Abstract
Aims
5‐FU is the backbone of most regimens in digestive oncology. Administration of standard 5‐FU leads to 15–30% of severe side effects, and lethal toxicities are regularly reported with fluoropyrimidine drugs. Dihydropyrimidine dehydrogenase (DPD) deficiency is a pharmacogenetic syndrome responsible for most cases of life‐threatening toxicities upon 5‐FU intake, and pre‐treatment checking for DPD status should help to reduce both incidence and severity of side effects through adaptive dosing strategies.
Methods
We have used a simple method for rapidly establishing the DPD phenotype of patients with cancer and used it prospectively in 59 routine patients treated with 5‐FU‐based therapy for digestive cancers. No patient with total DPD deficiency was found but 23% of patients exhibited poor metabolizer phenotype, and one patient was phenotyped as profoundly deficient. Consequently, 5‐FU doses in poor metabolizer patients were cut by an average 35% as compared with non deficient patients (2390 ± 1225 mg vs. 3653 ± 1371 mg, P < 0.003, t‐test).
Results
Despite this marked reduction in 5‐FU dosing, similar efficacy was achieved in the two subsets (clinical benefit: 40 vs. 43%, stable disease: 40 vs. 37%, progressive disease: 20% in both subsets, P = 0.893, Pearson's chi‐square). No difference in toxicities was observed (P = 0.104, Fisher's exact test). Overall, only 3% of early severe toxicities were recorded, a value markedly lower than the 15‐30% ones usually reported with 5‐FU.
Conclusions
This feasibility study shows how simplified DPD‐based adaptive dosing of 5‐FU can reduce sharply the incidence of treatment‐related severe toxicities while maintaining efficacy as part of routine clinical practice in digestive oncology.
Keywords: 5‐FU, adaptive dosing, digestive oncology, DPD deficency, efficacy, toxicity
What is Already Known About this Subject
5‐FU is the most‐widely prescribed anticancer drug.
Standard 5‐FU dosing claims 15–30% of severe toxicities and 1% of toxic death.
Dihydropyrimidine dehydrogenase (DPD) deficiency is a pharmacogenetic polymorphism responsible for most cases of severe/lethal toxicities because it affects the liver disposition of 5‐FU.
What this Study Adds
We have implemented a simple and rapid method to establish DPD status on a phenotypic basis in patients with digestive cancers.
This method was next used to tailor 5‐FU dosing according to DPD phenotype in routine cancer patients.
Reducing 5‐FU dosing in DPD deficient patients did not affect drug efficacy while it prevented severe toxicities.
The efficacy−toxicity balance of 5‐FU could be improved at low cost using this strategy.
Introduction
5‐FU is administered for treating a variety of solid tumours in adults, including digestive cancers. In patients with cancer of the lower digestive tract, 5‐FU is associated with other cytoxics such as irinotecan (a.k.a. Folfiri regimen) or oxaliplatin (a.k.a. Folfox), and can be further combined with the latest available targeted therapies such as anti‐VEGF or anti‐EGFR1 monoclonal antibodies, making this drug the backbone of most treatments in digestive oncology 1. Owing to the number of patients treated worldwide, limiting the occurrence of severe 5‐FU‐related side effects is a major issue, and it is known that patients with dihydropyrimidine dehydrogenase (DPD) deficiency, a pharmacogenetic syndrome resulting in partial or total loss of ability to detoxify 5‐FU in the liver (Figure 1), will experience severe/lethal toxicities 2. Depending on the regimen, administration of standard 5‐FU usually leads to 15–30% of severe toxicities, and about 1% of toxic deaths have been regularly reported in the literature 3. Historically, direct measurement of DPD enzymatic activity in lymphocytes with radiolabelled substrates has been first proposed to establish the DPD status in patients. However, it is now considered as a time‐consuming, costly and potentially biased method which can fail in predicting both the 5‐FU pharmacokinetic profile and pharmacodynamic endpoints 4, and a variety of surrogate methods have been tested for years 5 Upfront screening for DPD deficiency should help to improve the efficacy/toxicity balance of 5‐FU through adaptive dosing strategies. Various approaches have been proposed to address the issue of securing 5‐FU dosing, with noticeable results in improving clinical outcome 6. However, most of them require heavy pharmacokinetics and/or expensive phenotyping‐genotyping combined strategies with multiple sampling, that may not always meet the bedside requirements of implementation as a routine practice next 7, 8. We have therefore developed a cost‐effective and rapid surrogate method to establish, on a phenotypic basis using a single blood sample and standard h.p.l.c. equipment, the DPD status in patients scheduled for a 5‐FU‐based regimen 9 and to tailor dosing accordingly. Here, we present the clinical results of this rapid DPD‐driven adaptive dosing strategy over 1 year of routine clinical practice in the digestive oncology unit of our institute.
