Abstract
Background
Adverse drug reactions are sometimes described as being ‘non‐dose‐related’ because no relationship has been found between increasing doses and either the intensity of the response or the proportion of individuals in whom the response occurs; furthermore, hypersensitivity reactions are often regarded as being non‐dose‐related, even if different doses have not been studied. However, the law of mass action implies that all pharmacological effects are concentration related and should increase in intensity with increasing dose. We set out to explain this paradox.
Methods
We searched for published adverse drug reactions that have been described as non‐dose‐related and analysed them.
Results
We identified four categories of explanations that resolve the paradox: (i) the reaction is not real; it may have occurred by chance or there may be methodological problems, such as bias or confounding factors; (ii) the dose–response curve for the adverse effect reaches a maximum at doses lower than were studied (i.e. a hypersusceptibility reaction); this underpins the use of test doses to predict the possibility of an adverse reaction at therapeutic doses; (iii) susceptibility to the adverse reaction differs widely among individuals; and (iv) imprecision or inaccuracy in the measurement of either dose or effect obscures dose responsiveness. This last explanation encompasses: (a) reactions related to cumulative dose; (b) dissociation between dose and concentration through saturable pharmacokinetics; and (c) variability in the measurement of the effect.
Conclusions and implications
If an adverse drug reaction appears to be non‐dose‐related, the reasons should be sought, having these mechanisms in mind.
Keywords: dose–response relationship, drug‐related adverse effects, drug‐related adverse reactions, law of mass action
“…alein die Dosis macht daß ein Ding kein Gift ist” Paracelsus (Theophrastus von Hohenheim) Sieben Defensiones: Verantwortung über etliche Verunglimpfungen seiner Mißgönner, 1564
Introduction
There has until recently been wide acceptance of the idea that there is a class of adverse drug reactions that are non‐dose‐related, such reactions often being described using inappropriate terms such as ‘idiosyncratic’ (which actually means ‘occurring in a susceptible individual’) and ‘bizarre’ 1. However, it is a corollary of the law of mass action that all pharmacological effects are concentration related and should therefore increase with increasing dose. This is important in understanding the nature of adverse drug reactions, their management 2, and their prevention 3.
Here we offer explanations that resolve the paradox that some adverse drug reactions may appear to be non‐dose‐related in clinical practice, while the law of mass action predicts otherwise.
The law of mass action: defining concentration responsiveness
According to the law of mass action, the velocity of a reaction depends on the concentrations of the reactants; when a chemical reaction reaches equilibrium, the concentrations of the chemicals involved bear a constant relationship to each other, which is described by the equilibrium constant.
The pharmacological implications of this law 4, which were first described in relation to disinfection 5, can be seen in the concentration–effect curve 6. The representation of concentration–effect curves as dose–response curves implies that the administered dose predicts the concentration at the site of action.
The principle of the dose–response curve, stated in its simplest terms, is that:
no effect occurs in the absence of a drug;
the intensity of the effect increases when increasing concentrations of the drug are introduced;
there comes a point at which further increases in concentration produce no further appreciable increase in effect, that effect being regarded as a maximum.
In a very few cases, a further increase in concentration produces a down‐turning in effect, so‐called hormesis 7, but this phenomenon does not affect our general argument about dose‐relatedness.
Between the two extremes of no effect and maximum effect, the effect increases monotonically with increasing concentration. Although the exact shape of the function can vary (see Figure 1 8), it is its monotonicity that matters here, not its shape.
Figure 1.
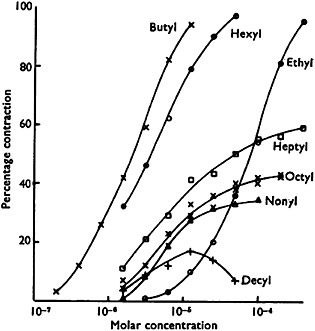
Concentration–effect relationships for a series of alkylated trimethylammonium salts causing contraction in guinea‐pig ileum in vitro. The data illustrate different potencies, different maximum efficacies, and, in one case, hormesis (reproduced from Stephenson 8)
What non‐dose‐relatedness would imply
It is commonly stated that a particular pharmacological effect, often an adverse drug reaction, is ‘non‐dose‐related’. The basis of such statements is usually that the effect has been studied at more than one dose of the drug, and that no relationship has been found between increasing doses and either the intensity of the response or the proportion of individuals in whom the response occurs. Furthermore, hypersensitivity reactions are sometimes described as non‐dose‐related, even though different doses have not been studied.
