Abstract
Aims
Using a selective α‐adrenoceptor blocker for medical expulsive therapy (MET) is an effective treatment approach widely used for ureteral stones. The aim of the review was to assess the efficacy and safety of silodosin in medical expulstion therapy compared with placebo and tamsulosin.
Methods
A systematic search was performed in PubMed, Cochrane Library and Embase to identify randomized controlled trials that compared silodosin with a placebo or tamsulosin for ureteral calculi.
Results
Eight publications involving a total of 1048 patients were used in the analysis, which compared silodosin with placebo and tamsulosin. We found that silodosin was effective in treating ureteral calculi in our meta‐analysis and was superior to tamsulosin in its efficacy. The expulsion rate of all ureteral stones (OR 1.59, 95% CI 1.08, 2.36, P = 0.02), the expulsion rate of distal ureteral stones (OR 2.82, 95% CI 1.70, 4.67, P < 0.0001) and the expulsion time (days) of distal ureteral stones (standard mean difference (SMD) −4.71, 95% CI −6.60, −2.83, P < 0.00001) indicated that silodosin was more effective than the placebo. Moreover, expulsion rate (OR 2.54, 95% CI 1.70, 3.78, P < 0.00001), expulsion time (days) (SMD −2.64, 95% CI −3.64, −1.64, P < 0.00001) and pain episodes (P < 0.00001) indicated that silodosin was more effective than the tamsulosin. Even though silodosin had a significant increase in abnormal ejaculation compared with tamsulosin, no significant differences were observed for complications (OR 1.00, 95% CI 0.58, 1.74, P = 1.00).
Conclusions
This meta‐analysis indicated that silodosin was superior to placebo or tamsulosin in the efficacy for distal ureteral calculi with better control of pain. The safety profile of silodosin was similar to tamsulosin though retrograde ejaculation was worse for silodosin use. We conclude that silodosin might have potential as a MET for ureteral stones.
Keywords: silodosin, ureteral calculi, urolithiasis, medical expulsion therapy, stone and randomized controlled trials
What is Already Known About This Subject
Using a selective α‐adrenoceptor blocker for medical expulsive therapy (MET) is an effective treatment approach widely used for ureteral stones.
What This Study Adds
Silodosin might have potential as a MET for ureteral stones, which is superior to placebo and tamsulosin.
Introduction
Urolithiasis is one of the most common disorders of theurinary tract affecting about 5%–10% of the population. The increasing prevalence of ureteric stones is a matter of concern in this era, and it may be linked to improved quality of life 1.
Minimally invasive therapies, such as extracorporeal shock wave lithotripsy and ureterolithotripsy, represent efficacious treatment modalities in almost all cases. Nevertheless, these procedures imply high costs and are not risk free 2. A watchful waiting approach has been reported to be associated with spontaneous stone expulsion in up to 50% of cases but some complications may occur such as urinary tract infections, hydronephrosis and colic events 2. The use of a watchful waiting approach has been extended as a result of advances in pharmacological therapy, which can reduce symptoms and facilitate stone expulsion 3, 4.
Medical expulsive therapy (MET) has now become an established method of treatment, and it involves the use of various drugs acting on the ureter by different mechanisms. The ureter is lined by α1‐adrenergic receptors, particularly the subtype α1D, which are found more in its distal third, and they play an important role in the lower ureteric physiology through an effect on detrusor and ureteric smooth muscle contraction 5. Use of a selective α‐adrenoceptor blocker for MET is a cost effective approach to treating ureteral stones, based on the growing body of evidence supporting its efficacy 6, 7. Both the American Urological Association (AUA) and the European Association of Urology include α‐adrenoceptor blockers in their treatment recommendations 7, 8.
The blocking of these receptors subsequently induces selective relaxation of the ureteric smooth muscle, which will result in ureteric lumen dilatation facilitating antegrade stone propagation 9, 10. Several studies have shown that tamsulosin, an α1A/1D‐adrenoceptor antagonist, facilitates ureteral stone expulsion 11, 12, 13, 14. Recently, we reported that α1A‐adrenoceptors are the main participant in phenylephrine‐induced ureteral contraction in the human isolated ureter 15. Therefore, silodosin, a recently introduced selective α1A‐adrenoceptor antagonist, has shown promising results with fewer side effects and a better efficacy 16.
The goal of the present study was to perform a meta‐analysis to evaluate the safety and efficacy of silodosin compared with placebo or tamsulosin in treating ureteral calculi, which may find that silodosin might have potential as MET for ureteral stones.
Methods
Data sources and searches
We carried out an electronic search of Cochrane Library (Issue 4, April 2015), PubMed (1966 to April 2015) and Embase (1974 to April 2015). The search strategy consisted of three parts (strategies for silodosin, calculi and a specific filter for clinical trials) using the following keywords in combination with both medical subject headings terms and text words: silodosin, ureteral calculi, urolithiasis, medical expulsion therapy, stone and randomized controlled trials. There was no limitation on publication status or language. We also searched the metaRegister and World Health Organization International Clinical Trials Registry Platform for ongoing studies. Reference lists of the included studies were checked manually to identify further studies.