Figure 1.
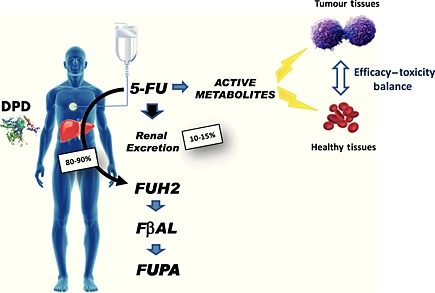
Dihydropyrimidine dehydrogenase (DPD) rapidly catabolizes more than 80% of the administered dose of 5‐FU to inactive metabolites. The remaining 5‐FU is distributed throughout the body and converted into nucleosides or deoxunucleosides then nucleotides or deoxynucleotides, including FdUMP, FdUTP and FUTP which display cytotoxic properties against TS, DNA and RNA respectively. DPD deficiency will increase the conversion of 5‐FU into active metabolites, thus triggering exposure of both healthy and tumor tissues
Methods
Patients
Any new adult patients admitted in the Digestive Oncology Unit of the La Timone University Hospital of Marseille, France and scheduled for any 5‐FU‐based regimen were considered. A total of 59 adult patients (34M/25F, mean age 63.3 , range 24–83 years old, 92.6% Caucasian, 5.7% northern African, 1.7% African) were hospitalized over 1 year of routine clinical practice in the unit. All patients were scheduled for a 5‐FU‐containing regimen for treating digestive cancer (e.g. colorectal, rectal, other) and none had received a fluoropyrimidine drug before. Signed informed consent was obtained prior to sampling the patients for DPD genetic status determination. Patients were scheduled for Folfox‐4 (36%), FolFiri (20%), LV‐5‐FU + CDDP (19%), LV‐5‐FU2 (15%) and other fluoropyrimidine‐containing chemotherapy combinations (10%).
Pre‐therapeutic screening for DPD
One 3 ml blood sample was withdrawn about 1 week before starting the treatment for DPD status evaluation. DPD deficiency was screened using a surrogate phenotyping test based upon the monitoring of the endogenous UH2 to U (UH2 : U) ratio in plasma after standard liquid–liquid extraction using a simple and time‐effective h.p.l.c.‐u.v. method, adapted from the method previously described 9, 10. Calculation of such a ratio permits the determination of DPD status as a continuous variable. Because no mathematical model was yet available, here we categorized patients depending on their UH2 : U ratio values. Patients were considered as not extensive metabolizers (EM) when the UH2 : U ratio was >4, and poor metabolizers (PM) when the UH2 : U ratio was <4 (i.e. mildly DPD deficient when the UH2 : U ratio was comprised between 2 and 4, intermediary DPD deficient when the U : UH2 ratio was comprised between 1 and 2, and profoundly DPD deficient when the UH2 : UH ratio was <1). Complete deficiency was defined as UH2 : U below 0.5 or when UH2 was not detectable at all upon h.p.l.c. analysis. Additional search for the canonical DPYD*2A allelic variant (IVS14 + 1G > A) was scheduled only in patients for whom profound or severe deficiency was evidenced, using HRM technology as described previously 9. To this end, a standardized personal informed consent form for germline genetic investigations was obtained following the French Biomedicine Agency guidelines.