For the sake of completeness, we note that in a few cases it has been observed that an effect occurs at a low dose and paradoxically not at a high dose. For example, in a survey of all Danish patients who sustained fractures during 1 year (n = 124 655) compared with age‐ and sex‐matched controls from the general population, low doses of oral β2‐adrenoceptor agonists were associated with a significantly increased risk of fracture but higher doses were not 9. This kind of effect is not what is typically referred to as a non‐dose‐related effect, and in any case such effects are most likely to be explained by confounding factors.
In some cases, bidirectional or paradoxical pharmacological effects can result in such unexpected relationships between dose or concentration and outcomes 10. For example, at low doses ketamine can cause nervous system excitation, and at higher doses sedation. A complete analysis of such reactions is beyond the scope of our discussion here. We merely point out that if such reactions are due to two separate mechanisms, one would expect each mechanism to show dose relatedness in the absence of the other.
Apparent non‐dose‐relatedness of adverse drug reactions
The idea that certain adverse drug reactions can be non‐dose‐related may have been first specifically suggested in 1973 by Levine, who distinguished dose‐related (‘toxic’ and ‘idiosyncratic’) reactions from non‐dose‐related (‘allergic’) reactions 11, but it had already been implied earlier, for example in 1958 by Wayne, who distinguished predictable effects (‘toxic effects … related to the main action of the drug or to its side effects’) and unpredictable effects (‘not related to the main or subsidiary pharmacological action of a drug’) 12. The idea was strengthened in 1977, when Rawlins and Thompson devised a system 13 in which adverse drug reactions were defined as being of one of two types, which they designated A and B, later adding the mnemonic tags, ‘augmented’ and ‘bizarre’ 14. They defined these two types of reaction as follows 15, 16:
Type A reactions: ‘the result of an exaggerated, but otherwise normal, pharmacological action of a drug given in the usual therapeutic doses’.
Type B reactions: ‘totally aberrant effects that are not to be expected from the known pharmacological actions of a drug when given in the usual therapeutic doses to a patient whose body handles the drug in the normal way’.
Type A reactions were characterized as usually predictable and dose related and Type B reactions as unpredictable and non‐dose‐related 16.
We have elsewhere detailed the difficulties that attend these definitions 1. However, aside from definitional problems, the fact that some adverse drug reactions have been labelled as ‘non‐dose‐related’ needs to be explained.
Resolving the paradox of apparently non‐dose‐related adverse drug reactions
Here, we describe four mechanisms that explain the paradox that some adverse drug reactions may appear to be non‐dose‐related in clinical practice, while the law of mass action predicts otherwise. The explanations are listed in Box 1, and we shall discuss them individually and illustrate them with examples drawn from the literature.
Box 1.
Four reasons that resolve the paradox of apparently nondose‐related adverse drug reactions
The effect is not real; for example, (a) it may have occurred by chance or (b) there may be methodological problems, such as bias or confounding factors;
The dose–response curve for the adverse effect reaches a maximum at doses lower than the authors studied (i.e. a hypersusceptibility reaction, whether immunological or not);
The susceptibility to the adverse reaction differs widely among individuals; and
Imprecision or inaccuracy in the measurement of either the dose or the effect obscures dose responsiveness; this encompasses: (a) reactions that are related to cumulative dose; (b) dissociation between dose and concentration through saturable pharmacokinetics; and (c) measurement variability.
The effect is not real
A putative effect of a drug, beneficial or adverse, may have been mistakenly attributed to the drug. This could happen, for example, by chance or when there are methodological problems, such as confounding by indication.
