Abstract
Aims
The aims of this study were to assess (1) the magnitude and temporality of decreased urinary citrate excretion in patients just starting topiramate and (2) the effect of alkali replacement on topiramate‐induced hypocitraturia.
Methods
Study 1 was a prospective, non‐intervention study in which patients starting topiramate for headache remediation provided pre‐ and post‐topiramate 24 h urine collections for measurement of urine citrate. Study 2 was a clinical comparative effectiveness study in which patients reporting to our stone clinic for kidney stones and who were treated with topiramate were prescribed alkali therapy. Pre‐ and post‐alkali 24 h urinary citrate excretion was compared.
Results
Data for 12 and 22 patients (studies 1 and 2 respectively) were evaluated. After starting topiramate, urinary citrate excretion dropped significantly by 30 days (P = 0.016) and 62% of patients had hypocitraturia (citrate <320 mg day–1). At 60 days, urine citrate was even lower than at baseline (P = 0.0032) and 86% of patients had developed hypocitraturia. After starting alkali, urine citrate increased in stone‐forming patients on topiramate (198 ± 120 to 408 ± 274 mg day–1; P = 0.042 for difference). 85% of patients were hypocitraturic on topiramate alone vs. 40% after adding alkali. The increase in urinary citrate was greater in patients provided ≥90 mEq potassium citrate.
Conclusions
Our study is the first to provide clinical evidence that alkali therapy can raise urinary citrate excretion in patients who form kidney stones while being treated with topiramate. Clinicians should consider alkali therapy for reducing the kidney stone risk of patients benefitting from topiramate treatment for migraine headaches or other conditions.
Keywords: alkali replacement, citrate, headache, nephrolithiasis, topiramate, urolithiasis
What is Already Known About this Subject
Topiramate has evidence supporting its use for the management of epilepsy, for migraine headache prophylaxis and for weight loss.
Though broadly successful in managing the above conditions and generally well tolerated, topiramate use is linked with acidosis, hypokalaemia, hypocitraturia and the development of calcium‐containing kidney stones.
Both the incidence and prevalence of kidney stones is higher in patients on topiramate than in those who are not.
What this Study Adds
Our study is the first to assess the effects of pharmacologic alkali therapy on urinary citrate excretion in patients who are taking topiramate.
Because of this study, urologists and nephrologists will have evidence for an additional clinical tool to reduce the stone risk of patients on topiramate.
By confirming the efficacy of potassium citrate to raise urinary citrate excretion, patients who benefit from topiramate may remain on the drug while experiencing reduced risk for kidney stone formation.
Introduction
Within recent years, topiramate has been linked with new onset urolithiasis 1. Topiramate is approved for seizure control in patients with epilepsy. It is also increasingly used for migraine headaches and chronic pain (off‐label use). Observations of weight loss up to 15–20% of body weight on moderate doses of topiramate (100–200 mg day–1) have led to its use for weight loss (off‐label) 2. With a relatively high prevalence of migraine headaches, estimated to be 16.2% in the US 3 and with obesity affecting more than one‐third of adults 4, the use of topiramate is rising.
Topiramate, a carbonic anhydrase inhibitor, interferes with renal bicarbonate retention in a reaction catalyzed by carbonic anhydrase, resulting in excessive urinary bicarbonate excretion. This in turn leads to systemic acidosis and increased urine pH. Systemic acidosis can promote excessive bone resorption of calcium and phosphorus, both of which are ultimately excreted in urine. To compensate renal citrate reabsorption is enhanced, making citrate available for conversion to bicarbonate. This leads to decreased urinary citrate excretion. The sum of these factors, high urine pH, high urine calcium and low urine citrate, increase the risk for calcium kidney stones, especially calcium phosphorus. Indeed, kidney stone incidence is estimated to be 1.5% for patients treated with topiramate 1, 5, and its prevalence may be as high as 54% in certain high risk populations 6.
Accordingly, patients treated for kidney stones are frequently advised to discontinue topiramate 7. However, an alternative approach, alkali replacement, has recently been suggested 8 and may already be endorsed by urologists and nephrologists who manage patients who take topiramate. Such rescue therapy, if effective, may be of value to those whose use of topiramate provides relief for the condition for which it was prescribed and improved health‐related quality of life (HRQOL). However, insufficient data to drive this practice are available.