DPD‐based adaptive dosing
Doses were tailored prospectively according to the recorded DPD status (Figure 2). 5‐FU was be precluded in patients displaying total DPD deficiency (i.e. UH2 : U < 0.5) due to previous observations of elevated risk of toxic death in those individuals 7. The geometric scale for dose tailoring was indicative, and could be further adjusted based upon the clinical experience of the team 10. Frail patients (e.g. elderly patients, poor performance status or patients presenting with several comorbidities) could have their 5‐FU dose further reduced, regardless of their DPD status, as part of the routine clinical practice of the unit. Comparison in dosing between DPD deficient and non‐DPD deficient subsets was performed using a standard t‐test (Sigma Stat 2.03, SPSS Inc, Germany).
Figure 2.
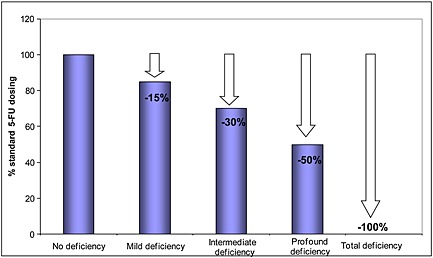
Geometric scale for tailoring 5‐FU dosing depending on the extent of DPD deficiency in PM patients. Because no mathematical model has yet been made available, DPD status established from UH2 : U ratio measurement in plasma was transformed as a discrete value and dose reduction was proposed using a linear geometric scale
Toxicity was monitored using standard CTC 2.0 grading. Special attention was paid to early toxicities (i.e. showing after the first and the second course of 5‐FU) because they are more likely to be attributed to inherited impaired DPD function. Delayed or cumulative toxicities (i.e. showing after the 3 day course) were monitored as well as part of routine care, despite being less likely to be attributed to a genetic polymorphism. Efficacy was evaluated at 3 months using the standard RECIST 1.1. criteria in digestive oncology. Clinical benefit was determined as the combined complete + partial responses.
Comparisons between groups were performed using Pearson's chi‐square test or Fisher's exact test depending on data distribution (Sigma Stat 2.03, SPSS Inc, Germany).
Results
DPD‐phenotype screening
UH2 : U ratios among our patients were not distributed following a normal law (P > 0.05, Kolmogorov Smirnov testing) and normal Kernel analysis confirmed indeed a multi‐modal distribution (data not shown). Fifteen out of 59 patients (i.e. 25%) were found to be PM and displayed mild (eight patients, i.e. 13.6%), intermediary (six patients, i.e. 10.2%) and profound (one patient, i.e. 1.7%) DPD deficiency. No patient with total DPD deficiency was found. Accordingly, no patient bearing the IVS14 + 1G > A polymorphism was found (data not shown). No difference in age (69.3 ± 9.8 vs. 61.4 ± 13.6 years), gender (9 M/6F vs. 25 M/19F) or ethnicity was observed between PM patients and EM patients.
DPD‐based dose tailoring
In the subset of DPD deficient patients (subset 1), 5‐FU dosage was cut by 15–50%. Consequently, mean 5‐FU total doses were 2390 ± 1225 mg in DPD deficient patients (subset 1, n = 15) and 3653 ± 1371 mg in patients with no DPD deficiency (subset 2, n = 44), i.e. an average 35% reduction in dosing (P = 0.003, t‐test).
Table 1 encapsulates both toxicity and efficacy figures in the two subsets. No early severe toxicities (i.e. > grade 3) were observed in the subset of PM patients with tailored dosage, whereas two EM patients (i.e. 5% of subset 2 representing 3% of the all patients) treated with standard Folfiri and LV‐5‐FU + CDDP regimens showed grade 3 diarrhoea after the first and second course of treatment. Severe delayed/cumulative toxicities showed after the third course (i.e. more than 42 days after the first 5‐FU administration) in three EM patients (one grade 3 nausea/vomiting, one grade 3 diarrhoea and one grade 3 neutropenia) and in one PM patient with reduced dosing (grade 3 neutropenia). Because of zero values in the early severe toxicities category in subset 1, it was not possible to run a chi‐square test. Fisher's exact test was performed instead and found no statistical difference in toxicities between the subsets (P = 0.104). For subsets 1 and 2, the clinical benefit (CR + PR) was 40% and 43%, stable disease 40% and 37% respectively and progressive disease 20% in both subsets. No statistical difference in response was found between the groups (P = 0.893, Pearson's chi‐square test).