Studies selection
We included randomized controlled trials that compared silodosin vs. placebo or tamsulosin for ureteral calculi. The patients in this study were limited to the population diagnosed with a single, unilateral, symptomatic, ureteric stone of 10 mm or smaller in the largest dimension in the distal ureter visible. The patients were evaluated with plain X–ray, ultrasonography and unenhanced computed tomography (CT) scans whenever they were necessary. The stone size was calculated on the first plain X‐ray or CT by using a digital ruler and the greatest dimension of the stone was taken into consideration as the stone size. Studies that examined the use of silodosin in special population groups (people with renal insufficiency, urinary tract infections, high grade hydronephrosis, previous therapies for the stone, solitary kidney, history of ureteral surgery or previous endoscopic procedures, concomitant calcium antagonists or corticosteroids medications, ureteric strictures, cardiovascular diseases, incomplete data) were excluded. The primary outcomes for this study were the stone expulsion rate and complication. The secondary outcomes included the stone expulsion time (days), pain episodes and abnormal ejaculation. Trials were eligible if one of these outcome measures was reported. Study eligibility was independently determined by two authors. The authors evaluated the eligibility of the remaining studies by examining the titles, abstracts and full articles progressively. Discrepancies were resolved by discussion.
Data extraction and quality assessment
Data were extracted independently by two authors using a standard form. Data extracted included study characteristics (title, publication time and patient numbers), patient characteristics (age, the location of the stone and size), intervention, control, method (randomization, blinding and loss to follow‐up) and outcomes (estimates, standard error and P value). Discrepancies were resolved by discussion. The authors of original studies were consulted for missing information where necessary.
Both methodological quality and quality of the evidence were assessed independently by two authors. The methodological quality of included studies was appraised with the Cochrane Collaboration bias appraisal tool 17. Based on the quality assessment criteria, each study was rated and assigned to one of the three following quality categories: A) if all quality criteria were adequately met, the study was deemed to have a low risk of bias, B) if one or more of the quality criteria was only partially met or was unclear, the study was deemed to have a moderate risk of bias or C) if one or more of the criteria were not met, or not included, the study was deemed to have a high risk of bias.
Data synthesis and analysis
The comparative effects were initially analyzed by the traditional pairwise meta‐analysis method using Cochrane Collaboration review manager software (RevMan v.5.1.0). We estimated the relative risk for dichotomous outcomes and the standardized mean difference (SMD) for continuous outcomes pooled across studies by using the DerSimonian & Laird random effects model 18. We used a 95% confidence interval (CI). If the result of analysis showed P > 0.05, we considered the studies homogeneous and so chose a fixed effect model for meta‐analysis. Otherwise, a random effect model was used. We quantified inconsistency using the I2 statistic, which describes the proportion of heterogeneity across studies that is not due to chance, thus describing the extent of true inconsistency in results across trials 19. I2 <25% reflects a small level of inconsistency and I2 >50% reflects significant inconsistency.
Results
Search results and study characteristics
The literature search yielded 798 citations, of which 747 were excluded after review of titles and abstracts. The full texts of 13 remaining citations were screened, and finally eight studies 5, 20, 21, 22, 23, 24, 25, 26 including 1048 patients were included (Figure 1). Of these, four studies 21, 22, 23, 25 were included to assess the efficacy and safety of silodosin in MET compared with placebo and five studies 5, 20, 23, 24, 26 were included to assess silodosin compared with tamsulosin. The baseline characteristics of the studies included in our meta‐analysis are listed in Table 1.
Figure 1.
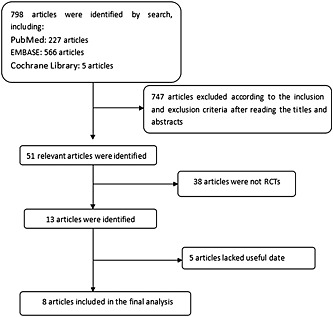
The flow diagram of the study selection
Table 1.
