Abstract
Aims
Clinical trials have reported conflicting results about whether celecoxib plus chemotherapy improves outcomes over chemotherapy alone in patients with advanced non‐small cell lung cancer.
Methods
We performed a meta‐analysis comparing the primary and secondary endpoints of treatment with celecoxib plus chemotherapy vs. chemotherapy alone in patients with advanced non‐small cell lung cancer.
Results
Six eligible trials (1181 patients) were selected from the 206 studies that were identified initially. A significant difference, favouring celecoxib plus chemotherapy over chemotherapy alone, was observed in the overall response rate [odds ratio (OR) 1.34; 95% confidence interval (CI) 1.08, 1.67; P = 0.009). However, there was no difference in the 1‐year survival rate (OR 1.08; 95% CI 0.86, 1.35; P = 0.512), clinical benefit (OR 1.05; 95% CI 1.88, 1.25; P = 0.613), complete response (OR 0.77; 95% CI 0.39, 1.51; P = 0.446) or partial response (OR 1.22; 95% CI 0.92, 1.63; P = 0.163). Toxicity did not differ significantly with the exception of the occurrence of leucopenia and thrombocytopenia.
Conclusions
Celecoxib plus chemotherapy appeared to improve the overall response rate compared with chemotherapy alone in the treatment of patients with advanced non‐small cell lung cancer. Further prospective randomized controlled trials are now needed.
Keywords: celecoxib, meta‐analysis, non‐small cell lung cancer
Introduction
To date, lung cancer still represents the leading cause of cancer‐related death all over the world. The majority of patients have advanced non‐small cell lung cancer (NSCLC) at stage ШB or IV at diagnosis and are treated palliatively 1. For patients with NSCLC, chemotherapy has reached its plateau in efficacy, and the search for new treatment strategies is urgently needed. There is growing evidence for a link between cancer and inflammation. Inflammation in the tumour microenvironment has tumour‐promoting effects 2. The presence of a systemic inflammatory response in patients with inoperable lung cancer seems to be associated with a poorer quality of life and shorter survival 3, 4. One target currently studied in the treatment of lung cancer is cyclooxygenase‐2 (COX‐2), an enzyme expressed in inflammatory and neoplastic tissue 5, 6. Increased expression of COX‐2 has been found in lung cancer and has been associated with a worse prognosis 6, 7. Preclinical studies have shown that COX‐2 inhibitors inhibit the growth of human lung cancer cells as single agents as well as in combination with chemotherapy 6, 8. Clinical trials have suggested that a combination of COX‐2 inhibitors with chemotherapy might have a better effect in NSCLC than chemotherapy alone 9, 10.
Several clinical trials have compared the efficacy and toxicity of celecoxib plus chemotherapy with chemotherapy alone in patients with advanced NSCLC. However, individually, these trials found that response rates, survival rates and toxicity were statistically inconsistent. The purpose of the current literature‐based meta‐analysis was to evaluate the efficacy [response rate, clinical benefit (CB), and 1‐year survival rate (SR)] and the toxicity profile of celecoxib plus chemotherapy as compared with chemotherapy alone for the treatment of patients with advanced NSCLC.
Methods
The meta‐analysis was performed according to a prospectively written protocol and analysis plan.
Definition of outcome
Efficacy was assessed using overall response rate (ORR), CB and 1‐year SR as the primary outcomes. The secondary endpoints were the rate of clinical complete (CR) and partial (PR) response, and the rate of grade 3 and grade 4 toxicity. ORR is defined as the percentage of patients who have a complete or partial tumour response; 1‐year SR is defined as the percentage of patients who remain alive 1 year after randomization, and CB as the proportion of patients in each arm with a CR, PR or stable disease according to the World Health Organization criteria. Regarding toxicity, we considered both haematological (leucopenia, thrombocytopenia and anaemia) and non‐haematological (nausea/vomiting, diarrhoea, gastric ulcer, cardiotoxicity, asthenia) grade 3 and grade 4 side effects of treatment.
Selection of trials
All published randomized controlled trials comparing the efficacy and toxicity of celecoxib plus chemotherapy with chemotherapy alone in patients with advanced NSCLC were selected for evaluation.
Search strategy
A literature search was carried out in March 2015 to identify all published randomized trials. Several databases, including Medline, Embase, China National Knowledge Infrastructure (CNKI), China Biomedicine Database disc (CBMdisc) and the Cochrane Central Register of Controlled Trials, were searched comprehensively up to December 2014 by using the following terms: ‘celecoxib’, ‘cyclooxygenase‐2 inhibitor’, ‘COX‐2 inhibitor’, ‘lung cancer’ and ‘lung carcinoma’. When two or more articles reported the same data, the most recently updated data were included. References from the identified articles were also checked and principal investigators were asked if they were aware of other trials.