Chance
In a dose‐ranging study of indacaterol in 436 patients with persistent asthma, headache was a reported adverse event in up to 7.9% of patients compared with 2.8% of those taking placebo; this effect was reportedly not dose‐related 17. However, in a much larger study of β2‐adrenoceptor agonists in 9441 patients, headache was not significantly more frequent with indacaterol than with placebo, except with the lowest of four doses of indacaterol in a subgroup of 449 patients 18, a finding that could be attributed to chance. The original finding, in a similarly small number of individuals, can therefore also be attributed to chance.
In pooled data from nine placebo‐controlled studies of the effects of irbesartan in patients with hypertension, it was reported that there was no relationship between dose and adverse reactions 19. However, rates of adverse events and discontinuation were almost identical in those who took irbesartan and those who took placebo. One can therefore conclude that the apparently non‐dose‐related adverse reactions that were reported in those studies actually occurred by chance and were not attributable to the drug.
Confounding by indication
In a study of 44 alcohol‐dependent patients, nine were reported to have had non‐dose‐related subclinical hepatotoxicity after taking disulfiram for 3 weeks 20. The problem of confounding by indication when attributing hepatotoxicity to a drug in patients with a high risk of liver damage (in this case due to alcohol) is obvious. Disulfiram may well cause hepatotoxicity, but it would be difficult to establish the dose relationship of such a reaction in a population of patients in whom liver damage is expected for another reason.
The effect has been studied in the wrong range of doses to detect a dose‐related effect
If the dose–response curve for the adverse reaction is sufficiently separated from the dose–response curve for the beneficial effect, it may be possible to delineate a dose relationship over one of those curves while failing to delineate it over the other. If we restrict ourselves to considering adverse drug reactions that appear to be non‐dose‐related, this is most likely to happen in hypersusceptibility reactions, in which the dose required to produce a maximal adverse reaction is below any dose that is normally used to produce a beneficial effect (Figure 2). [In technical terms, the dose–response curve for the adverse effect is to the left of the curve for the beneficial effect.] This is why test doses that are used to predict the likelihood of a hypersensitivity reaction (e.g. amphotericin 21) are chosen to be sufficiently small that any adverse reaction that occurs is correspondingly small, being intended to be at the lower end of the adverse dose–response curve (Figure 2, blue arrow). If the adverse reaction was not dose related, a test dose would be expected to produce as large a reaction as a normally beneficial dose. This is also the basis of desensitization protocols, which always start with very small doses.
Figure 2.
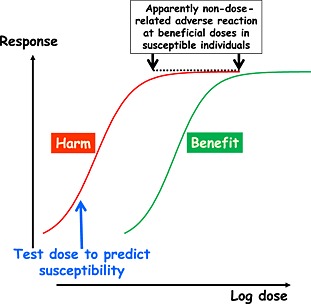
Theoretical concentration–effect relationships for a beneficial effect (right‐hand curve) and an adverse reaction (left‐hand curve); if the resultant adverse reaction is elicited at doses in the usually beneficial range, it may be severe and will appear to be non‐dose‐related (black arrows); however, a very low test dose (blue arrow) will elicit a milder adverse reaction, which would not be mild if the reaction was truly non‐dose‐related at all doses
Some hypersusceptibility reactions are immunologically mediated (e.g. penicillin hypersensitivity), while others are not (e.g. haemolysis due to oxidizing agents in glucose‐6‐phosphate dehydrogenase deficiency). Examples of immunological reactions that are clearly dose related include hay fever in response to pollen 22, the immunogenic response to hepatitis B vaccine 23, desensitization by the use of increasing doses of antigen (e.g. cephalosporins) 24; and type IV hypersensitivity skin reactions 25. When an immunological reaction is collateral (i.e. occurs within the same range of doses or concentrations as the beneficial response), there is no doubt about dose responsiveness. Examples include lupus‐like syndrome due to hydralazine 26 and methyldopa‐associated haemolytic anaemia 27.