We previously documented a progressive decline in citrate excretion over time 9, 10. We also noted a rise in urinary citrate excretion in a patient who discontinued topiramate, suggesting reversibility of hypocitraturia. An increasing number of patients using topiramate, largely for migraine headaches, have come to our clinic in recent years. Some of these patients were tried on but were unresponsive to other headache medications. As many patients, if not most, have expressed a desire to remain on topiramate despite kidney stone risk, the purpose of our study was to assess (1) the magnitude and temporality of decreased urinary citrate excretion in patients just starting topiramate and (2) the effect of alkali replacement in the form of potassium citrate as rescue therapy for topiramate‐induced hypocitraturia.
Methods
To determine the effect of topiramate on urinary citrate excretion and other urinary parameters (prevalence study), we identified adult patients between September 2011 and April 2012 from the headache clinic at our institution who were being prescribed topiramate. The study was approved by the institutional review board at our institution (M‐2009–1433) and subjects provided written consent. Patients were not eligible to participate if they were diagnosed with acidosis or currently taking another carbonic anhydrase inhibitor such as acetazolamide or zonisamide. There were no other exclusion criteria. No patients were on a medication to reverse acidosis. Eligible patients were enrolled if they agreed to provide a 24 h urine sample before initiating topiramate. Patients were then asked to complete a second 24 h urine collection after they had been on topiramate for 30 days and a third 24 h urine collection at 60 days.
To determine the effect of potassium citrate on topiramate‐induced hypocitraturia and stone risk (potassium citrate study), we assembled a convenience cohort of patients from our stone clinic who had been prescribed potassium citrate after presenting with topiramate‐induced hypocitraturia and/or exacerbated stone formation. The study was approved by our institutional review board (2011–0674). Results of patients' 24 h urine collections while on topiramate alone were compared with 24 h urine collections acquired during clinical follow‐up after starting potassium citrate.
Results of 24 h urine parameters related to stone risk were compared within and between individuals for all time points available. Urinary creatinine excretion was used to assure adequate/accurate 24 h urine collections. Group means for patients on topiramate before and after starting potassium citrate were calculated. Student's t‐tests (two‐tailed) were used to assess for differences within individuals over time and between groups. Paired t‐tests were used when patients had both pre‐ and post‐intervention measures. We evaluated plots of the data. Where variances were thought to be unequal, we used a t‐test assuming unequal variance.
Results
Prevalence of hypocitraturia: patients
Eighteen subjects were recruited from the headache clinic (13 female, five male). No patients had a history of urinary stones. Of these, 12 (eight female, four male) provided evaluable 24 h urine collections. Results hereafter refer to these patients. Age (38.3 ± 14 SD years; range 21–62 years) was not different between men and women (40 and 37 years, respectively). BMI (30.5 ± 3.9 kg m−2; range 24.4–39.0 kg m−2) was not different between men and women (30.0 and 30.8 kg m–2, respectively). At the headache clinic, patients were prescribed 100–200 mg of topiramate each and 50% underwent dose modification by headache clinic providers during the study. Subjects (75%) completed an investigator‐designed written survey about their adherence to topiramate treatment after it was initiated. Most (67%) reported ‘never’ missing a dose of topiramate. The remainder reported missing a dose ‘one–two times per month’. After 60 days on topiramate, subjects lost 1.2 kg (range, 0–4 kg). Men lost twice as much weight as women, 1.7 vs. 0.86 kg, respectively.
Prevalence of hypocitraturia: urine citrate
Urinary citrate excretion declined after starting topiramate (Table 1). Before starting topiramate, urine citrate was <320 mg day–1 (the risk cutoff for hypocitraturia) in two of 12 subjects (250 ± 38.9 mg day–1 vs. 582 ± 274 mg day–1 for those with urine citrate above the risk cutoff). After 30 days on topiramate, 10 subjects completed 24 h urine collections. Using urinary creatinine excretion >2000 and <650 mg as cutoffs, one was excluded as an over‐collection and one as an under‐collection. Results for the eight remaining subjects revealed that urinary citrate excretion decreased by an average of 247 mg day–1 (Table 1). While a decrease was observed in seven subjects, urine citrate increased in one (355 vs. 277 mg day–1 at baseline). Overall, 62% of subjects (five of eight) had hypocitraturia at 30 days compared with only 17% (two of 12) at baseline. After 60 days on topiramate, seven subjects completed 24 h urine collections. Of these, six (86%) had hypocitraturia. Mean urinary citrate excretion at 60 days was 218 ± 82 mg day–1 (Table 1). The difference between urinary citrate excretion at 60 days was not statistically different than at 30 days (P = 0.27) but was nevertheless lower (Table 1). A paired comparison of subjects (n = 6) who provided at least two of the 24 h urine collections is shown (Figure 1). Urine pH increased significantly from baseline to 30 days and plateaued. Details of 24 h urinary risk parameters are shown (Table 1).