Study and patient characteristics
| Sample size | |||||||
|---|---|---|---|---|---|---|---|
| Study | Therapy in experimental group | Therapy in control group | Country | Experimental | Control | Duration of treatment | Inclusion population |
| Itoh et al. 21 | Silodosin 8 mg daily | Tlacebo | Japan | 56 | 56 | 4 weeks | male patients with symptomatic calculi and had unilateral distal ureteral calculi of less than 10 mm in diameter |
| Rathi et al. 23 | Silodosin 8 mg daily | Placebo | India | 29 | 28 | 4 weeks | patients with symptomatic calculi and had unilateral distal ureteral calculi of less than 10 mm in diameter |
| Rathi et al. 23 | Silodosin 8 mg daily | Tamsulosin 0.4 mg daily | India | 29 | 30 | 4 weeks | Patients with symptomatic calculi and had unilateral distal ureteral calculi of less than 10 mm in diameter |
| Kumar et al. 5 | Silodosin 8 mg daily | Tamsulosin 0.4 mg daily | India | 90 | 90 | 4 weeks | Patients aged ≥ 18 years with a ureteral stone of 5‐10mmin size situated below the common iliac vessels |
| Imperatore et al. 20 | Silodosin 8 mg daily | Tamsulosin 0.4 mg daily | Italy | 50 | 50 | 4 weeks | Aged ≥ 18 years with a single, unilateral, symptomatic, distal ureteric stone of 10 mm or smaller |
| Itoh et al. 22 | Silodosin 8 mg daily | Placebo | Japan | 95 | 92 | 8 weeks | Patients with unilateral ureteral calculi of less than 10 mm in diameter |
| Gupta et al. [24] | Silodosin 8 mg daily | Tamsulosin 0.4 mg daily | India | 50 | 50 | 4 weeks | Patients unilateral, non–impacted, uncomplicated middle or lower ureteral stones which were </= 1 cm |
| Sur et al. 25 | Silodosin 8 mg daily | Placebo | USA | 115 | 117 | 4 weeks | Patients aged ≥18 years with a unilateral calculus ≥4 mm and ≤10 mm in any location of the ureter visible within 7 d |
| Dell'Atti et al. 26 | Silodosin 8 mg daily | Tamsulosin 0.4 mg daily | Italy | 66 | 67 | 3 weeks | Patients with renal colic, a single, unilateral, radiopaque, distal ureteral stone (range 4–10 mm in size) |
Quality of individual studies
All nine RCTs were double‐blind and all described the randomization processes that they had used. All included a power calculation to determine the optimal sample size (Table 2). The level of quality of each identified study was A to B (Table 2). The funnel plot provided a qualitative estimation of publication bias of the studies, and no evidence of bias was found (Figure 5).
Table 2.
Quality assessment of individual study
| Study | Allocation sequence generation | Allocation concealment | Blinding | Loss to follow‐up | Calculation of sample size | Statistical analysis | Intention‐to‐treat analysis | Level of quality |
|---|---|---|---|---|---|---|---|---|
| Itoh et al. 21 | A | B | B | 0 | Yes | Student's t‐test or Mann–Whitney U‐test and the χ2 test | Yes | B |
| Rathi et al. 23 | A | A | A | 0 | Yes | Student's t‐test or Mann–Whitney U‐test and the χ2 test | Yes | A |
| Kumar et al. 5 | A | A | A | 6 | Yes | Bonferroni or Kruskal–Wallis test, and Mann–Whitney U test | Yes | A |
| Imperatore et al. 20 | A | A | B | 0 | Yes | Student's t‐test and the χ2 test | Yes | B |
| Itoh et al. 22 | A | B | B | 0 | Yes | Student's t‐test or Mann–Whitney U‐test and χ2 test | Yes | B |
| Gupta et al. [24] | A | A | A | 0 | Yes | Student's t‐test and χ2 test | Yes | A |
| Sur et al. 25 | A | A | A | 6 | Yes | Logistic regression, or Wilcoxon test and Kaplan–Meier analysis | Yes | A |
| Dell'Atti et al. 26 | A | B | A | 0 | Yes | Mann–Whitney and the Wilcoxon tests, or χ2 test | Yes | B |
A all quality criteria met (adequate): low risk of bias. B one or more of the quality criteria only partly met (unclear): moderate risk of bias.
C one or more criteria not met (inadequate or not used): high risk of bias.
Figure 5.
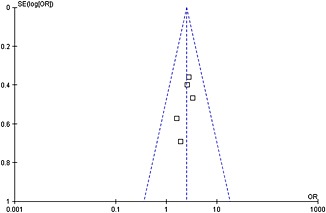
Funnel plot of the studies represented in our meta‐analysis
Silodosin compared with placebo
Expulsion rate in all cases
A total of two RCTs including 413 participants (204 in the silodosin group and 209 in the placebo group) (Figure 2) contributed to the analysis of the expulsion rate of all ureteral stones. According to our analysis, no heterogeneity was found among the trials, and a fixed effects model was thus chosen for the analysis. Compared with placebo, silodosin was associated with a significantly higher expulsion rate (OR 1.59, 95% CI 1.08, 2.36, P = 0.02). The expulsion rate in all cases with silodosin was about 8%–16% higher than with placebo.
Figure 2.
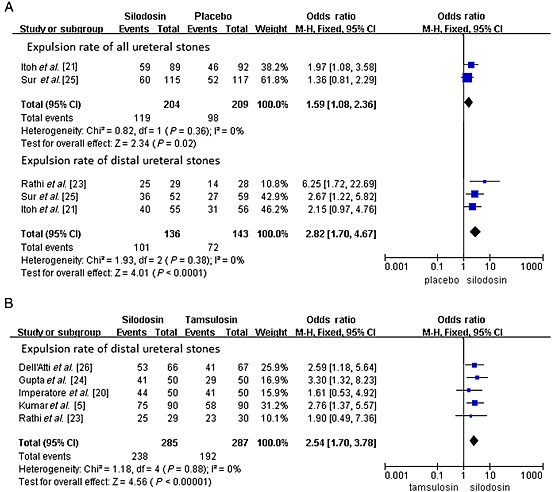
Expulsion rate. (A) expulsion rate of all ureteral stones; expulsion rate of distal ureteral stones (silodosin vs. placebo) and (B) expulsion rate of distal ureteral stones (silodosin vs. tamsulosin)
Expulsion rate of distal ureteral stones
A total of three RCTs including 279 participants (136 in the silodosin group and 143 in the placebo group) (Figure 2) contributed to the analysis of the expulsion rate of distal ureteral stones. Compared with placebo, silodosin was associated with a significantly higher expulsion rate (OR 2.82, 95% CI 1.70, 4.67, P < 0.0001). The expulsion rate of distal ureteral stones with silodosin ranged from 69% to 86%, which is about 24%–31% higher than with placebo.
