Introduction
Workers are commonly exposed to multiple factors and substances that are hazardous to health. For instance, in 2005, approximately 30% of the European workers reported being exposed to noise during at least a quarter of the time spent in their work environment, 11% reported inhaling vapors such as solvents and thinners; 19% reported exposure to smoke, fumes, powder, or dust; and 14% reported handling chemical substances.1 Although occupational noise exposure has long been recognized in the United States and in Europe as the most deleterious factor to hearing2,3 the impact of chemical-induced hearing loss on workers should not be underestimated.4 A cursory survey of the literature for the last three decades reveals that concern about the effects of chemicals on hearing has grown steadily. The proliferation of work-related substances and medicinal drugs (mostly antitumor drugs and aminoglycosides) has been accompanied by an equivalent increase in the number of scientific publications on the hearing risks encountered by chemical-exposed persons. Cause for concern is even greater when the synergistic risks of co-exposures are considered. For example, physiological factors may increase the severity of a chemical’s effect on hearing. Today, robust evidence also confirms that the effects of ototoxic substances on ear function can be aggravated by noise, which remains a well-recognized cause of hearing impairment. In an expert forecast supervised by the European Agency for Safety and Health at Work5 (EU-OSHA, 2009), a “combined exposure to noise and ototoxic substances” was rated as an emerging risk.
Industrial ototoxic chemicals have predominantly been assessed through animal studies, which are supported by data from epidemiologic studies on human workers from various industries. Bergström and Nyström (1986) published the seminal results of a 20-year longitudinal study performed with 319 Swedish employees from different industrial sectors.2 This study began in 1958 and involved testing workers’ hearing regularly. Its findings showed that a large proportion (23%) of the employees working in a chemical division suffered from hearing impairment, despite their exposure to lower noise levels than other divisions. Based on this type of evidence, researchers have long hypothesized that industrial solvents pose an additive risk to hearing. Scientific findings in animals are generally considered qualitatively relevant to human health, provided no substantial difference in biological response (e.g. metabolism) exists between test animals and humans. Despite these findings, current research is limited by the following:
a lack of detailed exposure histories;
the presence of confounding factors (ototoxic drugs, tobacco, alcohol consumption, aging, and exposures outside the workplace);
and the fact that chemical exposure scenarios used in experimental investigations are qualitatively different from real-world occupational settings.
In this extensive, but not exhaustive, review of the literature, the authors hope to share the evidence indicating that noise is not the only source of work-related hearing damage and to draw greater attention to the matter of chemically induced hearing loss. The present publication intends to provide occupational physicians and other health professionals with a clear picture of what is known about how chemical substances may affect hearing ability in the general population, and more specifically, in workers. The article describes the following:
chemicals that can be noxious to the inner ear;
work areas where exposure to ototoxic substances are likely;
and the basic features of the physiological mechanisms leading to hearing impairment.
This article also addresses the limitations of pure-tone air-conduction audiometry when assessing chemical-induced hearing loss and proposes a more complete approach to evaluate the auditory neurosensory hearing receptor and the neural pathways involved in the stapedial reflex. With the proposed battery of tests, physicians will be able to evaluate both ototoxicity and neurotoxicity. Throughout this manuscript, emphasis is placed on the need for stronger links between primary care and occupational physicians. This could help preserve patients’ hearing status as a part of their overall health.
Ototoxic drugs
It is well documented that some medicinal drugs, such as powerful antibiotics and anti-neoplastic drugs used against life-threatening diseases can cause auditory and/or vestibular dysfunction in patients. Functional damage can include permanent hearing loss and tinnitus. In several countries, administration of these drugs is monitored, and guidelines are available online (AAA, 2009, available online at http://www.audiology.org/resources/documentlibrary/Pages/OtotoxicityMonitoring.aspx). A brief review of these substances is presented in the section Which substances can be considered ototoxic?, but first we review some of the terms used to describe how they affect hearing and their definitions.
Definitions
Noise is a physical factor that causes mostly mechanical and metabolic damage to the peripheral auditory receptor, the cochlea, and more rarely, to the auditory neural pathways.6–8 In contrast, chemicals in the bloodstream go through either the blood-labyrinth barrier into the cochlea or the blood–brain barrier to reach the eighth cranial nerve and the central nervous system.9–13 As a result, chemical-induced hearing loss can be the result of effects on several sites within the hearing system. Ototoxic chemicals may affect the structures and/or the function of the inner ear (auditory and vestibular apparatus) and the connected neural pathways. From a general point of view, both cochleotoxicants and vestibulotoxicants can be defined as ototoxicants.
A cochleotoxicant can affect different cochlear structures, including the auditory sensory cells (“hair cells”), the fluid-producing cell layer on the outer wall of the cochlear duct (“stria vascularis”), and the spiral ganglion cells. In most cases, cochlear hair cells are the primary targets of cochleotoxicants (Figs. 1–5).
Fig. 1.
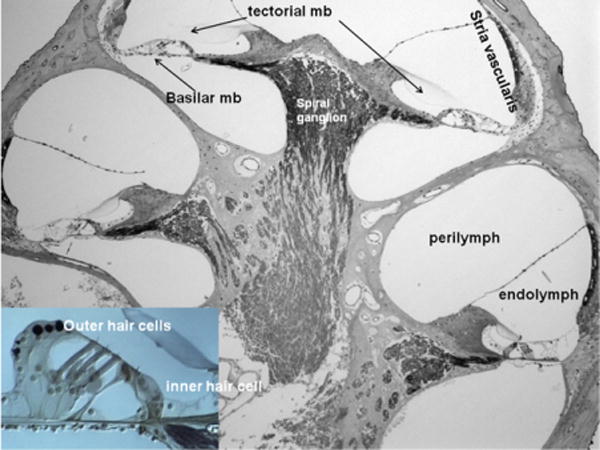
Cross section of the cochlea. mb, membrane. Left bottom panel: organ of Corti. (Color version of the figure is available online.)