Adverse reactions to which not all individuals are susceptible
When only a small proportion of the population is susceptible to an adverse reaction, those who are not susceptible will be able to take any dose of the drug without developing the adverse reaction; thus, the relationship between dose and response is imprecise and dose responsiveness will be hard to detect. If the adverse event can occur independently of the drug in nonsusceptible individuals, then the signal‐to‐noise ratio depends on the background incidence of the event. If the background incidence of the event (e.g. myocardial infarction) is high, it will be even more difficult to detect a dose–response relationship in those who are susceptible. For example, in order to demonstrate that the risk of myocardial infarction in patients taking rofecoxib was dose related, it was necessary to study a large number of patients 28.
Imprecision or inaccuracy in the measurement of either the dose or the effect
There are several ways in which the dose or the effect may be inaccurately or imprecisely measured. We have identified three varieties of this effect:
Reactions that are related to cumulative dose
If the measure of dose used is, for example, the amount that is given every day, then effects that are related to cumulative doses may misleadingly appear to be non‐dose‐related. For example, in a study of 51 patients with jaundice associated with flucloxacillin, it was reported that there was no relationship between the daily dose of flucloxacillin and the risk of jaundice 29. However, it was also reported that the risk was associated with the duration of treatment. This clearly implies that the risk was related to the cumulative dose.
Dissociation between dose and concentration through saturable pharmacokinetics
In theory, saturable absorption of a drug from the gut would obscure any relationship between dose and plasma concentration at doses exceeding the threshold of saturability, and any adverse reactions that were due to absorbed drug would appear to be non‐dose‐related. In this case, the dose would not be an accurate indicator of the concentration at the site of action. We have not identified any real‐life examples of this theoretical mechanism.
Variability in the measurement of effect
The fewer measurements of a variable that are made at each dose of a drug, the more uncertain the estimate of the mean effect size will be. Thus, when few subjects are studied or when few observations are made in an individual subject, dose responsiveness may be obscured by large variability. In other words, in such cases there is insufficient power to detect a dose‐related effect. An example is shown in Table 1 and Figure 3. ARIES‐1 and ‐2 (the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double‐Blind, Placebo‐Controlled, Multicenter, Efficacy Studies) were concurrent double‐blind studies in which a total of 394 patients with pulmonary arterial hypertension were randomized to placebo or ambrisentan orally once a day for 12 weeks (5 mg or 10 mg in ARIES‐1 and 2.5 mg or 5 mg in ARIES‐2) 30. The frequencies of a range of adverse events in the four groups were tabulated, and Table 1 shows the frequencies of two of those events, nasal congestion and abdominal pain. Although the numbers of subjects studied were small, it appears that there was a clear relationship between the dose of ambrisentan and the percentage incidence of nasal congestion. By contrast, the incidence of abdominal pain appeared to be significantly increased at all three doses of ambrisentan compared with placebo, without a clear dose relationship. However, Figure 3 shows the confidence intervals of the mean frequencies of abdominal pain in each group, calculated using the binomial rule, and it can be seen that there was very large variability in the frequency estimates. There are three possible interpretations of these data (Figure 4). The first is that the variability hides a probable dose–response relationship (the dotted curve in Figure 4); the second is that there is a dose–response relationship, but that the maximum effect is reached at doses below the lowest dose used in the study, giving the appearance of a non‐dose‐related adverse reaction (solid curve in Figure 4); the third is that there is no significant dose–response relationship (shaded area in Figure 4), in which case the event is not causally linked to the drug. In this case the paucity of data makes interpretation impossible.
Table 1.
The frequencies of two different adverse events in four treatment groups in the ARIES‐1 and ‐2 studies
| Placebo | Ambrisentan | |||
|---|---|---|---|---|
| (n = 132) | 2.5 mg (n = 64) | 5 mg (n = 130) | 10 mg (n = 67) | |
| Nasal congestion | 2 (1.5%) | 1 (1.6%) | 7 (5.4%) | 7 (10.4%) |
| Abdominal pain | 1 (0.8%) | 2 (3.1%) | 4 (3.1%) | 2 (3.0%) |
Figure 3.
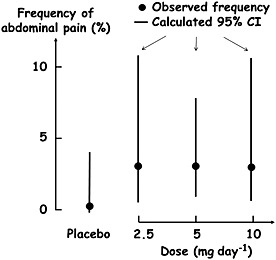
The frequencies of abdominal pain in the combined ARIES‐1 and ‐2 studies 30; the dots are the original data points, the vertical lines are the calculated 95% confidence intervals (CI)
Figure 4.