Data collection
Data abstraction was performed by two independent observers, who extracted the data from the respective trials and verified the results by comparison. The following data were collected from the identified trials: first author's name, year of publication, number and age of patients, treatment schedule, treatment outcomes and percentage of patients who experienced toxicity. The methodological quality of the trials was assessed using the modified Jadad score (seven‐point) for randomization, concealment of allocation, double‐blinding, withdrawals and dropouts. In this score, trials scoring 1–3 points are considered to be of low quality and 4–7 points as high quality 11.
Statistical methods
Celecoxib plus chemotherapy was considered as the investigational treatment, and chemotherapy alone was used as the control treatment. The outcomes were represented by dichotomous variables; the response rate, 1‐year SR and CB analysis were each calculated by applying an intent‐to‐treat analysis. Grade 3 and grade 4 toxicity analysis was performed by considering the number of patients evaluable for toxicity. Heterogeneity was assessed by the chi‐square test. A fixed‐effects model was adopted unless there was evidence of significant unexplained heterogeneity, in which case a random‐effects model was used. The Z test was used to compare the overall effects of the treatment group with those of the control group, and differences were considered to be statistically significant when P < 0.05. Publication bias is a common concern in meta‐analyses, and is related to the tendency of journals to favour the publication of large and positive studies. Funnel plot asymmetry was assessed using Egger's linear regression test. For the possible publication bias, we used the trim‐and‐fill method to evaluate the influence on the result 12, 13. All calculations were performed using the meta‐analysis function in Stata software (Stata, version 10; Stata Corporation, College Station, TX, USA).
Results
Selected trials
Sixty‐four potentially eligible trials were identified from a total of 206 randomized trials. Of the 64 trials, seven were included for more detailed evaluation. Finally, six trials were gathered for the meta‐analysis, and one was excluded because of repeated publication (Figure 1) 14, 15, 16, 17, 18, 19. The main characteristics of the six trials are listed in Table 1. Three trials were published in Chinese journals, and the others in English journals between 2006 and 2012, and included 1181 patients (592 in the celecoxib plus chemotherapy group and 589 in the chemotherapy group). The quality of the trials was assessed using the modified Jadad scale (Table 2). One trial achieved a score of 7 17, two were awarded a score of 3 15, 18 and three were given a score of 2 14, 16, 19. Among the six trials, only one described concealment of treatment allocation and blinding methods.
Figure 1.
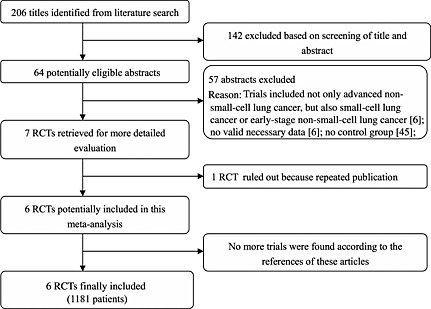
Flowchart of trial selection process. RCT, randomized controlled trial
Table 1.
Continued
| Characteristic | Lilenbaum et al. 14 | Zhou et al. 15 | Xiong et al. 16 | Koch et al. 17 | Groen et al. 18 | Liu and Huang 19 | |
|---|---|---|---|---|---|---|---|
| Interventions | Arm A | Celecoxib 400 mg po bid + DTX 35 mg m–2 or GEM 1000 mg m–2 + CPT‐11 60 ~ 100 mg/m2 ivgtt day 1 and 8 day, q3w | Celecoxib 400 mg po bid day 1–12 + NVB 25 mg m–2 iv qd day 1 and 8 + DDP 75 mg m–2 ivgtt qd days 1 and 2, q3w | Celecoxib 400 mg po bid + NVB 25 mg m–2 iv qd day 1 and 8 + DDP 70 mg m–2 ivgtt qd days 1–3, q3w | Celecoxib 400 mg po bid + GEM or NVB + CBP or DDP, ivgtt q3w * | Celecoxib 400 mg po bid + DTX 75 mg m–2 ivgtt qd day 1 + CBP ivgtt qd day 1, q3w† | Celecoxib 400 mg po bid days 1–5 + DTX 75 mg m–2 ivgtt qd day 1 + DDP 100 mg m–2 ivgtt qd day 1, q3w |