Fig. 5.
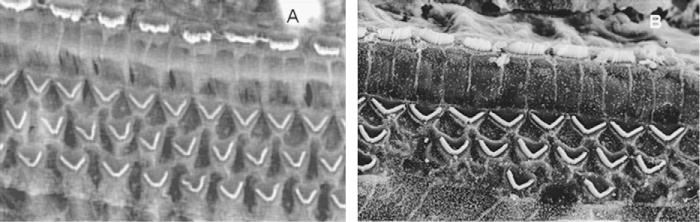
Scanning electron micrograph of a rat organ of Corti prior to- (A) and post- (B) toluene exposure. When present, the stereocilia of the hair cells look normal in both images.
Which substances can be considered ototoxic?
This article only reviews chemicals for which the strongest evidence of ototoxicity exists. A substance was included if human data are available, or if two well-documented animal studies reported clear and reliable findings in a coherent manner. If supporting human data were lacking, species-specific features should not contradict extrapolation of animal findings to humans. For the sake of clarity, data on potentially ototoxic substances are not reported in the present article.
Aminoglycosides
The aminoglycosides, such as streptomycin, kanamycin, neomycin, gentamicin, tobramycin, amikacin, and netilmicin are commonly used to treat gram-negative bacterial infections. Unfortunately, the clinical benefits of these agents can be outweighed by their toxicity, which includes cochlear damage. The incidence of hearing loss ranges from a few percent to up to 33% of the aminoglycoside-treated patients. Aminoglycoside-induced hearing loss spreads from high to low frequencies depending on the duration of treatment and the dose. After systemic administration, the antibiotics rapidly go through the blood–labyrinth barrier at the level of the stria vascularis to penetrate the cochlea. The drug reaches the perilymph and thereby the outer hair cells, which are particularly sensitive to antibiotics.14–17 They can remain in the cochlea for several months after discontinuing the treatment.18,19 Because of this long clearance time from the inner-ear liquids, occupational physicians must take aminoglycoside treatment into account as, after hospitalization, an employee could return to work with aminoglycoside-contaminated cochlea, which would make them particularly vulnerable to noise. An innocuous noise exposure can become noxious with aminoglycosides.20 Consequently, good communication between primary care and occupational physicians is required to avoid dangerous co-exposures to noise and aminoglycosides. Before their return to work, the company physician should examine anyone who has been hospitalized, or anyone who was treated with antibiotics to determine the nature of the drugs. Unfortunately, monitoring the serum aminoglycoside level is not sufficient to prevent a likely co-exposure since these antibiotics take longer to clear from the perilymph (inner-ear liquids) than from the blood. A safer approach would be to protect employees from an unexpected co-exposure to noise and aminoglycosides for at least 3 months after the end of the treatment.
Mechanism of aminoglycoside ototoxicity
Aminoglycosides penetrate the outer hair cells from the endolymph. They do not cause lipid peroxidation by themselves; however, they form an aminoglycoside–iron complex that interacts with polyphosphoinositides. This reaction increases membrane permeability and generates the release of reactive oxygen species (ROS) through the oxidation of arachidonic acid (breakdown products) in membranes. Superoxide, hydroxyl radical, and hydrogen peroxide then activate the c-Jun N-terminal kinase (JNK) pathway, which can translocate to the nucleus to activate genes involved in the cell death pathway. The products of these genes translocate to the mitochondria, where they induce the release of cytochrome c, which triggers apoptosis via caspases. Cell death may also result from caspase-independent mechanisms.21
Anti-neoplastic drugs
Platinum-derivates such as cisplatin and carboplatin are anticancer drugs that have ototoxic side effects.22–24 The ototoxicity induced by platinum-derivates is characterized by the loss of cochlear hair cells and cells of the spiral ganglion and by degeneration of the stria vascularis.25 As with aminoglycosides, hearing impairment induced by platinum-derivates covers the range from high to low frequencies.26 An exacerbation of cisplatin ototoxicity was observed with exposure to concomitant moderate to high noise levels in chinchilla and guinea pigs.27,28
Loop diuretics
Ethacrynic acid, furosemide, and bumetanide are three loop diuretics widely used to increase the volume of urine excretion as part of the treatment for high blood pressure and swelling due to congestive heart failure. They act on the ascending limb of the loop of Henle, inhibiting sodium- and chloride-ion reabsorption in the kidney. In the mean time, diuretics act on the sodium- and potassium-transporters/pumps located within the stria vascularis, disturbing the ionic concentration of endolymph. Therefore, there is a parallel effect of diuretics on kidney and ear. Hearing loss is a temporary side effect that lasts only during the treatment. The effect is characterized by a sudden high-frequency hearing loss due to dysfunctions of the stria vascularis. These dysfunctions affect the ionic gradients between the endolymph and the perilymph, decreasing endocochlear potential and thereby sensorial transduction.29–31
Toxic interactions of loop diuretics and other known ototoxic drugs have been well documented, particularly with aminoglycosides. Dysfunctions at the level of the stria vascularis may result in a massive entry of aminoglycosides into the inner-ear fluid.32 Thus, the effect of loop diuretics on the stria vascularis facilitates the toxic effects of aminoglycosides on the hair cells. Similarly, ethacrynic acid is known to interact with cisplatin to increase its ototoxic effects.33 Finally, results from a study in rats showed that the ototoxic effect of cadmium may be potentiated by furosemide.34
Acetyl salicylic acid