Table 1.
Comparison of 5‐FU dosing, clinical response and drug‐related toxicities between subset 1 and subset 2. Despite a marked reduction in dosing in subset 1, no difference in clinical outcome was observed between the groups
| Subset 1 (n = 15) PM patients Tailored 5‐FU | Subset 2 (n = 44) EM patients Standard 5‐FU | ||
|---|---|---|---|
| 5‐FU total dose (mg) | 2390 +/− 1225 | 3653+/− 1371 | P = 0003 (t‐test) |
| Clinical benefit (CR + PR) | 40% | 43% | P = 0893 (Pearson's chi‐square) |
| Stable disease | 40% | 37% | |
| Progressive disease | 20% | 20% | |
| Early severe toxicities | 0% | 5% | P = 0104 Fisher's exact test |
| Early G1‐G2 toxicities | 80% | 86% | |
| No early toxicities | 20% | 9% | |
| Delayed severe toxicities | 7% | 7% |
CR complete response; PR partial response.
Discussion
Standard 5‐FU admittedly leads to 10–30% of severe toxicities, including 0.5 up to 4% of toxic‐deaths, depending on the regimen 3, 9. DPD deficiency is a pharmacogenetic syndrome associated with increased risk of developing life‐threatening toxicities in patients receiving a 5‐FU‐containing regimen. Admittedly, 30 to 80% of the severe toxicities recorded after 5‐FU intake could be attributable to impaired DPD activity in the liver 2, 11 and DPD deficiency is an issue in patients undergoing oral capecitabine as well 9, 12. In this respect, developing strategies to anticipate treatment‐related toxicities by the pre‐identification of DPD deficient patients and subsequent dose tailoring should improve the efficacy–toxicity balance of this widely prescribed anticancer drug 7. Defining the best strategy to evaluate the actual DPD status is a controversial issue, and the utility of screening patients on the DPYD genotype remains widely debated today 2, 13. Several variants (i.e. IVS14 + 1G > A, 464 T > A, 1679C > T, 2846 T > A and 2194G > A to name but a few) have been proposed as genetic determinants to anticipate 5‐FU‐induced toxicities 14 and novel mutations are regularly reported 15, 16. Of note, a growing number of meta‐analyses are now published but do not systematically identify the same DPYD variants 17, 18, 19. Here, DPD status was primarily evaluated on a phenotype basis. Because this work was taken from the actual routine practice of the Digestive Oncology Unit of our institute, it encapsulates a wide range of 5‐FU based regimens (up to four main regimens: FolFox, FolFiri, LV‐5FU2, LV‐5FU + CDDP), so as to present our strategy in the actual diversity of real‐life clinical settings. Here, simple functional testing for DPD activity requiring a single pre‐treatment blood sample and a standard h.p.l.c. procedure proved to be sufficient to identify PM patients on a phenotypic basis and to propose next a simple geometric dose reduction strategy. Of note, we identified 25% of patients at risk of impaired detoxification, a figure markedly higher than the incidence of DPD deficiency usually reported from DPYD gene‐candidate studies, but in line with our previous functional study in head and neck patients 10. Although most of these PM patients (eight out of 15, i.e. >50% of the cases) were identified with mild impairment only and that no complete DPD deficiency was observed. This apparent discrepancy with the literature illustrates how the actual incidence of DPD impairment all depends on the technique used to screen it, and is probably higher actually than what gene‐candidate studies have suggested thus far. Indeed, most genetic studies based upon the search of the canonical DPYD*2A allelic variant or the combo c.1905 + 1G > A, c.1679 T > G, c.2846A > T polymorphisms 19, 20, 21 may fail in identifying all the PM individuals 2, not to mention the ones subjected to exogenous causes for impaired DPD such as drug–drug interactions, for example with DPD‐inhibiting antivirals 22. Importantly, and despite the fact that this clinical practice was performed in a single unit on a small number of patients which should prompt us to interpret the findings cautiously, this observational study strongly suggests that a rapid, simple