| Arm B | DTX 35 mg m–2 or GEM 1000 mg m–2 + CPT‐11 60 ~ 100 mg m–2 ivgtt day 1 and 8 day, q3w | NVB 25 mg m–2 iv qd days 1 and 8 + DDP 75 mg m–2 ivgtt qd days 1 and 2, q3w | NVB 25 mg m–2 iv qd days 1 and 8 + DDP 70 mg m–2 ivgtt qd days 1–3, q3w | Placebo + GEM or NVB + CBP or DDP, ivgtt q3w | Placebo + DTX 75 mg m–2 ivgtt qd day 1 + CBP ivgtt qd day 1, q3w | DTX 75 mg m–2 ivgtt qd day 1 + DDP 100 mg m–2 ivgtt qd day 1, q3w | |
| Study period | Feb 2002 to Sept 2003 | June 2004 to June 2005 | Jan 2003 to Jan 2006 | May 2003 to May 2006 | July 2003 to Dec 2007 | Jan 2006 to May 2011 | |
| Sample size | 133 (67/66) | 65 (32/33) | 60 (30/30) | 316 (158/158) | 561 (281/280) | 46 (24/22) | |
| Age (years) | 37–84 | 40–77 | 33–70 | 37–85 | 33–84 | 49–76 | |
| ECOG PS or Karnofsky score | ECOG 0–1 | ECOG 0–2 | ECOG 0–2 | ECOG 0–2 | ECOG 0–2 | Karnofsky ≧80 | |
| Median OS (months) | Arm A | 6.31 | 12 | 9.8 | 8.9 | 8.2 | Not reported |
| Arm B | 8.99 | 9.2 | 9.5 | 7.9 | 8.2 | Not reported | |
| Median PFS (months) | Arm A | 1.81 | Not reported | 5.2 | 6.1 | 4.5 | Not reported |
| Arm B | 2.09 | Not reported | 5 | 6.5 | 4 | Not reported | |
| CR (%) | Arm A | 24 | 3 | 0 | Not reported | 0 | 4 |
| Arm B | 30 | 0 | 0 | Not reported | 1 | 0 | |
| PR (%) | Arm A | 3 | 31 | 43 | Not reported | 37 | 42 |
| Arm B | 6 | 24 | 40 | Not reported | 29 | 32 | |
| Overall response rates (%) | Arm A | 27 | 34 | 43 | 36 | 37 | 46 |
| Arm B | 36 | 25 | 40 | 31 | 30 | 32 | |
| Clinical benefit (%) | Arm A | 48 | 84 | 73 | 78 | 82 | 83 |
| Arm B | 61 | 61 | 70 | 74 | 80 | 55 | |
| 1‐year overall survival (%) | Arm A | 24 | 58 | Not reported | 36 | 35 | 67 |
| Arm B | 36 | 37 | Not reported | 34 | 32 | 36 | |
| Grade 3 and grade 4 toxicity (%) | |||||||
| Leucopenia | Arm A | 28 | 13 | 20 | 37 | 36 | Not reported |
| Arm B | 20 | 9 | 23 | 25 | 30 | Not reported | |
| Thrombocytopenia | Arm A | 24 | 3 | 0 | 23 | 5 | Not reported |
| Arm B | 9 | 0 | 0 | 18 | 3 | Not reported | |
| Anaemia | Arm A | 9 | Not reported | 3 | 3 | Not reported | Not reported |
| Arm B | 3 | Not reported | 0 | 1 | Not reported | Not reported | |
| Nausea | Arm A | Not reported | 9 | 17 | Not reported | 3 | 17 |
| Arm B | Not reported | 3 | 13 | Not reported | 5 | 18 | |
| Diarrhoea | arm A | 9 | Not reported | Not reported | Not reported | 3 | Not reported |
| Arm B | 2 | Not reported | Not reported | Not reported | 4 | Not reported | |
| Gastric ulcer | Arm A | Not reported | Not reported | Not reported | 1 | 1 | Not reported |
| Arm B | Not reported | Not reported | Not reported | 1 | 1 | Not reported | |
| Asthenia | Arm A | 0 | Not reported | 10 | Not reported | 5 | 17 |
| Arm B | 5 | Not reported | 7 | Not reported | 6 | 14 | |
| Cardiotoxicity | Arm A | Not reported | Not reported | Not reported | 1 | 2 | Not reported |
| Arm B | Not reported | Not reported | Not reported | 1 | 1 | Not reported | |
| Neurotoxicity | Arm A | Not reported | 3 | 0 | Not reported | Not reported | Not reported |
| Arm B | Not reported | 0 | 0 | Not reported | Not reported | Not reported | |
bid, twice daily; CBP, carboplatin; CPT‐11, irinotecan; CR, complete response; d, day; DDP, cisplatin; DTX, docetaxel; iv, intravenously; ECOG PS, Eastern Cooperative Oncology Group performance status; GEM, gemcitabine; ivgtt, ?; KPS, Karnofsky performance status; NVB, vinorelbine; OS, overall survival; PFS, progression‐free survival; po, orally; PR, partial response; q, every; w, weeks;
The dose of chemotherapeutic agents was not mentioned in the trial;
The dose of carboplatin was not mentioned in the trial.