High doses of acetyl salicylic acid (aspirin;>2.5 g/day) may induce a temporary auditory threshold shift and sometimes tinnitus.35,36 In general, recovery of normal auditory sensitivity occurs within 2 or 3 days of discontinuing salicylate administration. The exact mechanism of salicylate-induced hearing impairments remains incompletely known. The ototoxic effects seem to result from a combination of several reversible cochlear disturbances.37 Like aspirin, many other painkillers and antipyretics (quinine and chloroquine) are recognized for their potential ototoxic side effects. Salicylate-induced temporary threshold shifts may exacerbate the temporary effects of noise, hamper speech discrimination, and increase difficulty in detecting an alarm sounding in a noisy environment.38,39
Work-related substances
In many industries workers are exposed to both noise and chemicals, these include printing, painting, boat building, construction, glue manufacturing, metal products, chemicals, petroleum, leather products, furniture making, agriculture, and mining.1 In addition, some public safety workers, like firefighters, are also exposed to both noise and chemicals. Environmental exposure studies have determined the concentration of combustion products and various toxic agents in the smoke to which firefighters may be exposed.40–44 According to these studies, smoke contains numerous harmful chemicals such as acrolein, benzene, methylene chloride, polyaromatic hydrocarbons, perchloroethylene, carbon monoxide, toluene, trichloroethylene, trichlorophenol, xylene, and formaldehyde. In addition, combustion products include metals such as lead, cadmium, mercury, and arsenic. Common sources of these metals in structural and vehicle fires include lead (old paint and PVC), cadmium (batteries and PVC), mercury (thermostats, automotive switches and thermometers), and arsenic (pressure-treated wood).45,46 Many of the compounds detected in smoke from fires are also known to be neurotoxic or ototoxic.
Aromatic solvents
Toluene and styrene are the most widely and extensively used aromatic solvents in industry.47 Toluene is a major component of adhesives, paints, lacquers, varnishes, printing inks, degreasers, fuel additives, glues and thinners, whereas styrene is present when manufacturing plastics, rubber articles, and glass fibers.48
Chronic exposure to these aromatic solvents can affect the central nervous system49,50,11 and also the inner ear.51,52 The cochleotoxic effects of aromatic solvents such as toluene, styrene, ethylbenzene, p-xylene, various methylstyrenes, allylbenzene, and n-propylbenzene have been repeatedly demonstrated in animal experiments.53–57 Long-duration exposures to aromatic solvents have been shown to cause irreversible hearing impairment, with the cochlear hair cells as the first targets.58,59 Most of these animal studies were performed with rats, whose cochleae are sensitive to aromatic solvents.60,61 Because of their similar metabolism, rats are considered comparatively good animal models for investigating the potential ototoxic properties of aromatic solvents in humans.62
Mechanisms of aromatic solvent ototoxicity
Aromatic solvents most likely affect hearing through chemical poisoning of hair cells, resulting in disorganization of their membranous structures.54 An acute effect may be caused by the direct action of solvents on the cells of the organ of Corti, whereas chronic ototoxic effects may be explained by the formation of chemically and biologically reactive intermediates. These intermediates include reactive oxygen species, which may trigger the death of these cells.63 They can also provoke dysfunctions of transmembrane K+ fluxes, which play a determining role in the physiology of hair cells during acoustic stimulation.64 During acoustic stimulation, K+ enters massively into the hair cells; efflux of this K+ from the hair cells into the outer tunnel of the organ of Corti increases the ionic level in the extracellular fluid. If this K+ is allowed to accumulate in the tunnel fluid, it would cause outer hair cell cytotoxicity. Clearly, K+ recycling from the organ of Corti back to the endolymph or to the stria vascularis plays an important role, preventing an adverse local effect on the hair cells and allowing K+ ions to be saved.65 Solvents transit from the outer sulcus into the organ of Corti, where they likely disrupt the role played by the Hensen and Tectal cells, thereby affecting K+ recycling.
Neuropharmacological effects of solvents
Nearly a century ago, volatile chemicals were thought to produce non-specific effects on the central nervous system (CNS). More recently, clear evidence has emerged regarding how inhaled solvents act specifically on several types of ion channels expressed in neurons.66 Many abused inhalants such as toluene may enhance inhibitory synaptic responses through their action on specific sites or through similar mechanisms of action to other CNS depressants, like anesthetics.67,68 For instance, N-methyl-D-aspartic acid,69 δ-aminobutyric acid, glycine,70 adenosyl triphosphate,71 serotonin,72 and nicotinic acetylcholine receptors73 are all involved in CNS function and are sensitive to toluene. Recently, Lataye et al.74 and Maguin et al.75 showed that toluene could alter acetylcholine receptors and voltage-dependent Ca2+ channels function in in vivo studies. Consequently, toluene, like most aromatic solvents, can affect the middle-ear acoustic reflex (the contraction of the middle-ear muscles that occurs in response to high-intensity sound stimuli). This reflex is driven by a cholinergic efferent system.76 This type of effect suggests that the hearing loss observed in solvent-exposed subjects might be due to dysfunctions beyond the peripheral auditory system, which could at least partially explain the synergistic effects of co-exposure to noise and aromatic solvents.
Co-exposure
What is the evidence from animal experiments?
Experiments with rats have shown that combined exposure to noise and solvents (such as toluene, styrene, and ethylbenzene) induces synergistic adverse effects on hearing.77–81 In most of these investigations, high concentrations of solvents were used for short intervals of time, conditions which do not accurately reflect occupational exposure conditions.