Table 1.
Prevalence of hypocitraturia in patients starting topiramate. Data show mean ± SD for 24 h urinary parameters related to stone risk. Time points shown are before topiramate (baseline) and at 30 and 60 days after starting topiramate. P values‡ for differences between time points are shown at the right for each parameter. Significant differences (P ≤ 0.05) are in bold and denoted with an asterisk
| Baseline | 30 days | 60 days | Baseline vs. 30 days | Baseline vs. 60 days | 30 vs. 60days | |
|---|---|---|---|---|---|---|
| Volume (l) | 1.33 ± 0.67 | 1.57 ± 0.98 | 1.64 ± 0.84 | 0.52 | 0.37 | 0.88 |
| pH | 6.13 ± 0.44 | 6.60 ± 0.50 | 6.53 ± 0.27 | *0.046 | *0.022 | 0.71 |
| Calcium (mg) | 160 ± 116 | 180 ± 109 | 197 ± 131 | 0.71 | 0.52 | 0.78 |
| Oxalate (mg) | 28 ± 6.2 | 25 ± 8.6 | 32 ± 15 | 0.42 | 0.37 | 0.26 |
| Uric acid (mg) | 474 ± 198 | 514 ± 178 | 470 ± 135 | 0.65 | 0.96 | 0.58 |
| Citrate (mg) | 526 ± 280 | 279 ± 121 | 218 ± 82 | *0.016 | *0.0032 | 0.27 |
| Sodium (mEq) | 136 ± 82 | 141 ± 63 | 155 ± 63 | 0.88 | 0.58 | 0.66 |
| Sulfate (mmol) | 15 ± 8.2 | 15 ± 6.9 | 16 ± 6.8 | 0.97 | 0.94 | 0.91 |
| Phosphorus (mg) | 925 ± 366 | 791 ± 275 | 759 ± 305 | 0.39 | 0.30 | 0.83 |
| Magnesium (mg) | 94 ± 42 | 91 ± 48 | 107 ± 67 | 0.89 | 0.59 | 0.59 |
| Ammonium (mEq) | 26 ± 11 | 26 ± 7.7 | 27 ± 13 | 0.97 | 0.80 | 0.82 |
| Potassium (mEq) | 50 ± 28 | 47 ± 18 | 46 ± 18 | 0.80 | 0.68 | 0.86 |
| Creatinine (mg) | 1381 ± 518 | 1371 ± 445 | 1411 ± 544 | 0.96 | 0.90 | 0.88 |
| CaOx SS† | 1.70 ± 0.70 | 1.61 ± 0.74 | 1.99 ± 1.2 | 0.78 | 0.50 | 0.46 |
| Brushite SS | 2.07 ± 1.3 | 2.95 ± 0.88 | 2.76 ± 1.2 | 0.11 | 0.24 | 0.72 |
| Struvite SS | 2.00 ± 2.6 | 5.23 ± 3.5 | 3.59 ± 2.0 | 0.040 | 0.18 | 0.27 |
| Uric acid SS | 1.77 ± 1.7 | 0.86 ± 1.1 | 0.52 ± 0.34 | 0.16 | *0.029 | 0.42 |
SS, relative supersaturation.
Where data plots revealed unequal variance, a t‐test assuming unequal variance was used to assess statistical difference.
Figure 1.
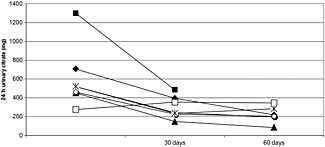
Paired comparison of 24 h urinary citrate excretion for six patients in study 1 (prevalence of hypocitraturia while on topiramate) who completed more than one 24 h urine collection. Values shown represent urine citrate at baseline (before starting topiramate) and then 30 and 60 days after starting topiramate
Effects of potassium citrate therapy: subjects
Patients from our stone clinic who happened to also be taking topiramate were identified (n = 43; 34 female, nine male; 45 ± 13 years). Topiramate dosages varied (range 25–400 mg day–1; mean 131 mg; median 100 mg). If warranted, based on 24 h urinary citrate excretion and considering other factors related to stone pathology, patients were prescribed alkali therapy. Potassium citrate dosages ranged from 30 to 90 mEq daily (mean 58 mEq; median 60 mEq). Twenty‐two patients completed 24 h urine collections in clinical follow‐up (19 female, three male). Data hereafter refer only to these. Some patients (n = 13; 59%) had a history of urolithiasis, but none had known hypocitraturia prior to starting topiramate and none was taking any medications to control kidney stones.