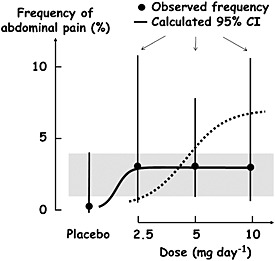
Three possible interpretations of the data in Figure 3: (i) the dotted curve is a dose–response relationship that fits the data, with the maximum reached at the highest dose used in the study; (ii) the solid curve is an example of a dose–response relationship with the maximum reached at the lowest dose used in the study; (iii) the shaded area is the region that is common to the 95% confidence ranges of all the mean frequencies, including placebo, showing that the adverse event need not be an adverse reaction at all. CI, confidence interval
Conclusions
We have outlined four major categories of reasons that explain apparent non‐dose‐relatedness of adverse drug reactions, reconciling clinical observations with the law of mass action, which predicts dose responsiveness. In most cases, we have been able to find examples of these mechanisms in published reports.
We suggest that whenever an adverse drug reaction appears to be non‐dose‐related, the reasons should be sought, bearing in mind the mechanisms that we have described.
Competing Interests
Both authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; JKA is a member of a Technology Appraisal Committee of NICE, Editor of Meyler's Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions, Editor of the Side Effects of Drugs Annuals, and a member of the Editorial Advisory Board of the Adverse Drug Reactions Bulletin. REF is a member of the Appeals Panel of NICE, Editor of the Adverse Drug Reactions Bulletin, and Director of the MHRA Yellow Card Centre West Midlands; they declare no other relationships or activities that could appear to have influenced the submitted work. The views expressed in this article are those of the authors and are not necessarily shared by these institutions or others associated with them.
Aronson, J. K. , and Ferner, R. E. (2016) The law of mass action and the pharmacological concentration–effect curve: resolving the paradox of apparently non‐dose‐related adverse drug reactions. Br J Clin Pharmacol, 81: 56–61. doi: 10.1111/bcp.12706.
References
- 1. Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf 2005; 28: 851–70. [DOI] [PubMed] [Google Scholar]
- 2. Aronson JK, Price D, Ferner RE. A strategy for regulatory action when new adverse effects of a licensed product emerge. Drug Saf 2009; 32: 91–8. [DOI] [PubMed] [Google Scholar]
- 3. Aronson JK, Ferner RE. Preventability of drug‐related harms. Part 2: proposed criteria, based on frameworks that classify adverse drug reactions. Drug Saf 2010; 33: 995–1002. [DOI] [PubMed] [Google Scholar]
- 4. Paton WDM, Payne JP. Pharmacological Principles and Practice, 1st edn (reprinted with corrections). London: J & A Churchill Limited, 1969; 3. [Google Scholar]
- 5. Chick H. An investigation of the laws of disinfection. J Hyg (Lond) 1908; 8: 92–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tallarida RJ, Jacob LS. The Dose–Response Relation in Pharmacology. Heidelberg: Springer‐Verlag, 1979. [Google Scholar]
- 7. Calabrese EJ, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol 2005; 202: 289–301. [DOI] [PubMed] [Google Scholar]
- 8. Stephenson RP. A modification of receptor theory. Br J Pharmacol Chemother 1956; 11: 379–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with chronic lung diseases treated with bronchodilator drugs and inhaled and oral corticosteroids. Chest 2007; 132: 1599–607. [DOI] [PubMed] [Google Scholar]
- 10. Smith SW, Hauben M, Aronson JK. Paradoxical and bidirectional drug effects. Drug Saf 2012; 35: 173–89. [DOI] [PubMed] [Google Scholar]
- 11. Levine RR. Factors modifying the effects of drugs in individuals In: Pharmacology: Drug Actions and Reactions. Boston, MA: Little, Brown and Co, 1973; 261–91. [Google Scholar]