Another difficulty with recreating real-life conditions stems from the fact that humans are generally exposed to solvents in combination with a multitude of other factors (several exposures, physical demands, etc.); whereas animal experiments typically involve isolated solvent exposures. In studies with experimental animals, researchers have demonstrated that when other stressors are added (such as impact noise or carbon monoxide), or when the animals are active during the chemical exposure, the lowest concentration at which solvents elicited an auditory effect was reduced.79 Another complication in determining the concentration needed for hearing loss is that workers are often not aware of the concentration to which they are exposed, and many factors can interact to cause effect. However, cases of hearing loss have been observed after exposures that were within permissible European limits, these will be detailed in the next session.
Lataye et al.79 found interactive effects of noise and styrene with quite low levels of noise: A time-weighted average (TWA) of 85 dB and low concentration of styrene: 400 ppm, 6 h per day, 5 days per week for 4 weeks. In this investigation, solvent absorption was increased by forcing animals to walk in a special wheel during the exposure; as this reflects more closely human exposure scenarios where subjects are rarely stationary.
Recently, it has been reported that some aromatic solvents reduce the protective role played by the middle-ear acoustic reflex.76 A dysfunction of this reflex would increase risks to hearing by allowing higher acoustic energy levels to penetrate the inner ear.75,82 This would make co-exposure more dangerous than exposure to noise or to styrene alone.
What is the evidence from epidemiological studies?
In general, clinical or epidemiological studies are designed to test hypotheses, such as the hypothesis that certain chemicals are ototoxic. Several clinical and epidemiological studies confirmed an association between exposure to solvents (styrene, toluene, xylenes, solvent mixtures, and jet fuels) in the workplace and increased prevalence of hearing loss, as well as poor hearing thresholds beyond the traditional 4 kHz noise-related audiometric notch.83–92 For an extensive review of human studies, see Johnson and Morata93 (available online at https://gupea.ub.gu.se/handle/2077/23240).
To examine dose–response relationships, large populations and detailed exposure information would be needed, which can be very challenging to obtain. Thus, the limitations of existing studies (e.g., study designs, insufficient characterization of the exposure levels for chemicals and noise, and lack of details on whether and how other risk factors were accounted for) do not allow us to identify the following: (1) the type of interaction between the agents and how their results can be used to estimate dose–response relationships or (2) the lowest concentrations necessary for an effect to be detected for the solvents covered in the present document. Overall, studies conducted with experimental animals provide the most robust evidence regarding mechanisms and dose–effect relationships between agents and effects on the auditory function or physiology.
Given the difficulties of extrapolating animal findings and analyzing the data obtained from human studies, those in charge of prevention policy must consider both experimental and epidemiological studies.
The approaches adopted in different countries have been described in two recent reviews.5,93 Overall, with combined exposure to noise and organic solvents, interactive effects may be observed depending on the parameters of noise (intensity and impulsiveness) and the solvent-exposure concentrations. In case of concomitant exposures, solvents can exacerbate noise-induced impairments even with a noise intensity below the European permissible limit value.
From a mechanistic point of view, solvent-induced hearing impairment in humans would suggest the involvement of both the inner ear and the central nervous system.
Non-aromatic solvents
What do animal experiments show?
Trichloroethylene is a cleaning and degreasing agent that can also be used for extractions in different chemical processes. Exposure to high concentrations of trichloroethylene has been shown to disrupt cochlear sensory hair and spiral ganglion cells as well the auditory nerve pathways within the cochlea.94–98
In contrast, carbon disulfide (used as a solvent for lipids, sulfur, rubber, phosphorus, oils, resins and waxes), as well as n-hexane (used as a cleaning agent for textiles, furniture, and leather) have been shown to affect the auditory nervous pathway beyond the cochlea in animals99–103; for review see Vyskocil et al.104 These two solvents are associated with retrocochlear dysfunctions.
Nitriles
Nitriles are mainly used for the preparative synthesis of carboxylic acids. However, acetonitrile is used as a solvent, benzonitrile as an initial compound for melamine resins, and acrylonitrile as a monomer for polyacrylonitrile. The ototoxic effects of these compounds have been demonstrated in animal experiments only. Cis-2-pentenenitrile, 3-butenenitrile, cis-crotononitrile, and 3,3′-iminodipropionitrile were shown to cause cochlear hair cell losses and spiral ganglion cell losses, respectively, in rats, mice, guinea pigs, and frogs.13,105,53 In contrast, acute exposure to acrylonitrile in rats produces a transient reduction in auditory sensitivity in the high-frequency range.106
After administration of acrylonitrile, noise-induced hearing impairment was significantly potentiated in rats.106 Under test conditions in which neither acrylonitrile nor noise exposures caused any permanent hearing loss, combined exposure caused permanent hearing impairment and significant losses of the outer hair cells. Thus, hearing loss may occur at low noise intensities if simultaneous exposure to acrylonitrile occurs. Pouyatos et al.107 hypothesized that acrylonitrile increases the risk of noise-induced oxidative damage to the inner ear by impairing the antioxidant’s defense mechanisms of the cells.
Asphyxiants
Carbon monoxide and hydrogen cyanide (salts: cyanides) are two well-known ototoxic asphyxiants. The first is found in exhaust fumes when combustion processes are incomplete in motor vehicles, poorly ventilated stoves and furnaces, acetylene welding, or in enclosed areas (mines and tunnels). Hydrogen cyanide is used as an intermediate product in the organic synthesis of carboxylic acids, pharmaceuticals, dyes and pesticides; relatively large quantities are also required for the surface treatment of metals, galvanizing, and the cyanide leaching process.
What do animal experiments show?