Table 2.
Quality assessment of trials by modified Jadad scale*
| Author | Randomization | Allocation concealment | Blinding | Withdrawals and dropouts | Score | Quality |
|---|---|---|---|---|---|---|
| Lilenbaum et al. 14 | 1 | 0 | 0 | 1 | 2 | low |
| Zhou et al. 15 | 2 | 0 | 0 | 1 | 3 | low |
| Xiong et al. 16 | 2 | 0 | 0 | 0 | 2 | low |
| Koch et al. 17 | 2 | 2 | 2 | 1 | 7 | high |
| Groen et al. 18 | 2 | 0 | 0 | 1 | 3 | low |
| Liu and Huang 19 | 2 | 0 | 0 | 0 | 2 | low |
There are four items in the Jadad scale: randomizations; allocation concealment; double blinding; withdrawals and dropouts. If the item was not described in the study, the score would be 0; otherwise it was 1. If the method of the item was described and it was appropriate, the score would be 2; if the item of “withdrawals and dropouts” was not described, the score would be 0; if the item of “withdrawals and dropouts” was described and it was appropriate, the score would be 1. Randomized controlled trials were considered to be of high quality if the score was 4–7, and of low quality if the score was 1–3.
Combined analysis
All the obtained results are displayed in Table 3 (primary endpoints) and Table 4 (secondary endpoints); primary endpoint plots are depicted in Figure 2.
Table 3.
Primary endpoint analysis
| Outcome | RCTs | Patients | OR | 95% CI | P | Heterogeneity (P) |
|---|---|---|---|---|---|---|
| ORR | 6 | 1181 | 1.34 | 1.08, 1.67 | 0.009 | 0.401 |
| CB | 6 | 1181 | 1.05 | 1.88, 1.25 | 0.613 | 0.832 |
| 1‐year SR | 5 | 1121 | 1.08 | 0.86, 1.35 | 0.512 | 0.346 |
CB, clinical benefit; CI, confidence interval; OR, odds ratio; ORR, overall response rate; RCTs, randomized clinical trials; SR, survival rate.
Table 4.
Secondary endpoint analysis
| Outcome | RCTs | Patients | OR | 95% CI | P | Heterogeneity (P) |
|---|---|---|---|---|---|---|
| CR | 5 | 865 | 0.77 | 0.39, 1.51 | 0.446 | 0.196 |
| PR | 5 | 865 | 1.22 | 0.92, 1.63 | 0.163 | 0.867 |
| Grade 3 and grade 4 toxicity | ||||||
| Leucopenia | 5 | 1135 | 1.29 | 1.01, 1.65 | 0.042 | 0.885 |
| Thrombocytopenia | 6 | 1181 | 1.49 | 1.02, 2.19 | 0.039 | 0.632 |
| Anaemia | 3 | 509 | 2.69 | 0.99, 7.28 | 0.052 | 0.873 |
| Nausea | 4 | 732 | 0.89 | 0.47, 1.66 | 0.708 | 0.549 |
| Diarrhoea | 2 | 694 | 1.23 | 0.57, 2.66 | 0.592 | 0.073 |
| Gastric ulcer | 2 | 877 | 1 | 0.25, 4.01 | 0.998 | 0.469 |
| Cardiotoxicity | 2 | 877 | 1.94 | 0.62, 6.06 | 0.254 | 0.998 |
| Asthenia | 4 | 800 | 0.82 | 0.45, 1.49 | 0.511 | 0.384 |
CI, confidence interval; CR, complete response; OR, odds ratio; PR, partial response; RCTs, randomized clinical trials.
Figure 2.