Expulsion time (days) of distal ureteral stones
A total of two RCTs including 168 participants (84 in the silodosin group and 84 in the placebo group) (Figure 3) contributed to the analysis of the expulsion time (days) of distal ureteral stones. Network meta‐analysis demonstrated that silodosin was associated with a significant decrease in expulsion time of distal ureteral stones compared with placebo (SMD –4.71; 95% CI −6.60 to −2.83, P < 0.00001). The mean expulsion time (days) of distal ureteral stones with silodosin was approximately 8.3 days.
Figure 3.
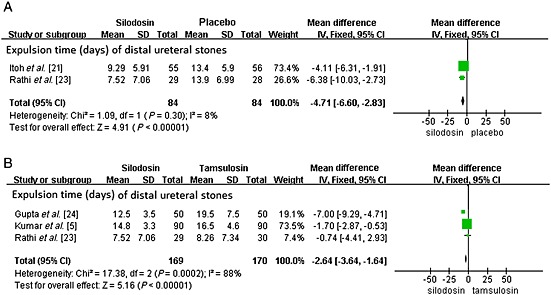
Expulsion time (days). (A) expulsion time of distal ureteral stones compared with placebo and (B) expulsion time of distal ureteral stones compared with tamsulosin
Silodosin compared with tamsulosin
Expulsion rate of distal ureteral stones
A total of five RCTs including 572 participants (285 in the silodosin group and 287 in the tamsulosin group) (Figure 2) contributed to the analysis of the expulsion rate of distal ureteral stones. According to our analysis, no heterogeneity was found among the trials, and a fixed effects model was thus chosen for the analysis. Compared with tamsulosin, silodosin was associated with a significantly higher expulsion rate (OR 2.54, 95% CI 1.70, 3.78, P < 0.0001). The expulsion rate of distal ureteral stones with silodosin was about 17% higher than with tamsulosin.
Expulsion time (days) of distal ureteral stones
A total of three RCTs including 339 participants (169 in the silodosin group and 170 in the tamsulosin group) (Figure 3) contributed to the analysis of the expulsion time (days) of distal ureteral stones. Network meta‐analysis demonstrated that silodosin was associated with a significant decrease in expulsion time of distal ureteral stones compared with tamsulosin (SMD –2.64, 95% CI −3.64, −1.64, P < 0.00001). The expulsion time (days) of distal ureteral stones with silodosin was about 3 days less than with tamsulosin.
Pain episodes
A total of two RCTs including 239 participants (119 in the silodosin group and 120 in the tamsulosin group) (Figure 4) contributed to the analysis of the pain episodes. Network meta‐analysis demonstrated that silodosin was associated with a significant decrease in pain episodes compared with tamsulosin (SMD –0.55, 95% CI −0.77, −0.33, P < 0.00001).
Figure 4.
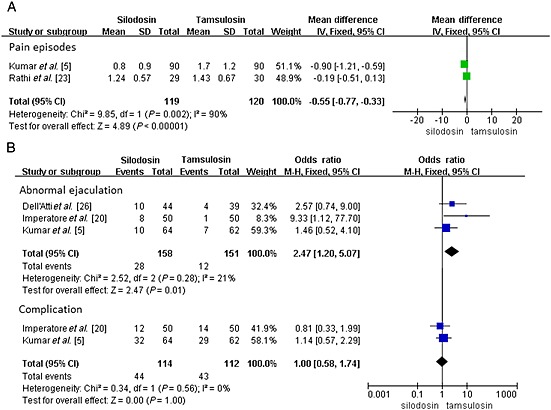
(A) changes in pain episodes and (B) changes in abnormal ejaculation and the total complication
Abnormal ejaculation
A total of three RCTs including 309 participants (158 in the silodosin group and 151 in the tamsulosin group) (Figure 4) contributed to the analysis of the abnormal ejaculation episodes. Compared with tamsulosin, silodosin was associated with a significantly higher abnormal ejaculation episodes (OR 2.47, 95% CI 1.20, 5.07, P = 0.01).
Complications
A total of two RCTs including 226 participants (114 in the silodosin group and 112 in the tamsulosin group) (Figure 4) contributed to the analysis of the complication episodes. The adverse events caused by silodosin were generally mild and did not require cessation of therapy in any patient. Dizziness, postural hypotension, headache, nasal congestion, backache, diarrhoea and abnormal ejaculation were the most common reported adverse events. Based on our analysis, the pooled estimate of OR was 1.00 and the 95% CI was 0.58, 1.74 (P = 1.00). This result suggests that silodosin showed no significant difference in complication episodes compared with tamsulosin (Figure 5).