and technically easily affordable pre‐therapeutic screening for DPD deficiency can sharply reduce the risk of drug‐related toxicities, through subsequent cuts in dosing. Here, only two out of 59 patients (i.e. 3%) showed early severe toxicities, none among those with PM phenotype, whereas 15% of grade 3–4 toxicities and 0.9% of toxic deaths had been previously reported in our institute upon the first administration of standard 5‐FU 9. Of note, only 5% of severe toxicities were recorded in the subset of EM patients who were administered standard 5‐FU, despite the presence of elderly patients up to 83 years old. This suggests that most of the 15–30% of patients who usually display severe side effects upon 5‐FU intake as reported in the literature are probably the PM individuals administered with standard dosage of the drug when no preliminary screening for DPD deficiency is undertaken. Importantly, and despite the average 35% lower doses administered to patients with DPD deficiency, no loss in efficacy was observed, most probably because the cut in dosing was balanced by the reduced liver clearance of 5‐FU in these very patients. Despite its small sample size and the heterogeneity of the treatment modalities, this study advocates for the pre‐therapeutic screening for DPD status in patients scheduled for 5‐FU‐based regimen. Here, our data suggest that no additional expensive DPYD genotype support was needed for sharply reducing the risk of 5‐FU‐induced severe toxicities. The relevance of UH2 : U monitoring as a surrogate for DPD deficiency testing remains widely debated, when not openly questioned 23, and alternative and promising approaches such as exogenous oral uracil intake 24 or a saliva test 25 have been recently proposed, among the variety of genotypic and phenotypic strategies published thus far to address the issue of upfront detection of DPD deficiency 6. Regardless of the functional test chosen eventually, and owing to the complexity of picturing DPYD genetic and epigenetic regulations, establishing DPD phenotype remains a quick, cost‐effective and convenient method to secure the administration of fluoropyrimidine drugs. Beside 5‐FU, this approach could be extended to oral capecitabine, because life‐threatening toxicities have been already reported in DPD deficient patients upon capecitabine intake 25, 26. Additionally, because 5‐FU exposure levels have been repeatedly associated with clinical outcome 7, 27, the issue of ultra‐metabolizer patients prone to suboptimal dosing and detected with a phenotypic approach is a rising concern with 5‐FU 7. Here, six out of the 59 patients (i.e. 11%) displayed abnormally high UH2 : U values, thus suggesting a particularly elevated conversion rate of 5‐FU towards inactive dihydro‐5‐FU. Of note, two of them (33%) had progressive disease, although the number of observations was too small to draw any conclusion regarding the relevance of increasing 5‐FU dosing so as to achieve higher efficacy in the future. Finally, beyond its small size one of the weaknesses of this study was the geometric scale used to customize dosing which was highly empirical and relied mostly upon the past clinical expertise of our laboratory and our medical team in the field of DPD deficiency. Because phenotyping DPD generates a continuous variable, developing a dedicated PK/PD model integrating DPD status as a covariate to calculate precisely the individual dosing should achieve better results in terms of reduction of toxicities and clinical efficacy in the future, provided that PK‐population studies are performed. Several models for describing 5‐FU pharmacokinetics in DPD deficient patients have already been proposed 28, 29, 30. When combined with DPD status used as a covariate, and because 5‐FU is always administered as long‐lasting infusions (i.e. 24 up to 96 h), such PK‐pop models could be further used to tailor in real‐time 5‐FU dosing, based upon a priori DPD status information, population parameters and early blood samples collected as soon as 5‐FU reaches its steady‐state.