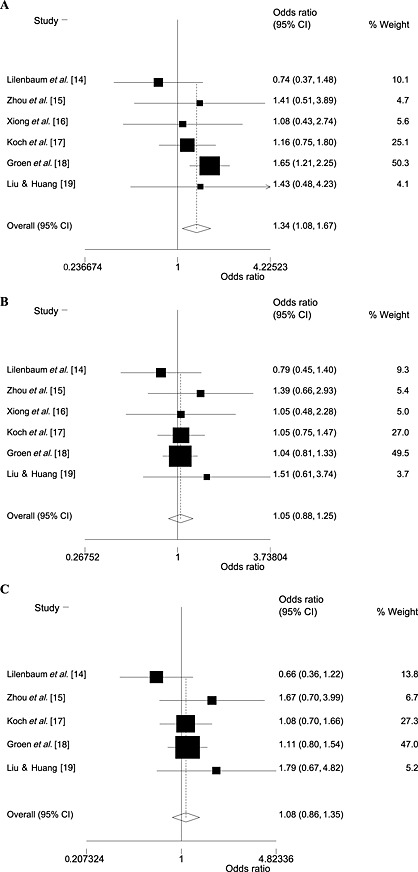
Overall response rate (A), clinical benefit (B) and 1‐year survival rate (C) of celecoxib plus chemotherapy compared with chemotherapy alone in advanced non‐small cell lung cancer therapy. CI, confidence interval
Primary endpoints
All the trials reported ORR and CB, representing a total of 1181 patients. The ORR was increased for celecoxib plus chemotherapy (250/592 = 42%) compared with chemotherapy alone (184/589 = 31%). The pooled results also showed celecoxib plus chemotherapy to have a statistically significantly greater effect than chemotherapy alone on ORR [odds ratio (OR) 1.34; 95% confidence interval (CI) 1.08, 1.67; P = 0.009; heterogeneity: χ2 = 5.12; P = 0.401] (Table 3 and Figure 2A). The CB was 77% (454/592) and 73% (431/589) for celecoxib plus chemotherapy and chemotherapy alone, respectively. The pooled results demonstrated no statistical difference in CB between celecoxib plus chemotherapy and chemotherapy alone (OR 1.05; 95% CI 0.88, 1.25; P = 0.613; heterogeneity: χ2 = 2.12; P = 0.832; Table 3 and Figure 2B).
The number of patients achieving 1‐year survival was available in five eligible trials (1121 patients) 14, 15, 17, 18, 19. In the overall population, the 1‐year SR was found not to be significantly higher for patients receiving celecoxib plus chemotherapy than in those receiving chemotherapy alone (OR 1.08; 95% CI 0.86, 1.35; P = 0.512), without significant heterogeneity (P = 0.346; Table 3 and Figure 2C).
Secondary endpoints
The number of patients who achieved a CR was available in five trials (865 patients) 14, 15, 16, 18, 19. In the overall population, the number achieving a CR was no different for patients receiving celecoxib plus chemotherapy than for those receiving chemotherapy alone (OR 0.77; 95% CI 0.39, 1.51; P = 0.446), without significant heterogeneity (P = 0.196; Table 4).
The number of patients who achieved a PR was available in five trials (865 patients) 14, 15, 16, 18, 19, and was found to be similar in the two groups (OR 1.22; 95% CI 0.92, 1.63; P = 0.163), without significant heterogeneity (P = 0.867; Table 4).
Although toxicity, including both haematological and non‐haematological grade 3 and grade 4 side effects of treatment, differed among all trials, celecoxib plus chemotherapy was associated with a higher incidence of haematological toxicity compared with chemotherapy alone, with the exception of anaemia. In the evaluable population, no significant difference between celecoxib plus chemotherapy and chemotherapy alone was found for grade 3 and grade 4 non‐haematological toxicity (Table 4).
Publication bias
The result of the publication bias analysis for 1‐year SR was significant (t = −4.21; P = 0.019); the funnel plots also showed asymmetry. No obvious publication bias was observed in the ORR (t = 1.24; P = 0.319) or CBR (t = 0.56; P = 0.947) analyses (Figure 3). Using the trim‐and‐fill method showed that three studies were trimmed for ORR, one for CB and none for 1‐year SR, and the values of the original estimate did not significantly change after the adjustment, indicating the stability of our results (Figure 4).
Figure 3.
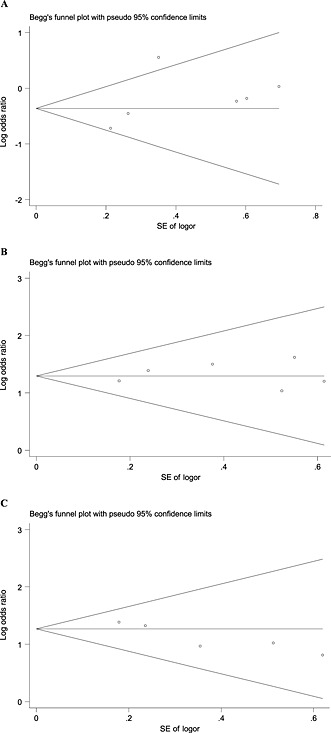
Funnel plot of publication bias for overall response rate (A), clinical benefit (B) and 1‐year survival rate (C). SE, standard error; pseudo 95% confidence limits, 95% confidence interval; SE logor, SE log odds ratio
Figure 4.