Discussion
Ureteral colic, which is mainly due to ureterolithiasis, represents 1 to 2% of the hospital emergency admissions. MET has recently emerged as an alternative strategy for the initial management of selected patients with distal ureteric stones 27. The role of adrenergic receptors in the human ureter was first described in 1970 28. It was shown that the α‐adrenergic receptor agonists had a stimulatory effect on the ureteral smooth muscle, whereas the α‐adrenergic receptor agonists had an inhibitory effect 29. They prevent the uncoordinated muscle activity which is seen in renal colic, while maintaining ureteral peristalsis, which might facilitate a spontaneous stone passage 27. The α‐adrenoceptor blockers mainly produce relaxation of the distal human ureter by reducing the ureteric smooth muscle tone rather than completely ablating its activity. In 2005, Sigala et al. found that α1D‐ and α1A‐adrenoceptors were expressed in significantly larger amounts than α1B‐adrenoceptors in the human ureter 27. These authors also demonstrated that the distal ureter expressed a greater amount of α1‐adrenoceptor mRNA than the proximal and medial ureter. Itoh et al. reported that α1D‐adrenoceptor mRNA is more highly expressed than α1A‐adrenoceptor mRNA in each region of the ureter 30. It was shown that the α‐adrenergic receptors were classified into three different subtypes of α1A, α1B and α1D, of which the distribution in the human ureter was α1D >α1A >α1B (Table 3) 30. According to their results, an α1D‐adrenoceptor blocker maybe expected to be more effective for the expulsion of ureteral stones than an α1A‐adrenoceptor blocker. However, Tomiyama et al. reported that, in the hamster ureter, ureteral contraction was mediated mainly by α1A‐adrenoceptors, even though α1D‐adrenoceptors were more prevalent 31. Besides, Tsuzaka et al. reported that an α1A‐adrenoceptor blocker was more effective than an α1D‐adrenoceptor blocker with respect to stone expulsion rate, suggesting more clinical usefulness of α1A‐adrenoceptor blockers 16. Sasaki et al. found that among α1‐adrenoceptors, the α1A subtype played the major role in contraction in the human ureter 15. Accordingly, α1A‐adrenoceptor antagonists could become a useful medication for stone passage in urolithiasis patients. We speculate that α1A‐adrenoceptors and α1D‐adrenoceptors both have effect on the contraction in the human ureter and the selectivity of drugs with different α1‐receptor subtypes is different (Table 4AA) 32, 33, 34. In Table 4AA, we set at 1 for α1B subtype in order to compare the selectivity of drugs with α1A subtype and α1D subtype, but in reality they are all different. In addition, the binding affinities (pKi) for various α1 adrenergic receptor antagonists at α1‐adrenergic receptor subtypes is also different (Table 4AB), which is a composite from different studies 32, 33, 34.
Table 3.
Distribution of α1‐adrenoceptor (AR) subtypes in the the body's tissues 30
| Ureteral | Urethral | Vascular | |
|---|---|---|---|
| α1A‐AR | 26% | 70% | 3% |
| α1D‐AR | 51% | 27% | 12% |
| α1B‐AR | 23% | 3% | 85% |
Table 4A.
| Silodosin | Tamsulosin | Doxazosin | Terazosin | Alfuzosin | |
|---|---|---|---|---|---|
| α1A‐AR | 162 | 9.5 | 0.4 | 0.4 | 0.3 |
| α1D‐AR | 3.2 | 3 | 1.2 | 1.1 | 0.6 |
| α1B‐AR * | 1 | 1 | 1 | 1 | 1 |
we set at 1 for α1B subtype in order to compare the selectivity of drugs with α1A subtype and α1D subtype, but in reality they are all different
Tamsulosin, which is a selective α1A/α1D‐adrenergic receptor antagonist, has been widely studied in the context of MET for patients with distal ureteric stones smaller than 10 mm. It has been proved that tamsulosin increases stone expulsion rates, decreases pain, reduces mean time to stone expulsion and decreases analgesic usage when compared with placebo 35, 36, 37, 38, 39. Although most of the studies used tamsulosin, the efficacies of the other α‐adrenoceptor blockers such as doxazosin, terazosin, alfuzosin and naftopidil were also indicated 40, 41, 42. Silodosin, which is a highly selective α1A and selective α1D‐adrenoceptor antagonist, has been supported by trials is at least as effective as other α‐adrenoceptor blockers. The selectivity of silodosin for the α1D subtype is similar to tamsulosin (3.2 vs. 3 Table 4AA, 8.6 vs. 8.5 Table 4BB) but the selectivity of silodosin for the α1A subtype was approximately 17‐fold greater than that for tamsulosin (162 vs. 9.5 Table 4AA). In other words, silodosin has a higher affinity for the α1A subtype than tamsulosin (10.5 vs. 9.2 Table 4BB). Then, will silodosin have a greater effect on MET than tamsulosin?