In conclusion we have implemented in routine clinical practice a simple method to detect patients with DPD PM status so as to tailor dosing of 5‐FU. Although adaptive dosing was performed on an empirical and intuitive geometric basis (the lower the UH2 : U ratio, the deeper the reduction), clinical monitoring in 59 patients with digestive cancers showed that reducing dosing by an average 35% in PM individuals did not affect efficacy. Of note, only 3% of severe toxicities were recorded, a value markedly lower than the 15–30% of treatment‐related toxicities described with 5‐FU. Overall, and although to be considered as a feasibility study, this work demonstrates that basic DPD‐based adaptive dosing of 5‐FU achieves better efficacy–toxicity balance in patients with digestive cancer and can be implemented in routine clinical practice. Developing a proper PK/PD/PGx model should help in the future to tailor more precisely 5‐FU dosing, so as to achieve a better optimization of the efficacy–toxicity balance of 5‐FU.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Launay, M. , Dahan, L. , Duval, M. , Rodallec, A. , Milano, G. , Duluc, M. , Lacarelle, B. , Ciccolini, J. , and Seitz, J.‐F. (2016) Beating the odds: efficacy and toxicity of dihydropyrimidine dehydrogenase‐driven adaptive dosing of 5‐FU in patients with digestive cancer. Br J Clin Pharmacol, 81: 124–130. doi: 10.1111/bcp.12790.
Principal investigator
References
- 1. Aklilu M, Eng C. The current landscape of locally advanced rectal cancer. Nat Rev Clin Oncol 2011; 8: 649–59. [DOI] [PubMed] [Google Scholar]
- 2. Ciccolini J, Gross E, Dahan L, Lacarelle B, Mercier C. Routine dihydropyrimidine dehydrogenase testing for anticipating 5‐fluorouracil‐related severe toxicities: hype or hope? Clin Colorectal Cancer 2010; 9: 224–8. [DOI] [PubMed] [Google Scholar]
- 3. Tsalic M, Bar‐Sela G, Beny A, Visel B, Haim N. Severe toxicity related to the 5‐fluorouracil/leucovorin combination (the mayo clinic regimen): a prospective study in colorectal cancer patients. Am J Clin Oncol 2003; 26: 103–6. [DOI] [PubMed] [Google Scholar]
- 4. Etienne MC, Lagrange JL, Dassonville O, Fleming R, Thyss A, Renée N, Schneider M, Demard F, Milano G. Population study of dihydropyrimidine dehydrogenase in cancer patients. J Clin Oncol 1994; 12: 2248–53. [DOI] [PubMed] [Google Scholar]
- 5. Mercier C, Ciccolini J. Profiling dihydropyrimidine dehydrogenase deficiency in patients with cancer undergoing 5‐fluorouracil/capecitabine therapy. Clin Colorectal Cancer 2006; 6: 288–96. [DOI] [PubMed] [Google Scholar]
- 6. van Staveren MC, Guchelaar HJ, van Kuilenburg AB, Gelderblom H, Maring JG. Evaluation of predictive tests for screening for dihydropyrimidine dehydrogenase deficiency. Pharmacogenomics J 2013; 13: 389–95. [DOI] [PubMed] [Google Scholar]
- 7. Gamelin E, Delva R, Jacob J, Merrouche Y, Raoul JL, Pezet D, Dorval E, Piot G, Morel A, Boisdron‐Celle M. Individual fluorouracil dose adjustment based on pharmacokinetic follow‐up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol 2008; 26: 2099–105. [DOI] [PubMed] [Google Scholar]
- 8. Ibrahim T, Di Paolo A, Amatori F, Mercatali L, Ravaioli E, Flamini E, Sacanna E, Del Tacca M, Danesi R, Amadori D. Time‐dependent pharmacokinetics of 5‐fluorouracil and association with treatment tolerability in the adjuvant setting of colorectal cancer. J Clin Pharmacol 2012; 52: 361–9. [DOI] [PubMed] [Google Scholar]