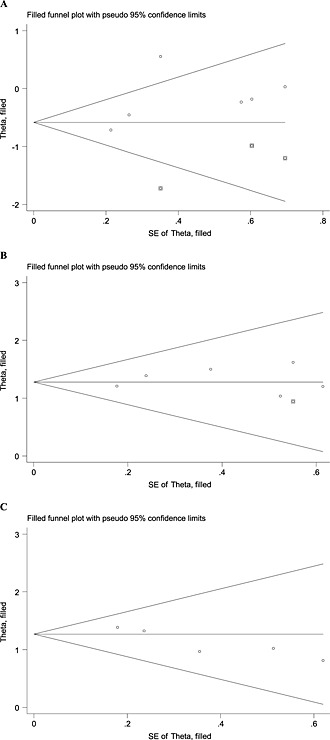
Funnel plot of publication bias for overall response rate (A), clinical benefit (B) and 1‐year survival rate (C), adjusted using the trim–and‐fill method. SE, standard error; pseudo 95% confidence limits, 95% confidence interval
Discussion
COX‐2 inhibitors have been shown to induce apoptosis of NSCLC cell lines and to enhance the activity of standard chemotherapeutic agents, providing the rationale for combining celecoxib with chemotherapy in the treatment of NSCLC 20. Recently, six trials have focused on the difference in efficacy and toxicity between celecoxib plus chemotherapy and chemotherapy alone regimens in the treatment of patients with advanced NSCLC. Four trials reported similar response rates and survival with the two regimens 14, 16, 17, 18. However, two trials showed significant improvements in response rate and survival with celecoxib plus chemotherapy 15, 19 compared with chemotherapy alone. Incidences of toxicity for the two regimens were reported inconsistently in most studies. To assess comprehensively the advantages and disadvantages of celecoxib for patients with advanced NSCLC, we undertook a meta‐analysis.
Our meta‐analysis indicated a significantly increased ORR with celecoxib plus chemotherapy over chemotherapy alone. However, we could not determine whether celecoxib plus chemotherapy was more effective than chemotherapy in the treatment of patients with advanced NSCLC. A possible explanation for this might have been the difference in chemotherapeutic regimens or dosage used among the trials. In two trials, patients treated with vinorelbine plus cisplatin were included, but each trial used a different dose of cisplatin 15, 16. In the other four trials, patients treated with four different chemotherapeutic regimens were included 14, 17, 18, 19. One study revealed that the cytotoxicity of various chemotherapeutic agents in NSCLC cells could be enhanced by the adjunctive use of a COX‐2 inhibitor but the synergistic effects varied considerably 21. The use of a COX‐2 inhibitor in combination with irinotecan and docetaxel resulted in more NSCLC cells undergoing apoptosis than with etoposide and cisplatin 21. Furthermore, another study demonstrated that a COX‐2 inhibitor antagonized the cytotoxicity and proapoptotic activity of a chemotherapeutic agent in human gastric cancer cells by decreasing intracellular accumulation 22. These results might partly explain the different efficacies of celecoxib plus chemotherapy in the treatment of patients with advanced NSCLC. The heterogeneity in data regarding chemotherapeutic regimens in the meta‐analysis reflects the selection bias of the available trials, and contributes to publication bias. Moreover, we could not carry out a systematic review on overall survival because of the lack of data. With regard to tolerability, only data on haematological (leucopenia, thrombocytopenia and anaemia) and non‐haematological (nausea/vomiting, diarrhoea, gastric ulcer, cardiotoxicity and asthenia) were available in all trials, so no definitive conclusion could be drawn. The rate of severe haematological (leucopenia, thrombocytopenia) toxicity significantly increased when celecoxib was added to chemotherapy. This might have been associated with the increased expression of COX‐2 in tumour bone marrow cells, and the role played by COX‐2 in the recovery of the bone marrow after chemotherapy, which could be a possible explanation for the higher frequency of haematological toxicity in the celecoxib plus chemotherapy group 23, 24, 25.
There were some limitations to our approach. First, our meta‐analysis was limited to trials that were randomized, controlled and published only in Chinese and English. Three trials included in this analysis were from China 15, 16, 19 and the other three from Western countries 14, 17, 18. Second, our meta‐analysis was limited to a small number of trials, and not based on individual patient data. Meta‐analyses based on published data tend to overestimate treatment effects compared with individual data analysis. Therefore, we should interpret the results with care, especially for a positive result. Third, only one trial in our meta‐analysis described the concealment of treatment allocation and blinding methods. Therefore, we were unable to draw firm conclusions from the data, and confirmation must await investigation in future trials. Finally, two studies in our meta‐analysis did not report how they handled patients who were lost to follow‐up, the percentage of patients lost to follow‐up and whether these patients were censored in the analysis. There exists the possibility of censoring which could bias our findings, and our analysis would tend toward underestimation of any effects of this potential bias.
