetterberg H, Ziegelitz D, Wikkelso C. Spinal cerebrospinal fluid volume in healthy elderly individuals. Clin Anat 2011; 24: 733–40. [DOI] [PubMed] [Google Scholar]
- 19. Meythaler JM, Steers WD, Tuel SM, Cross LL, Haworth CS. Continuous intrathecal baclofen in spinal cord spasticity. A prospective study. Am J Phys Med Rehabil 1992; 71: 321–7. [DOI] [PubMed] [Google Scholar]
- 20. Grabb PA, Guin‐Renfroe S, Meythaler JM. Midthoracic catheter tip placement for intrathecal baclofen administration in children with quadriparetic spasticity. Neurosurgery 1999; 45: 833–6; discussion 836‐7. [DOI] [PubMed] [Google Scholar]
- 21. Proost JH, Schiere S, Eleveld DJ, Wierda JM. Simultaneous versus sequential pharmacokinetic‐pharmacodynamic population analysis using an iterative two‐stage Bayesian technique. Biopharm Drug Dispos 2007; 28: 455–73. [DOI] [PubMed] [Google Scholar]
- 22. Fleuren JF, Voerman GE, Erren‐Wolters CV, Snoek GJ, Rietman JS, Hermens HJ, Nene AV. Stop using the Ashworth scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 2010; 81: 46–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Observed baclofen concentration vs. individual predicted baclofen concentration.
Figure S2 Residuals (observed minus individual predicted baclofen concentration) vs. time after administration.
Figure S3 Residuals (observed minus individual predicted baclofen concentration) vs. individual predicted baclofen concentration.
Figure S4 Observed MAS vs. population predicted MAS.
Figure S5 Observed MAS vs. individual predicted MAS.
Figure S6 Residuals (observed MAS minus population predicted MAS) vs. time after administration.
Figure S7 Residuals (observed MAS minus individual predicted MAS) vs. time after administration.
Figure S8 Residuals (observed MAS minus population predicted MAS) vs. population predicted MAS.
Figure S9 Residuals (observed MAS minus individual predicted MAS) vs. individual predicted MAS.
Supporting info item