- 9. Ciccolini J, Mercier C, Evrard A, Dahan L, Boyer JC, Duffaud F, Richard K, Blanquicett C, Milano G, Blesius A, Durand A, Seitz JF, Favre R, Lacarelle B. A rapid and inexpensive method for anticipating severe toxicity to fluorouracil and fluorouracil‐based chemotherapy. Ther Drug Monit 2006; 28: 678–85. [DOI] [PubMed] [Google Scholar]
- 10. Yang CG, Ciccolini J, Blesius A, Dahan L, Bagarry‐Liegey D, Brunet C, Varoquaux A, Frances N, Marouani H, Giovanni A, Ferri‐Dessens RM, Chefrour M, Favre R, Duffaud F, Seitz JF, Zanaret M, Lacarelle B, Mercier C. DPD‐based adaptive dosing of 5‐FU in patients with head and neck cancer: impact on treatment efficacy and toxicity. Cancer Chemother Pharmacol 2011; 67: 49–56. [DOI] [PubMed] [Google Scholar]
- 11. Van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5‐fluorouracil. Eur J Cancer 2004; 40: 939–50. [DOI] [PubMed] [Google Scholar]
- 12. Mercier C, Ciccolini J. Univ severe or lethal toxicities upon capecitabine intake: is DPYD genetic polymorphism the ideal culprit? Trends Pharmacol Sci 2007; 28: 597–8. [DOI] [PubMed] [Google Scholar]
- 13. Bocci G, Di Paolo A, Barbara C, Masi G, Fornaro L, Loupakis F, Allegrini G, Falcone A, Del Tacca M, Danesi R. Pharmacokinetics, a main actor in a many‐sided approach to severe 5‐FU toxicity prediction. Br J Clin Pharmacol 2009; 67: 132–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amstutz U, Froehlich TK, Largiadèr CR. Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5‐fluorouracil toxicity. Pharmacogenomics 2011; 12: 1321–36. [DOI] [PubMed] [Google Scholar]
- 15. Thomas F, Hennebelle I, Delmas C, Lochon I, Dhelens C, Garnier Tixidre C, Bonadona A, Penel N, Goncalves A, Delord JP, Toulas C, Chatelut E. Genotyping of a family with a novel deleterious DPYD mutation supports the pretherapeutic screening of DPD deficiency with dihydrouracil/uracil ratio. Clin Pharmacol Ther 2015; Aug 11. doi:. doi:10.1002/cpt.210. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Del Re M, Michelucci A, Di Leo A, Cantore M, Bordonaro R, Simi P, Danesi R. Discovery of novel mutations in the dihydropyrimidine dehydrogenase gene associated with toxicity of fluoropyrimidines and viewpoint on preemptive pharmacogenetic screening in patients. EPMA J 2015; 6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terrazzino S, Cargnin S, Del Re M, Danesi R, Canonico PL, Genazzani AA. DPYD IVS14 + 1G>A and 2846A>T genotyping for the prediction of severe fluoropyrimidine‐related toxicity: a meta‐analysis. Pharmacogenomics 2013; 14: 1255–72. [DOI] [PubMed] [Google Scholar]
- 18. Li Q, Liu Y, Zhang HM, Huang YP, Wang TY, Li DS, Sun HZ. Influence of DPYD genetic polymorphisms on 5‐fluorouracil toxicities in patients with colorectal cancer: a meta‐analysis. Gastroenterol Res Pract 2014; 2014: 827989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Rosmarin D, Palles C, Church D, Domingo E, Jones A, Johnstone E, Wang H, Love S, Julier P, Scudder C, Nicholson G, Gonzalez‐Neira A, Martin M, Sargent D, Green E, McLeod H, Zanger UM, Schwab M, Braun M, Seymour M, Thompson L, Lacas B, Boige V, Ribelles N, Afzal S, Enghusen H, Jensen SA, Etienne‐Grimaldi MC, Milano G, Wadelius M, Glimelius B, Garmo H, Gusella M, Lecomte T, Laurent‐Puig P, Martinez‐Balibrea E, Sharma R, Garcia‐Foncillas J, Kleibl Z, Morel A, Pignon JP, Midgley R, Kerr D, Tomlinson I. Genetic markers of toxicity from capecitabine and other fluorouracil‐based regimens: investigation in the QUASAR2 study, systematic review, and meta‐analysis. J Clin Oncol 2014; 32: 1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee SY, McLeod HL. Pharmacogenetic tests in cancer chemotherapy: what physicians should know for clinical application. J Pathol 2011; 223: 15–27. [DOI] [PubMed] [Google Scholar]