The ototoxicity of asphyxiants is believed to be a consequence of effective oxygen deprivation (hypoxia) within the cochlea. In animal experiments, exposure to carbon monoxide or cyanide has reversible auditory effects at low concentrations and impairs cochlear function under severe exposure conditions.108–110 These experimental studies showed that the two asphyxiants predominantly affect hearing for high-frequency tones, and suggested that while cyanides induce dysfunction of the stria vascularis,110 carbon monoxide produces excessive glutamate release (glutamatergic excitotoxicity) in the synaptic area below the inner hair cells.111
Both carbon monoxide and cyanides have been found to potentiate permanent noise-induced hearing loss in animals and humans.106,112–118 This potentiation may be the result of a reduction in the cell’s ability to repair noise-induced damage due to carbon monoxide poisoning.112,113
What do epidemiological studies show?
In a large epidemiological investigation conducted by Lacerda et al.,118 the hearing thresholds of employees working in noisy occupational environments with combined carbon monoxide exposures were compared with the hearing thresholds of employees working in noisy occupational environments without carbon monoxide. The analysis was based on 9396 audiograms collected by the Quebec National Public Health Institute between 1983 and 1996. The results showed significantly higher hearing thresholds at high frequencies (3, 4 and 6 kHz) for the carbon monoxide-exposed group, with more pronounced effects observed as the duration of exposure increased (15–20 years of exposure).
For an extensive review of human studies with carbon monoxide using other designs, see Johnson and Morata.93 Even though further human studies are needed to clarify these findings, noise-induced hearing impairment also appears to be potentiated by carbon monoxide in humans.
Tobacco smoke
Although a few studies have found no association between smoking and hearing impairment,119,120 numerous epidemiological studies suggest a link.121–126 Tobacco smoke contains hydrogen cyanide among other compounds, and results from epidemiological investigations indicate that smokers may have an increased risk of noise-induced hearing impairment.121,127
Metals and metal compounds
Lead and mercury
Lead and mercury are metal compounds that are known to be neurotoxic, and therefore ototoxic.128,129 Lead and lead compounds can be found in the manufacture of lead–acid batteries and plastic, in ship breaking, in paint and petrol. Lead may also be present during car radiator repair, welding, plumbing, smelting, refining, and mining. Mercury can be found in the chlorine–alkali (chloralkali) industry; the industrial process is performed in mercury cells in which mercury acts as the cathode to produce chlorine and sodium hydroxide. Like lead, mercury compounds may be used in batteries (mercuric oxide) and found in pigments, catalysts, explosives (mercury fulminate), laboratory-based research, and in some pharmaceutical applications.
Several studies carried out with monkeys exposed long-term to lead, and epidemiological studies on lead-exposed workers suggest that lead has an ototoxic effect caused by a neurotoxic mechanism.130–134 Similarly, mercury compounds were shown to induce hearing-damaging effects both in laboratory animals (methyl mercury chloride and mercuric sulfide) and humans.135–138
A few studies did not confirm the effect of these metals on hearing,139,140 but, given the current evidence from human studies, lead and mercury do appear to be ototoxic.
Tin and organic compounds
Tri-n-alkyltins are phytotoxic agents used as powerful bactericides and fungicides. Trimethyltin and triethyltin induce hearing impairments in both rats and guinea pigs, the severity of which is dose-dependent.141–145 Acute limbic-cerebellar syndrome, which includes hearing impairment and involuntary eye movements (nystagmus), was detected in six industrial workers who inhaled trimethyltin.146
Germanium and manganese
Germanium and manganese are found in the manufacture of steel alloys, dry-cell batteries, electrical coils, ceramics, matches, glass, dyes, in fertilizers, welding rods, as oxidizing agents, and as animal food additives.
The administration per os of germanium dioxide (100 mg/kg/day for 4 weeks and 0.5% in food for 2 months) provoked hearing impairment in rats and guinea pigs due to degeneration of the stria vascularis and the cochlear supporting cells.147 Other authors assessed alterations of brainstem transmissions in similar tests.148
Nikolov149 reported in 1974 that the potential ototoxicity of manganese may be exacerbated by exposure to noise, and that workers exposed to both manganese and noise seemed to have accelerated hearing impairment compared with those exposed to manganese alone.
Halogenated hydrocarbons
Finally, there is a strong suspicion for an ototoxic potential of cadmium and arsenic compounds, as well as halogenated hydrocarbons [polychlorinated biphenyls (PCB), tetrabromobisphenol A, hexabromocyclododecane and hexachlorobenzene].150–152 Animal data suggest that halogenated hydrocarbon-induced hearing impairments are the sequelae of thyroid gland disorders caused by some of these substances.153,154 In addition, Powers et al.155 proposed in 2006 that polychlorinated biphenyls could have a direct adverse effect on the outer hair cell function.
Chemical intoxication and presbycusis
Presbycusis refers to a constellation of age-related physiological degenerations, causing a bilateral loss of hearing sensitivity ranging from high to low audiometric frequencies and a decreased ability to understand speech, particularly in the presence of background noise.156–158 From a histopathological point of view, four predominant types of presbycusis can be identified159:
Sensory presbycusis, which refers to the loss of sensory hair cells and supporting cells in the cochlea.
Neural presbycusis, which refers to degeneration of nerve fibers (Fig. 2) in the cochlea and central neural pathways. A large decrease occurs in the density of spiral ganglion cells and afferent fibers, especially evident in the apical turn of the spiral ganglion (Fig. 6, bottom panels).
Strial presbycusis, which results from degeneration of the stria vascularis in the cochlea. Strial presbycusis is characterized by vascular stenosis and reduced vascularization.
Mechanical presbycusis, which results from morphological changes of the basilar membrane of the cochlea.
Fig. 2.