Statistical heterogeneity may arise because of clinical differences or methodological differences between studies, and will lead to funnel plot asymmetry. For example, substantial benefit may be seen only in high‐risk patients, and these may be preferentially included in small studies. Or the intervention may have been implemented less thoroughly in larger studies, resulting in smaller effect estimates compared with smaller studies. Statistically significant ‘positive’ results are more likely to be published, and may also cause funnel plot asymmetry 26, 27. Although the quality of the trials included in the present meta‐analysis was low, systematic reviews and meta‐analyses such as the present one will help to specify where further improvements in the design and reporting of research conducted are needed. In addition, these studies were conducted at major academic institutions, in patients with adequate major organ function, and might not have reflected the general patient population in the community or patients with organ dysfunction.
In conclusion, we found evidence that celecoxib plus chemotherapy might improve the ORR for advanced NSCLC therapy. However, confirmation of these conclusions in rigorously controlled, randomized trials is required before firmer inferences about this therapy can be drawn.
Competing Interest
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Contributors
HLC, HF and XHB were responsible for the design of this meta‐analysis. XHB and HF performed independently trial selection. HLC and XHB were responsible for data acquisition and interpretation of the data. HF and XHB did the statistical analyses. HLC and XHB were involved in drafting the manuscript.
Hou, L.‐C. , Huang, F. , and Xu, H.‐B. (2016) Does celecoxib improve the efficacy of chemotherapy for advanced non‐small cell lung cancer?. Br J Clin Pharmacol, 81: 23–32. doi: 10.1111/bcp.12757.
References
- 1. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 2. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature 2008; 454: 436–44. [DOI] [PubMed] [Google Scholar]
- 3. Scott HR, McMillan DC, Brown DJ, Forrest LM, McArdle CS, Milroy R. A prospective study of the impact of weight loss and the systemic inflammatory response on quality of life in patients with inoperable non‐small cell lung cancer. Lung Cancer 2003; 40: 295–9. [DOI] [PubMed] [Google Scholar]
- 4. Koch A, Fohlin H, Sorenson S. Prognostic significance of C‐reactive protein and smoking in patients with advanced non‐small cell lung cancer treated with first‐line palliative chemotherapy. J Thorac Oncol 2009; 4: 326–32. [DOI] [PubMed] [Google Scholar]
- 5. Brabender J, Park J, Metzger R, Schneider PM, Lord RV, Hölscher AH, Danenberg KD, Danenberg PV. Prognostic significance of cyclooxygenase‐2 mRNA expression in non‐small cell lung cancer. Ann Surg 2002; 235: 440–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laskin JJ, Sandler AB. The importance of the eicosanoid pathway in lung cancer. Lung Cancer 2003; 41: S73–9. [DOI] [PubMed] [Google Scholar]
- 7. Edelman MJ, Watson D, Wang X, Morrison C, Kratzke RA, Jewell S, Hodgson L, Mauer AM, Gajra A, Masters GA, Bedor M, Vokes EE, Green MJ. Eicosanoid modulation in advanced lung cancer: cyclooxygenase‐2 expression is a positive predictive factor for celecoxib + chemotherapy‐cancer and leukemia group B trial 30203. J Clin Oncol 2008; 26: 848–55. [DOI] [PubMed] [Google Scholar]
- 8. Hida T, Kozaki K, Ito H, Miyaishi O, Tatematsu Y, Suzuki T, Matsuo K, Sugiura T, Ogawa M, Takahashi T, Takahashi T. Significant growth inhibition of human lung cancer cells both in vitro and in vivo by the combined use of a selective cyclooxygenase‐2 inhibitor, JTE‐522, and conventional anticancer agents. Clin Cancer Res 2002; 8: 2443–7. [PubMed] [Google Scholar]
- 9. Altorki NK, Keresztes RS, Port JL, Libby DM, Korst RJ, Flieder DB, Ferrara CA, Yankelevitz DF, Subbaramaiah K, Pasmantier MW, Dannenberg AJ. Celecoxib, a selective cyclooxygenase‐2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early‐stage non‐small cell lung cancer. J Clin Oncol 2003; 21: 2645–50. [DOI] [PubMed] [Google Scholar]