- 21. Falvella FS, Caporale M, Cheli S, Martinetti A, Berenato R, Maggi C, Niger M, Ricchini F, Bossi I, Di Bartolomeo M, Sottotetti E, Bernardi FF, de Braud F, Clementi E, Pietrantonio F. Undetected toxicity risk in pharmacogenetic testing for dihydropyrimidine dehydrogenase. Int J Mol Sci 2015; 16: 8884–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diasio RB. Sorivudine and 5‐fluorouracil; a clinically significant drug–drug interaction due to inhibition of dihydropyrimidine dehydrogenase. Br J Clin Pharmacol 1998; 46: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sistonen J, Büchel B, Froehlich TK, Kummer D, Fontana S, Joerger M, van Kuilenburg AB, Largiadèr CR. Predicting 5‐fluorouracil toxicity: DPD genotype and 5,6‐dihydrouracil:uracil ratio. Pharmacogenomics 2014; 15: 1653–66. [DOI] [PubMed] [Google Scholar]
- 24. van Staveren MC, Theeuwes‐Oonk B, Guchelaar HJ, van Kuilenburg AB, Maring JG. Pharmacokinetics of orally administered uracil in healthy volunteers and in DPD‐deficient patients, a possible tool for screening of DPD deficiency. Cancer Chemother Pharmacol 2011; 68: 1611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carlsson G, Odin E, Gustavsson B, Wettergren Y. Pretherapeutic uracil and dihydrouracil levels in saliva of colorectal cancer patients are associated with toxicity during adjuvant 5‐fluorouracil‐based chemotherapy. Cancer Chemother Pharmacol 2014; 74: 757–63. [DOI] [PubMed] [Google Scholar]
- 26. Ciccolini J, Mercier C, Dahan L, Evrard A, Boyer JC, Richard K, Dales JP, Durand A, Milano G, Seitz JF, Lacarelle B. Toxic death‐case after capecitabine + oxaliplatin (XELOX) administration: probable implication of dihydropyrimidine deshydrogenase deficiency. Cancer Chemother Pharmacol 2006; 58: 272–5. [DOI] [PubMed] [Google Scholar]
- 27. Mercier C, Ciccolini J. Severe or lethal toxicities upon capecitabine intake: is DPYD genetic polymorphism the ideal culprit? Trends Pharmacol Sci 2007; 28: 597–8. [DOI] [PubMed] [Google Scholar]
- 28. Di Paolo A, Lencioni M, Amatori F, Di Donato S, Bocci G, Orlandini C, Lastella M, Federici F, Iannopollo M, Falcone A, Ricci S, Del Tacca M, Danesi R. 5‐fluorouracil pharmacokinetics predicts disease‐free survival in patients administered adjuvant chemotherapy for colorectal cancer. Clin Cancer Res 2008; 14: 2749–55. [DOI] [PubMed] [Google Scholar]
- 29. Woloch C, Di Paolo A, Marouani H, Bocci G, Ciccolini J, Lacarelle B, Danesi R, Iliadis A. Population pharmacokinetic analysis of 5‐FU and 5‐FDHU in colorectal cancer patients: search for biomarkers associated with gastro‐intestinal toxicity. Curr Top Med Chem 2012; 12: 1713–9. [DOI] [PubMed] [Google Scholar]
- 30. van Kuilenburg AB, Häusler P, Schalhorn A, Tanck MW, Proost JH, Terborg C, Behnke D, Schwabe W, Jabschinsky K, Maring JG. Evaluation of 5‐fluorouracil pharmacokinetics in cancer patients with a c.1905 + 1G > a mutation in DPYD by means of a Bayesian limited sampling strategy. Clin Pharmacokinet 2012; 51: 163–74. [DOI] [PubMed] [Google Scholar]