Table 4B.
| Silodosin | Tamsulosin | Doxazosin | Terazosin | Alfuzosin | |
|---|---|---|---|---|---|
| α1A‐AR | 10.5 | 9.2 | 8.2 | 6.9 | 7.0 |
| α1D‐AR | 8.6 | 8.5 | 8.1 | 7.9 | 8.0 |
| α1B‐AR | 7.1 | 8.1 | 9.0 | 8.7 | 8.5 |
Our study reveals that silodosin was effective in the expulsion rate of all ureteral stones, in the expulsion rate of distal ureteral stones and in the expulsion time of distal ureteral stones compared with placebo. We found the expulsion rate of silodosin was 74.3% in the distal ureter, which was approximately 24% higher with silodosin than placebo. Itoh et al. 22 reported that there was no significant difference in expulsion rate of stones <5 mm between silodosin and placebo. We assumed that maybe because of the high likelihood of spontaneous passage for stones up to approximately 5 mm, MET is less likely to increase the stone‐free rate because of the high spontaneous expulsion rate 43. Therefore, it is important that administration of silodosin can facilitate expulsion of 5–10 mm distal ureteral stones, as compared with control. Our study also reveals that silodosin was more effective than tamsulosin in the expulsion rate of distal ureteral stones, in the expulsion time and in pain episodes. Stone expulsion rate in patients with distal ureteric stones treated with silodosin was 83.5% with a mean expulsion time of 11 days, which was superior to tamsulosin (66.9%, 14 days). The results of this study indicated that silodosin increases distal ureteric stone expulsion significantly along with better control of pain 5.
Adverse side effects commonly reported with different α1‐adrenoceptor blockers include dizziness, headache, asthenia, postural hypotension, syncope, rhinitis and sexual dysfunction 44, 45. Even though silodosin has a higher incidence of abnormal ejaculation than tamsulosin, no significant difference was observed in the incidence of total adverse events. Besides, we found that retrograde ejaculation were mild to moderate and was well tolerated. Those patients who experienced a retrograde ejaculation were followed‐up and they were found to have been relieved of this problem 24. No side‐effects that required cessation of the treatment with silodosin were encountered.
As the dose of silodosin was 8 mg daily, so we can conclude that silodosin 8 mg daily is an effective and well tolerated treatment for ureteral calculi. MET should be offered as a cost‐effective treatment for the patients with distal ureteral calculi sized 10 mm or smaller, who are amenable to a waiting management.
This meta‐analysis includes studies which are all findings from randomized double‐blind, placebo‐controlled trials. According to the quality assessment scale that we developed, the quality of the individual studies in the meta‐analysis was conforming. The results of this analysis have great importance from the scientific standpoint but also in everyday clinical practice. However the number of included studies was not many. The longer term safety, efficacy and persistence of silodosin cannot be extrapolated from this article. In addition, data from unpublished studies were not included in the analysis. These factors may have resulted in a bias. More high quality trials with larger samples are proposed to learn more about the efficacy and safety of the therapy on ureteral calculi. Further studies on MET for ureteral stones are needed to determine the superiority of α1A‐ vs. α1D‐adrenoceptor blockers.
Conclusion
This meta‐analysis indicates that silodosin was superior to placebo or tamsulosin in its efficacy for distal ureteral calculi with better control of pain. The safety profile of silodosin was similar to tamsulosin though retrograde ejaculation was worse with silodosin use. We conclude that silodosin might have potential as a MET for ureteral stones.
Competing Interests
The authors declare no conflict of interests. All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
This research did not receive any specific grant from any funding agency in the public, commercial or not‐for‐profit sector.
Huang, W. , Xue, P. , Zong,, H. , and Zhang, Y. (2016) Efficacy and safety of silodosin in the medical expulsion therapy for distal ureteral calculi: a systematic review and meta‐analysis. Br J Clin Pharmacol, 81: 13–22. doi: 10.1111/bcp.12737.
References
- 1. Colella J, Kochis E, Galli B, Munver R. Urolithiasis/nephrolithiasis: what's it all about? Urol Nurs 2005; 25: 427–48; 475, 449. [PubMed] [Google Scholar]
- 2. Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medicalexpulsive therapy for distal ureteral calculi. J Urol 2005; 174: 167–72. [DOI] [PubMed] [Google Scholar]
- 3. Borghi L, Meschi T, Amato F, Novarini A, Giannini A, Quarantelli C, Mineo F. Nifedipine and methylprednisolone in facilitating ureteral stone passage: a randomized, double‐blind, placebo‐ controlled study. J Urol 1994; 152: 1095–8. [DOI] [PubMed] [Google Scholar]
- 4. Porpiglia F, Destefanis P, Fiori C, Fontana D. Effectiveness of nifedipine and deflazacort in the management of distal ureter stones. Urology 2000; 56: 579–82. [DOI] [PubMed] [Google Scholar]
- 5. Kumar S, Jayant K, Agrawal MM, Singh SK, Agrawal S, Parmar KM. Role of tamsulosin, tadalafil, and silodosin as the medical expulsive therapy in lower ureteric stone: a randomized trial (a pilot study). Urology 2015; 85: 59–63. [DOI] [PubMed] [Google Scholar]
- 6. Bensalah K, Pearle M, Lotan Y. Cost‐effectiveness of medical expulsive therapy using alpha‐blockers for the treatment of distal ureteral stones. Eur Urol 2008; 53: 411–9. [DOI] [PubMed] [Google Scholar]
- 7. Management of ureteral calculi: EAU/AUA nephrolithiasis panel (2007). American UrologicalAssociationWebsite.http://www.auanet.org/education/guidelines/ureteral‐calculi.cfm. Accessed January 29, 2014.