- 10. Nugent FW, Mertens WC, Graziano S, Levitan N, Collea R, Gajra A, Marshall J, McCann J. Docetaxel and cyclooxygenase‐2 inhibition with celecoxib for advanced non‐small cell lung cancer progressing after platinum‐based chemotherapy: a multicenter phase II trial. Lung Cancer 2005; 48: 267–73. [DOI] [PubMed] [Google Scholar]
- 11. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaqhan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 12. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 13. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in metaanalysis. Biometrics 2000; 56: 455–63. [DOI] [PubMed] [Google Scholar]
- 14. Lilenbaum R, Socinski MA, Altorki NK, Hart LL, Keresztes RS, Hariharan S, Morrison ME, Fayyad R, Bonomi P. Randomized phase II trial of docetaxel/irinotecan and gemcitabine/irinotecan with or without celecoxib in the second‐line treatment of non‐small‐cell lung cancer. J Clin Oncol 2006; 24: 4825–32. [DOI] [PubMed] [Google Scholar]
- 15. Zhou SW, Zhou CC, Xu JF, Lv MJ. First‐line regimen of vinorelbine and cisplatin (NP) combined with cyclooxygenase‐2 inhibitor celecoxib in advanced non‐small‐cell lung cancer. J Tongji University (Med Sci) 2007; 28: 87–91. [Google Scholar]
- 16. Xiong JP, Xiang XJ, Zhang L, Zhong LX, Chen WY, Yu F. A phase II study of vinorelbine/cisplatin with or without COX‐2 inhibitor in first‐line treatment of non‐small cell lung cancer. Cancer Res Prev Treat 2008; 35: 201–3. [Google Scholar]
- 17. Koch A, Bergman B, Holmberg E, Sederholm C, Er L, Kosieradzki J, Lamberg K, Thaning L, Ydreborg SO, Sorenson S. Effect of celecoxib on survival in patients with advanced non‐small cell lung cancer: a double blind randomized clinical phase Ш trial (CYCLUS study) by the Swedish lung cancer study group. Eur J Cancer 2011; 47: 1546–55. [DOI] [PubMed] [Google Scholar]
- 18. Groen HJ, Sietsma H, Vincent A, Hochstenbag MM, van Putten JW, van den Berg A, Dalesio O, Biesma B, Smit HJ, Termeer A, Hiltermann TJ, van den Borne BE, Schramel FM. Randomized, placebo‐controlled phase III study of docetaxel plus carboplatin with celecoxib and cyclooxygenase‐2 expression as a biomarker for patients with advanced non‐small‐cell lung cancer: the NVALT‐4 study. J Clin Oncol 2011; 29: 4320–6. [DOI] [PubMed] [Google Scholar]
- 19. Liu GH, Huang JA. Clinical study of celecoxib combined with chemotherapy in the treatment of patients with advanced lung cancer. Chin J Cancer Prev Treat 2012; 19: 1661–3. [Google Scholar]
- 20. Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase‐2 inhibitors. Cancer Res 2000; 60: 1306–11. [PubMed] [Google Scholar]
- 21. Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, Sugiura T, Ogawa M, Takahashi T. Cyclooxygenase 2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non‐small cell lung cancer cell lines. Clin Cancer Res 2000; 6: 2006–11. [PubMed] [Google Scholar]
- 22. Chen MH, Yu L, Gu CP, Zhong DS, Wu SG, Liu SW. Celecoxib antagonizes the cytotoxic effect of cisplatin in human gastric cancer cells by decreasing intracellular cisplatin accumulation. Cancer Lett 2013; 329: 189–96. [DOI] [PubMed] [Google Scholar]
- 23. Lorenz M, Slaughter HS, Wescott DM, Carter SI, Schnyder B, Dinchuk JE, Car BD. Cyclooxygenase‐2 is essential for normal recovery from 5‐fluorouracil‐induced myelotoxicity in mice. Exp Hematol 1999; 27: 1494–502. [DOI] [PubMed] [Google Scholar]
- 24. Liu XW, He QT, Li ZQ, Ma HJ, Li J, Jia GR, Lu Y, Han HY, Li Z, Yun Y, Zhang DX. Expression of cyclooxygenase‐2 in bone marrow cells of leukemia patients and its association with angiogenesis. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2009; 17: 40–2. [PubMed] [Google Scholar]
- 25. Valcárcel M, Mendoza L, Hernández JJ, Carrascal T, Salado C, Crende O, Vidal‐Vanaclocha F. Vascular endothelial growth factor regulates melanoma cell adhesion and growth in the bone marrow microenvironment via tumor cyclooxygenase‐2. J Transl Med 2011; 9: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biljana M, Jelena M, Branislav J, Milorad R. Bias in meta‐analysis and funnel plot asymmetry. Stud Health Technol Inform 1999; 68: 323–8. [PubMed] [Google Scholar]
- 27. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]