- 12. Wayne EJ. Problems of toxicity in clinical medicine In: The Evaluation of Drug Toxicity, eds Walpole AL, Spinks A. London: J & A Churchill Ltd, 1958: 1–11. [Google Scholar]
- 13. Rawlins MD, Thompson JW. Pathogenesis of adverse drug reactions In: Textbook of Adverse Drug Reactions, ed Davies DM. Oxford: Oxford University Press, 1977: 44. [Google Scholar]
- 14. Rawlins MD, Thompson JW. Pathogenesis of adverse drug reactions In: Textbook of Adverse Drug Reactions, 2nd edn, ed Davies DM. Oxford: Oxford University Press, 1981: 11. [Google Scholar]
- 15. Rawlins MD. Clinical pharmacology: adverse reactions to drugs. Br Med J (Clin Res Ed) 1981; 282: 974–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rawlins MD, Thomas SHL. Mechanisms of adverse drug reactions In: Davies's Textbook of Adverse Drug Reactions, 5th edn, eds Davies DM, Ferner RE, De Glanville H. London: Chapman and Hall Medical, 1998: 40. [Google Scholar]
- 17. LaForce C, Alexander M, Deckelmann R, Fabbri LM, Aisanov Z, Cameron R, Owen R, Higgins M. Indacaterol provides sustained 24 h bronchodilation on once‐daily dosing in asthma: a 7‐day dose‐ranging study. Allergy 2008; 63: 103–11. [DOI] [PubMed] [Google Scholar]
- 18. Donohue JF, Singh D, Kornmann O, Lawrence D, Lassen C, Kramer B. Safety of indacaterol in the treatment of patients with COPD. Int J Chron Obstruct Pulmon Dis 2011; 6: 477–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnston CI. Pharmacology of irbesartan. Expert Opin Investig Drugs 1999; 8: 655–70. [DOI] [PubMed] [Google Scholar]
- 20. Goyer PF, Major LF. Hepatotoxicity in disulfiram‐treated patients. J Stud Alcohol 1979; 40: 133–7. [DOI] [PubMed] [Google Scholar]
- 21. Gilead Sciences Limited . AmBisome. Summary of Product Characteristics. Available at http://www.medicines.org.uk/emc, 2012 (last accessed 2 April 2015).
- 22. Frenz DA. Interpreting atmospheric pollen counts for use in clinical allergy: allergic symptomology. Ann Allergy Asthma Immunol 2001; 86: 150–7. [DOI] [PubMed] [Google Scholar]
- 23. Troisi CL, Heiberg DA, Hollinger FB. Normal immune response to hepatitis B vaccine in patients with Down's syndrome. A basis for immunization guidelines. JAMA 1985; 254: 3196–9. [PubMed] [Google Scholar]
- 24. Kelkar PS, Li JT. Cephalosporin allergy. N Engl J Med 2001; 345: 804–9. [DOI] [PubMed] [Google Scholar]
- 25. Friedmann PS, Moss C, Shuster S, Simpson JM. Quantitative relationships between sensitising dose of DNCB and reactivity in normal subjects. Clin Exp Immunol 1983; 53: 709–15. [PMC free article] [PubMed] [Google Scholar]
- 26. Cameron HA, Ramsay LE. The lupus syndrome induced by hydralazine: a common complication with low dose treatment. Br Med J (Clin Res Ed) 1984; 289: 410–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carstairs KC, Breckenridge A, Dollery CT, Worlledge SM. Incidence of a positive direct Coombs test in patients on alpha‐methyldopa. Lancet 1966; 2: 133–5. [DOI] [PubMed] [Google Scholar]
- 28. McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2006; 296: 1633–44. [DOI] [PubMed] [Google Scholar]
- 29. Fairley CK, McNeil JJ, Desmond P, Smallwood R, Young H, Forbes A, Purcell P, Boyd I. Risk factors for development of flucloxacillin associated jaundice. BMJ 1993; 306: 233–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ, Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double‐Blind, Placebo‐Controlled, Multicenter, Efficacy Studies (ARIES) Group . Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double‐blind, placebo‐controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008; 117: 3010–19. [DOI] [PubMed] [Google Scholar]