Effects of potassium citrate therapy: urine citrate
Twenty patients completed the topiramate only (pre‐alkali) urine collection prior to their initial appointment in our stone clinic. These were collected at various time frames from initiating topiramate (mean, 19 ± 16 months; median, 17 months; range 0.90–49 months); 17 of 20 patients were hypocitraturic. Duration of time on topiramate was not correlated with urinary citrate excretion (r 2 = 0.002). The dose of topiramate was only weakly inversely correlated with urinary citrate excretion (r 2 = −0.19). Post‐alkali urine collections were unavailable for five of the 22 patients and seven did not start potassium citrate or were not taking it at follow‐up; 24 h urine collections for these patients were not included in the post‐alkali analysis. Thus, a total of 10 post‐alkali urine collections were evaluated. Because patients' 24 h urine collections were scheduled as part of clinical follow‐up, the time from starting potassium citrate to obtaining the post‐alkali 24 h urine collection was not standardized (9.7 ± 4.1 months post‐alkali; range 4.5–17 months). Urinary citrate excretion increased after adding potassium citrate. All patients had higher urine citrate (Table 2). Four of eight patients (50%) who were hypocitraturic while on topiramate alone were above the risk cutoff after adding potassium citrate.
Table 2.
Effects of potassium citrate therapy on topiramate‐induced hypocitraturia. Data show mean ± SD for urinary parameters related to urinary stone risk. Time points shown are from 24 h urine collections while on topiramate alone (dosage mean, 131 ± 111 mg; median, 100 mg day–1) for 9.7 ± 4.1 months and from 24 h urine collections after adding potassium citrate (dosage mean, 58 ± 21 mEq day–1; median, 60 mEq day–1). P values for differences between urinary parameters are shown. ‡Significant differences (P ≤ 0.05) are in bold and denoted with an asterisk
| Topiramate alone | Topiramate plus potassium citrate | P value | |
|---|---|---|---|
| Volume (l) | 1.36 ± 0.55 | 1.89 ± 0.98 | 0.14 |
| pH | 6.65 ± 0.55 | 7.25 ± 0.78 | *0.04 |
| Calcium (mg) | 138 ± 73 | 175 ± 81 | 0.23 |
| Oxalate (mg) | 33 ± 15 | 40 ± 24 | 0.37 |
| Uric acid (mg) | 492 ± 164 | 508 ± 151 | 0.81 |
| Citrate (mg) | 198 ± 120 | 408 ± 274 | *0.04 |
| Sodium (mEq) | 131 ± 78 | 154 ± 38 | 0.28 |
| Sulfate (mmol) | 12 ± 6.7 | 13 ± 5.4 | 0.49 |
| Phosphorus (mg) | 643 ± 289 | 633 ± 224 | 0.92 |
| Magnesium (mg) | 89 ± 58 | 108 ± 68 | 0.46 |
| Potassium (mEq) | 47 ± 25 | 70 ± 44 | 0.08 |
| Creatinine (mg) | 1182 ± 311 | 1126 ± 355 | 0.68 |
| CaOx SS† | 2.16 ± 0.92 | 1.99 ± 1.6 | 0.76 |
| Brushite SS | 2.78 ± 1.4 | 2.99 ± 2.8 | 0.83 |
SS, relative supersaturation.
Where data plots revealed unequal variance, a t‐test assuming unequal variance was used to assess statistical difference.
The change in urinary citrate within patients in study 2 who provided both pre‐ and post‐alkali urine collections (n = 8) is shown in Figure 2. The increase in urinary citrate excretion was not correlated with the dosage of potassium citrate prescribed (r 2 = 0.036 for correlation with dose as a continuous variable). However, patients prescribed 90 mEq of potassium citrate had a higher increase in urine citrate (227 ± 33 mg day–1), even though they started with lower urine citrate on topiramate alone (108 ± 73 mg day–1), than those prescribed <90 mEq. For the latter, there was a lower increase in urine citrate (106 ± 60 mg day–1). Urine pH increased significantly after addition of potassium citrate, but other urinary risk factors for urolithiasis did not change (Table 2), including urinary supersaturation indices for calcium oxalate and calcium phosphate.