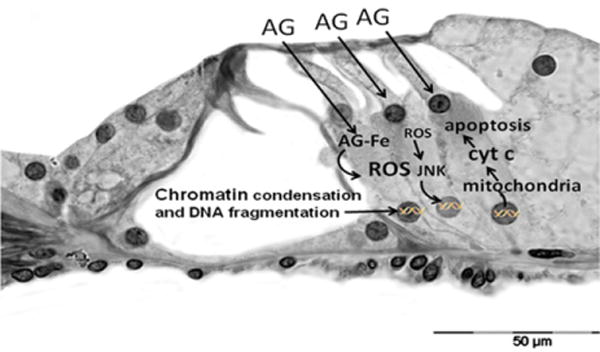
Mechanisms of aminoglycoside-induced outer hair cell damage. AG, aminoglycoside; AG–Fe, aminoglycoside– iron complex; ROS, reactive oxygen species; JNK, c-Jun N-terminal kinase; Cyt C, cytochrome C. Adapted from Rybak and Ramkumar.21
Fig. 6.
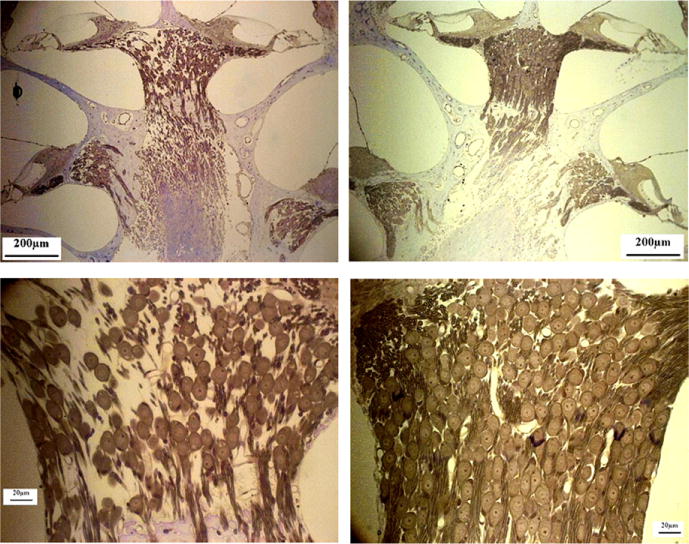
Neural presbycusis. Mid-modular cut cochlea. The left panels correspond to a 25-month-old rat, whereas the right panels correspond to a 5-month-old rat. (Color version of the figure is available online.)
At younger ages (<50 years), the first effects of presbycusis are normally concealed by central counterbalancing mechanisms. Indeed, after an acoustic trauma or an ototoxic intoxication, the central nervous system can increase the spontaneous firing rate of neurons in the dorsal cochlear nucleus160,161 or decrease gabanergic inhibition in the inferior colliculus.162,163 This neural hyperactivity maintains a suitable level of excitability of the auditory nerve cells: the phenomena are called “up-regulation” in the dorsal cochlear nucleus and “down-regulation” in the inferior colliculus. In addition to these up- and down-regulations within the auditory central nuclei, younger subjects have more cochlear hair cells than necessary for assuring normal hearing. As a result, a limited loss of hair cells can be sustained without leading to significant hearing deficits.164 For all these reasons, the effects of ototoxic substances in an exposed population cannot be fully determined by pure-tone audiology (PTA) alone.
What are the consequences of trauma or poisoning on the first manifestations of presbycusis?
Noise exposure (even at moderate intensity) or chemical intoxication can exacerbate age-related hearing loss and lead to early presbycusis, due to exhaustion of the compensatory strategies developed by the different features of the hearing system.165 Like noise, the effects of ototoxic agents are insidious and hard to correlate with the duration of exposure. Hearing damage is generally identified after several years of exposure when it is too late to take preventive action. An early diagnosis of presbycusis is definitely an admission of failure. Preventive action must be taken when it is still possible to preserve workers’ hearing. As a result, we believe PTA is not the appropriate test to use for auditory follow-up when people are exposed to noise or ototoxic agents.
Although many people experience a decrease in hearing acuity with age, others do not, and it is not possible to predict who will and who will not develop hearing loss as they get older. The median hearing loss attributable to aging for a given age group cannot be generalized to all individuals in that age group. Thus, when calculating significant threshold shifts, age-correcting hearing thresholds will overestimate the expected hearing loss for some people and underestimate it for others.
The adjustment of audiometric thresholds to account for age-related effects has become a common practice in workers’ compensation litigation. The age-correction of audiograms measured as part of an occupational hearing loss prevention program is not recommended.166
Patient evaluation, diagnosis, prevention and treatment
Today, few health care providers inquire about hearing loss. In fact, among older adults, 60 years of age and older, only 15% of the primary care providers asked or screened patients for hearing loss.167 If hearing loss is rarely addressed among older adults, for whom many providers would expect hearing loss to be an issue, even fewer clinicians are likely to inquire about hearing loss and tinnitus among middle-aged persons who are exposed to ototoxic substance and/or loud noise in the work or home environment (hearing risks are not confined solely to workplace noise or chemical exposures). Each case history should investigate risk factors in all aspects of the subject’s daily activities, including occupational and nonoccupational tasks, and be followed by appropriate referral and/or counseling. In addition, if hearing is found to be impaired (regardless of etiology), this should not dissuade the physician from encouraging the patient to protect their residual hearing. Each consultation offers a chance for education that should not be wasted. Subjects who are at risk of hearing loss and tinnitus due to exposure to ototoxic substances and noise need to be fully informed about the risks they run and the protection strategies that can be implemented. They must assume responsibility for using personal protective equipment, and develop the habit of using it whenever they are exposed to loud noise (both on and off the job). Thus it is important for health professionals to make a point of seeking out risks to patients’ hearing and advising them appropriately. Many resources are available to help guide counseling activities, including online materials from professional organizations, advocacy groups, government agencies, and vendors of hearing-related health products. However, as the accuracy of information varies greatly among online resources a critical review should be undertaken before using the information.