- 8. Tiselius HG, Ackermann D, Alken P, Buck C, Conort P, Gallucci M. Working Party on Lithiasis, European Association of Urology. Guidelines on urolithiasis. European AssociationofUrologyWebsite. http://www.uroweb.org/fileadmin/user_upload/Guidelines/Urolithiasis.pdf. Accessed January 28, 2014. [DOI] [PubMed]
- 9. Ueno A, Kawamura T, Ogawa A, Takayasu H. Relation of spontaneous passage of ureteral calculi to size. Urology 1977; 10: 544–6. [DOI] [PubMed] [Google Scholar]
- 10. Porpiglia F, Vaccino D, Billia M, Renard J, Cracco C, Ghignone G, Scoffone C, Terrone C, Scarpa RM. Corticosteroids and tamsulosin in the medical expulsive therapy for symptomatic distal ureter stones: single drug or association? Eur Urol 2006; 50: 339–44. [DOI] [PubMed] [Google Scholar]
- 11. Cervenakov I, Fillo J, Mardiak J, Kopecny M, Smirala J, Lepies P. Speedy elimination of ureterolithiasis in lower part of ureters with the alpha 1‐blocker‐tamsulosin. Int Urol Nephrol 2002; 34: 25–9. [DOI] [PubMed] [Google Scholar]
- 12. Dellabella M, Milanese G, Muzzonigro G. Efficacy of tamsulosin in the medical management of juxtavesical ureteral stones. J Urol 2003; 170: 2202–5. [DOI] [PubMed] [Google Scholar]
- 13. Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, Hollenbeck BK. Medical therapy to facilitate urinary stone passage: a meta‐analysis. Lancet 2006; 368: 1171–9. [DOI] [PubMed] [Google Scholar]
- 14. Parsons JK, Hergan LA, Sakamoto K, Lakin C. Efficacy of alpha blockers for the treatment of ureteral stones. J Urol 2007; 177: 983–7. [DOI] [PubMed] [Google Scholar]
- 15. Sasaki S, Tomiyama Y, Kobayashi S, Kojima Y, Kubota Y, Kohri K. Characterization of a (1)‐adrenoceptor subtypes mediating contraction in human isolated ureters. Urology 2011; 77: e13–7. [DOI] [PubMed] [Google Scholar]
- 16. Tsuzaka Y, Matsushima H, Kaneko T, Yamaguchi T, Homma Y. Naftopidil vs silodosin in medical expulsive therapy for ureteral stones: A randomized controlled study in Japanese male patients. Int J Urol 2011; 18: 792–5. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions, v.5.1 [updated March 2011]. Cochrane Collaboration Web site. http://www.cochrane‐handbook.org/.
- 18. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imperatore V, Fusco F, Creta M, Di Meo S, Buonopane R, Longo N, Imbimbo C, Mirone V. Medical expulsive therapy for distal ureteric stones: tamsulosin versus silodosin. Arch Ital Urol Androl 2014; 86: 103–7. [DOI] [PubMed] [Google Scholar]
- 21. Itoh Y, Okada A, Yasui T, Ando R, Tozawa K, Sasaki S, Kohri K. Administration of the selective alpha 1A‐adrenoceptor antagonist silodosin facilitates expulsion of size 5‐10 mm distal ureteral stones, as compared to control. Int Urol Nephrol 2013; 45: 675–8. [DOI] [PubMed] [Google Scholar]
- 22. Itoh Y, Okada A, Yasui T, Hamamoto S, Hirose M, Kojima Y, Tozawa K, Sasaki S, Kohri K. Efficacy of selective α1A adrenoceptor antagonist silodosin in the medical expulsive therapy for ureteral stones. Int J Urol 2011; 18: 672–4. [DOI] [PubMed] [Google Scholar]
- 23. Rathi S, Agarwal A, Patnaik P, Shaw D, Trivedi S, Dwivedi US. Evaluation of medical expulsive therapy for distal ureteral stone: A prospective randomized controlled study to compare silodosin versus tamsulosin. Indian J Urology 2014; 30 (Suppl. 1): S83–83. [Google Scholar]
- 24. Gupta S, Lodh B, Kaku Singh A, Somarendra K, Sholay Meitei K, Rajendra SS. Comparing the efficacy of tamsulosin and silodosin in the medical expulsion therapy for ureteral calculi. J Clin Diagn Res 2013; 7: 1672–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sur RL, Shore N, L'Esperance J, Knudsen B, Gupta M, Olsen S, Shah O. Silodosin to Facilitate Passage of Ureteral Stones: a Multi‐institutional, Randomized, Double‐blinded, Placebo‐controlled Trial. Eur Urol 2014; pii: S0302‐2838(14)01165‐8. [DOI] [PubMed] [Google Scholar]
- 26. Dell'Atti L. Silodosin versus Tamsulosin as medical expulsive therapy for distal ureteral stones: a prospective randomized study. Urologia 2015; 82: 54–7. [DOI] [PubMed] [Google Scholar]