Figure 2.
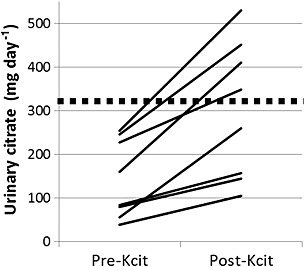
Urinary citrate excretion from the 24 h urine collections of eight patients in study 2 (effects of potassium citrate on topiramate‐induced hypocitraturia) who were on topiramate for 19 ± 16 months (range 0.90–49 months) and then added potassium citrate. Data for ‘pre‐Kcit’ represent urine citrate while on topiramate only. Data for ‘post‐Kcit’ represent urine citrate while on both topiramate and potassium citrate for 9.7 ± 4.1 months (range 4.5–17 months). The dashed line shows the risk cutoff for hypocitraturia (320 mg day–1). Increase in urinary citrate after potassium citrate was significant (paired t‐test, P = 0.0013)
Discussion
Our results confirm that patients taking topiramate have reduced urinary citrate excretion and increased urine pH within 30 days of starting topiramate. These changes persist at 60 days. Our results also demonstrate that alkali replacement can serve as a rescue agent for patients on topiramate with low urine citrate. All patients on topiramate whose post‐citrate urine collections were available showed increased urine citrate. The magnitude of increase varied. Patients prescribed 90 mEq of potassium citrate had larger increases in urine citrate even while starting with lower urine citrate. The variable increase in urinary citrate excretion after adding alkali suggests the existence of non‐responders or patients for whom a larger dose of potassium citrate may be required to achieve full therapeutic benefit.
Topiramate is effective in migraine prophylaxis 11, a disabling and frequently chronic condition. The use of topiramate was approved by the US Food and Drug Administration (FDA) in 2004 for preventing episodic migraine in adults 12. In 2012, the FDA approved its use for preventing migraine in adolescents 13. As migraine headaches, both episodic and chronic, can be debilitating and are associated with productivity loss, increased healthcare resource use and reduced HRQOL 14, effective management is paramount.
The aim of medical management for stones is to achieve the most therapeutic benefit while improving the patient's overall health and HRQOL. In the case of patients on topiramate, especially if it is successful in relieving the condition for which it was prescribed, strategies to reduce stone recurrence while remaining on topiramate are needed. Even with a prior history of stones, as with 59% of the patients in our study, many insist on the prioritization of their headache condition over the risk of stone recurrence.
Our data confirm that potassium citrate is effective rescue for topiramate‐induced hypocitraturia. It is generally well‐tolerated, though some experience gastrointestinal symptoms 15, and has few unwanted side effects (but may be contraindicated in those with significantly compromised renal function). It could be considered in patients starting topiramate who are at high risk for stone formation, i.e. as primary prevention. These include those with a prior stone history (incidental or symptomatic), strong family history for kidney stones, sarcoidosis, distal renal tubular acidosis or malabsorptive conditions. Studies assessing the interest of such patients in adding potassium citrate as prophylaxis for urolithiasis are warranted as are studies that assess whether stone incidence is affected.
Limitations
Our study was small, limiting generalizability. Dietary factors that could have contributed to altered urinary citrate excretion were not assessed. Plasma acid base parameters and electrolytes, which could have identified acidosis unrelated to topiramate, were not available for review. Patients' adherence to topiramate was self‐reported. We cannot confirm compliance with prescription regimens. Although increased urinary potassium excretion was observed (Table 2), confirming our assumptions about compliance with alkali therapy, it did not reach statistical significance (P = 0.080). Thus, while we assume potassium citrate was used as directed and responsible for the effects we observed, we are unable to confirm this unequivocally. Another limitation is the variable time frame between initiation of both topiramate and potassium citrate and the 24 h urine collections in the potassium citrate study. Thus, our results may not confirm absolute effect but nevertheless support clinical effectiveness. Finally, we were unable to account for the effect of family history for stones and the potential for inherited hypocitraturia. This could have provided clues as to the lower responsiveness of some patients vs. others, and may have affected our results as reported.