Evaluation and diagnosis
Dysfunctions of the central auditory nervous system following occupational exposures have been described on the basis of results from behavioral tests, despite some studies reporting normal hearing thresholds.168–173 This suggests that hearing loss caused by chemicals can affect a worker’s life more extensively than noise-induced hearing loss, because sounds are perceived not only as less loud, but also as distorted. Word recognition may also be compromised.
While pure-tone air-conduction audiometry (PTA) remains the most commonly used clinical test both in the United States and Europe to measure the extent of temporary and permanent hearing loss, it has been shown to be insufficient for examining hearing loss from mixed exposure to noise and ototoxic agents, because it does not allow the source of the problem to be diagnosed (i.e., cochlear vs. retrocochlear). In the case of central hearing loss, a PTA can indicate normal hearing, but a person can still have difficulty understanding speech, particularly in background noise, making it difficult to hold a conversation in a busy restaurant or at a party. Thus, for persons exposed to ototoxic chemicals in isolation or in combination with noise it is important to use tests that evaluate the auditory system more comprehensively, from the cochlea to the higher auditory pathways. These tests may help differentiate between the individual (or the combined) effects of noise and ototoxicants on hearing.
As a result, PTA should be complemented with supplementary prevention tools such as distortion product otoacoustic emissions (DPOAEs). Cubic DPOAEs detect inner-ear dysfunctions only, particularly those involving the outer hair cells sensitive to noise and ototoxicants. The inclusion of DPOAEs in a battery of tests to assess hearing would facilitate the distinction between sensory and neural hearing disorders. By combining DPOAE measurements with contra-lateral acoustic stimulation, the stapedial reflex can also be measured. This is important as ototoxicants were recently shown to affect the central nuclei driving the middle-ear acoustic reflex.76,82 Therefore, from a safety point of view, it would be more efficient to collect and measure performances both in the middle ear and in the inner ear. The acoustic reflex would allow chemically induced retrocochlear disorders to be evaluated. Equipment is being designed to offer an alternative to subjective PTA to detect early-stage hearing impairment. One example is the EchoScan, which can provide sensitive, objective, quick, and reliable measurements of both inner- and middle-ear performances, taking both the age and gender of subjects into account.174 This system does not require stringent acoustic conditions (it does not have to be carried out in a sound-proof booth, it can be carried out in a quiet room), making it very convenient for use in the workplace. It is therefore suitable for occupational medicine, especially for the auditory follow-up of people exposed to noise and/or ototoxic agents. The major advantage of EchoScan is that it facilitates detection of the middle-ear reflex, which is quite sensitive to the neuropharmacological effects of some ototoxic substances. As a result, EchoScan can assess both the inner-ear (amplitude of the distortion products) and the middle-ear reflex at the end of a working day. Fifteen minutes before and after the exposure are enough to go through all the tests and to obtain an idea of the cochlear and retrocochlear effects of suspected substances. This system is quite promising for early detection of effects in hearing conservation programs.
Although ideal, we acknowledge that the routine use of an extensive battery of audiological tests in primary care settings may be prohibitive because of time and cost constraints. In the face of such constraints, the selection of a minimum battery of tests with proven validity and reliability, as well as availability and ease of administration, becomes crucial for planning a protocol for routine evaluation.
Prevention
While hearing loss is one of the principal work-related disabilities among various groups of workers, a considerable number of losses could be prevented if exposure to known oto-toxic chemicals, alone or in combination with even mild to moderate noise, was included in risk assessment and management. The initial steps of hearing loss prevention programs are hazard assessment and control. It is important to learn whether and which hazardous exposures exist in a workplace. Whenever hazardous noise or chemicals exist in the workplace, measures to reduce exposure levels to protect exposed workers and to monitor the effectiveness of these interventions are required by law. The most effective way to prevent hearing loss due to noise or chemical exposure is to remove the source of risk from the workplace by engineering controls, finding alternatives to minimize exposure (such as reducing the duration of exposure), or requiring the use of personal protective equipment if engineering or administrative controls do not eliminate exposure. If the use of personal protective equipment is required, it should be worn as directed. Many professional organizations and government agencies have comprehensive sources of information to help guide your counseling activities.
Conclusions
Evidence of how hearing dysfunctions can be caused by chemicals and the combined effects of co-exposures to noise and ototoxic chemicals have emerged predominantly from animal tests in which these associations have been demonstrated. Animal studies have been used to assess the specific effects of several substances to be studied in controlled and appropriate experimental conditions. These findings are supported by cross-sectional epidemiological studies in workers from different industrial sectors exposed to styrene, toluene, solvent mixtures, carbon disulfide, or to a combination of noise and solvents such as toluene, styrene, and ethylbenzene. The risks encountered by workers exposed to chemicals or to chemicals and noise are real, and data obtained from animal models must not be neglected. Today, our scientific knowledge is robust enough to recommend at least precautionary measures for use in occupational environments where workers are exposed to chemicals.