- 27. Tzortzis V, Mamoulakis C, Rioja J, Gravas S, Michel MC, de la Rosette JJ. Medical expulsive therapy for distal ureteral stones. Drugs 2009; 69: 677–92. [DOI] [PubMed] [Google Scholar]
- 28. Malin JM Jr, Deane RF, Boyarsky S. Characterisation of adrenergic receptors in human ureter. Br J Urol 1970; 42: 171–4. [DOI] [PubMed] [Google Scholar]
- 29. Weiss RM, Bassett AL, Hoffman BF. Adrenergic innervation of the ureter. Invest Urol 1978; 16: 123–7. [PubMed] [Google Scholar]
- 30. Itoh Y, Kojima Y, Yasui T, Tozawa K, Sasaki S, Kohri K. Examination of alpha 1 adrenoceptor subtypes in the human ureter. Int J Urol 2007; 14: 749–53. [DOI] [PubMed] [Google Scholar]
- 31. Tomiyama Y, Kobayashi K, Tadachi M, Kobayashi S, Inada Y, Kobayashi M, Yamazaki Y. Expressions and mechanical functions of alpha 1 adrenoceptor subtypes in hamster ureter. Eur J Pharmacol 2007; 573: 201–5. [DOI] [PubMed] [Google Scholar]
- 32. Tatemichi S, Kobayashi K, Maezawa A, Kobayashi M, Yamazaki Y, Shibata N. α1‐adrenoceptor subtype selectivity and organ specificity of silodosin (KMD‐3213) [J]. Yakugaku Zasshi 2006; 126: 209–16. [DOI] [PubMed] [Google Scholar]
- 33. Kenny BA, Miller AM, Williamson IJ, O'Connell J, Chalmers DH, Naylor AM. Evaluation of the pharmacological selectivity profile of alpha 1 adrenoceptor antagonists at prostatic alpha 1 adrenoceptors: binding, functional and in vivo studies. Br J Pharmacol 1996; 118: 871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Forray C, Noble SA. Subtype selective alpha1‐adrenoceptor antagonists for the treatment of benign prostatic hyperplasia. Expert Opin Investig Drugs 1999; 8: 2073–94. [DOI] [PubMed] [Google Scholar]
- 35. Ahmed AF, Al‐Sayed AY. Tamsulosin versus Alfuzosin in the Treatment of Patients with Distal Ureteral Stones: Prospective, Randomized, Comparative Study. Korean J Urol 2010; 51: 193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yilmaz E, Batislam E, Basar MM, Tuglu D, Ferhat M, Basar H. The comparison and efficacy of three different alpha1‐adrenergic blockers for distal ureteral stones. J Urol 2005; 173: 2010–2. [DOI] [PubMed] [Google Scholar]
- 37. Al‐Ansari A, Al‐Naimi A, Alobaidy A, Assadiq K, Azmi MD, Shokeir AA. Efficacy of tamsulosin in the management of lower ureteral stones: a randomized double‐blind placebo‐controlled study of 100 patients. Urology 2010; 75: 4–7. [DOI] [PubMed] [Google Scholar]
- 38. Abdel‐Meguid TA, Tayib A, Al‐Sayyad A. Tamsulosin to treat uncomplicated distal ureteral calculi: a double blind randomized placebo‐controlled trial. Can J Urol 2010; 17: 5178–83. [PubMed] [Google Scholar]
- 39. Agrawal M, Gupta M, Gupta A, Agrawal A, Sarkari A, Lavania P. Prospective randomized trial comparing efficacy of alfuzosin and tamsulosin inmanagement of lower ureteral stones. Urology 2009; 73: 706–9. [DOI] [PubMed] [Google Scholar]
- 40. Pedro RN, Hinck B, Hendlin K, Feia K, Canales BK, Monga M. Alfuzosin stone expulsion therapy for distal ureteral calculi: a double‐blind, placebo controlled study. J Urol 2008; 179: 2244–7. [DOI] [PubMed] [Google Scholar]
- 41. Wang CJ, Huang SW, Chang CH. Efficacy of an alpha1 blocker in expulsive therapy of lower ureteral stones. J Endourol 2008; 22: 41–6. [DOI] [PubMed] [Google Scholar]
- 42. Sun X, He L, Ge W, Lv J. Efficacy of selective alpha1D‐blocker naftopidil as medical expulsive therapy for distal ureteral stones. J Urol 2009; 181: 1716–20. [DOI] [PubMed] [Google Scholar]
- 43. Türk C, Knoll T, Petrik A, Sarica K, Straub M, Seitz C. Guidelines on urolithiasis. European Assocociation of Urology, 2011. Available at http://www.uroweb.org/gls/pdf/urolithiasis%202010.pdf (Accessed July 2011). [Google Scholar]
- 44. Chapple CR. A comparison of varying alpha‐blockers and other pharmacotherapy options for lower urinary tract symptoms. Rev Urol 2005; 7 (Suppl 4): S22–30. [PMC free article] [PubMed] [Google Scholar]
- 45. Montorsi F. Profile of silodosin. Eur Urol Suppl 2010; 9: 491–5. [DOI] [PubMed] [Google Scholar]