In conclusion, while topiramate has provided success in alleviating chronic illnesses such as seizures and migraine headaches, it has been at the cost of increased risk for kidney stone formation via reduced urinary citrate excretion. Rather than encourage cessation of topiramate, which may be especially compromising in those whose lives are improved by it, our data support the use of alkali replacement in those forming stones while on topiramate. Additionally, we suggest that alkali replacement be considered for high risk patients at the time topiramate is started. As response to citrate replacement while on topiramate may vary, urine citrate should be continually monitored and adjustments to alkali replacement made as needed.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Contributors
RAJ co‐formulated the clinical research question, co‐designed the protocol, invited and enrolled subjects, assisted with data interpretation and analysis, and wrote the first draft of the manuscript. RAJ provided clinical care to patients as part of his role as nephrologist and director of the Metabolic Stone Clinic.
MLW assisted with data collection and analysis, human subjects regulatory compliance, and in the writing of the manuscript.
KLP co‐formulated the clinical research question, co‐designed the protocol, oversaw regulatory compliance, invited and enrolled subjects, analyzed data, and co‐wrote and revised the manuscript.
Allan Jhagroo, R. , Wertheim, M. L. , and Penniston, K. L. (2016) Alkali replacement raises urinary citrate excretion in patients with topiramate‐induced hypocitraturia. Br J Clin Pharmacol, 81: 131–136. doi: 10.1111/bcp.12751.
References
- 1. Maalouf NM, Langston JP, Van Ness PC, Moe OW, Sakhaee K. Nephrolithiasis in topiramate users. Urol Res 2011; 39: 303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two‐year sustained weight loss and metabolic benefits with controlled‐release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo‐controlled, phase 3 extension study. Am J Clin Nutr 2012; 95: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smitherman TA, Burch R, Sheikh H, Loder E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache 2013; 53: 427–36. [DOI] [PubMed] [Google Scholar]
- 4. Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007–2012. JAMA Intern Med 2015; 175: 1412–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matlaga BR, Shah OD, Assimos DG. Drug‐induced urinary calculi. Rev Urol 2003; 5: 227–31. [PMC free article] [PubMed] [Google Scholar]
- 6. Goyal M, Grossberg RI, O'Riordan MA, Davis ID. Urolithiasis with topiramate in nonambulatory children and young adults. Pediatr Neurol 2009; 40: 289–94. [DOI] [PubMed] [Google Scholar]
- 7. Assimos DG. Re: metabolic disturbances and renal stone promotion on treatment with topiramate: a systematic review. J Urol 2014; 191: 1810–1. [DOI] [PubMed] [Google Scholar]
- 8. Dell'Orto VG, Belotti EA, Goeggel‐Simonetti B, Simonetti GD, Ramelli GP, Bianchetti MG, Lava SA. Metabolic disturbances and renal stone promotion on treatment with topiramate: a systematic review. Br J Clin Pharmacol 2014; 77: 958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaplon DM, Penniston KL, Nakada SY. Patients with and without prior urolithiasis have hypocitraturia and incident kidney stones while on topiramate. Urology 2011; 77: 295–8. [DOI] [PubMed] [Google Scholar]
- 10. Penniston KL, Jhagroo RA, Nakada SY. Topiramate results in a rapid and progressive decline in urinary citrate over 60 days: a prospective study. J Urol 2013; 189: E920. [Google Scholar]
- 11. Modi S, Lowder DM. Medications for migraine prophylaxis. Am Fam Physician 2006; 73: 72–8. [PubMed] [Google Scholar]
- 12. Shamliyan TA, Choi JY, Ramakrishnan R, Miller JB, Wang SY, Taylor FR, Kane RL. Preventive pharmacologic treatments for episodic migraine in adults. J Gen Intern Med 2013; 28: 1225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Food and Drug Administration release . FDA approves Topamax for migraine prevention in adolescents. March 28, 2014. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm391026.htm (last accessed 11 June 2015).
- 14. Lanteri‐Minet M. Economic burden and costs of chronic migraine. Curr Pain Headache Rep 2014; 18: 385. [DOI] [PubMed] [Google Scholar]
- 15. Ngo TC, Assimos DG. Uric acid nephrolithiasis: recent progress and future directions. Rev Urol 2007; 9: 17–27. [PMC free article] [PubMed] [Google Scholar]