Recommendations
Recognizing the risk of occupational chemical exposure on hearing, the European noise legislation (Directive 2003/10 EC noise)175 as well as the U.S. Army Hearing Conservation guidelines already included ototoxic chemical exposure as a risk factor to be considered in risk assessment and prevention strategies for hearing. The U.S. Army Center for Health Promotion and Preventive Medicine176 also issued a recommendation to consider ototoxic chemical exposures for inclusion in a hearing conservation program and to provide annual audiograms for workers exposed to these chemicals, particularly in combination with marginal noise. The American College of Occupational and Environmental Medicine (ACOEM) guidance statement also indicates that exposure to ototoxicants should be considered when health professionals evaluate sensorineural hearing loss (ACOEM, 2012).177
The American Conference of Governmental Industrial Hygienists (ACGIH) stated in its Threshold Limited Values® and Biological Exposure Indices® publications that periodic audiograms are advised and should be carefully reviewed in occupational settings where exposure to toluene, lead, manganese, or n-butyl alcohol occurs.178 But none of these require clear hearing controls following exposure to ototoxic substances or to co-exposures with noise. In fact, no regulations require workers’ hearing to be monitored when they are exposed to ototoxic chemicals. Tests for ototoxicity should be standardized and incorporated into national, or even international guidelines. More frequent medical monitoring should be considered for workers exposed to ototoxic substances, irrespective of the noise exposure level, and workers’ health results should be recorded to allow early changes at individual and collective levels to be detected.
The audiological control of workers exposed to noise, chemicals, or both should go further than PTA or DPOAE measurements to take the main dimensions of hearing impairments into consideration. An assessment of the loss of communication skills, a middle-ear test (a quick measure of the stapedial reflex), and questionnaires on therapeutic treatments or exposure to chemicals should be included in the battery of audiological tests used to allow early detection and correctly evaluate the total effects on workers hearing. This battery of tests could also be used as an early indicator of neurotoxicity and would be helpful to evaluate the toxicity of industrial chemicals and establish occupational exposure limits.
Ideally, after hospitalization and before returning to work, employees should be interviewed by an occupational physician and potentially ototoxic drugs administered as part of the treatment should be listed. Because the precautionary principle of the EU Commission requires an adequate level of protection even when scientific data are insufficient or ambiguous, individual hearing protectors are recommended for at least 3 months post-treatment for exposures of 80 dBA and above (time-weighted average) in a complex occupational environment (noise plus chemical ototoxic substances). (The precautionary principle is based on evidence that is graded as fair and information from one or two studies that are judged to be reliable.) A special label for ototoxic substances may be considered for chemicals.
For too long, hearing loss prevention has been viewed as a specialized area for a few professionals who manage occupational hearing conservation programs. Hearing loss due to work-related exposure represents a substantial portion of all hearing impairments. Because most work-related hearing losses are permanent, the only way to reduce their burden on society is to prevent them. Without intervention, the financial and social costs of hearing loss also become significant. Physicians have the tools and the opportunity and are in a strategic position to advocate for healthy hearing practices and to facilitate hearing loss prevention. The public health nature of work-related hearing loss and the magnitude of the problem in today’s society require their increased involvement.
Fig. 3.
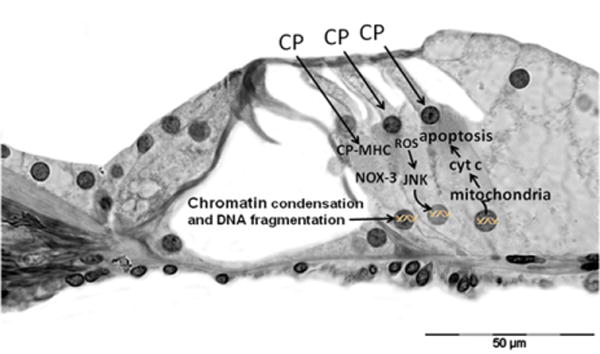
Mechanisms of anticancer drug toxicity. CP, cisplatin; MHC, monohydrate complex; ROS, reactive oxygen species; NOX-3, NADPH oxidase 3; Cyt C, cytochrome C. Adapted from Rybak and Ramkumar.21
Fig. 4.
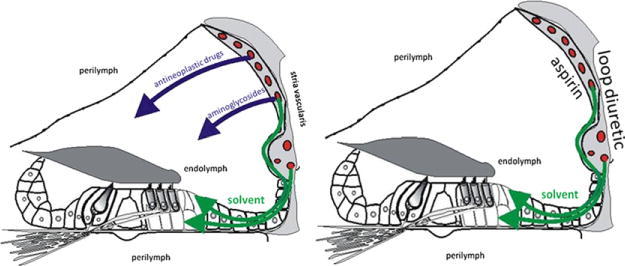
Intoxication route for different medicines and work-related substances. (A) Anti-neoplastic drugs and aminoglycosides are hydrophilic substances, whereas solvents are lipophilic substances. (B) Loop diuretics modify the activity of the ionic pumps, whereas aspirin affects blood fluidity within the stria vascularis. As a result, the ionic concentration of endolymph is disturbed until the molecules are cleared from the system. (Color version of the figure is available online.)
Footnotes
Disclaimer: Mention of any company or product does not constitute endorsement by the Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- 1.Parent-Thirion A, Fernández Macías E, Hurley J, Vermeylen G. Fourth European Working Conditions Survey, European Foundation for the Improvement of Living and Working Conditions. Dublin: 2007. [Google Scholar]
- 2.Bergström B, Nyström B. Development of hearing loss during long-term exposure to occupational noise—a 20-year follow-up study. Scand Audiol. 1986;15:227–234. doi: 10.3109/01050398609042148. [DOI] [PubMed] [Google Scholar]
- 3.Daniell W, Swan S, McDaniel M, Camp J, Cohen M, Stebbins J. Noise exposure and hearing loss prevention programmes after 20 years of regulations in the US. Occup Environ Med. 2006;63:343–351. doi: 10.1136/oem.2005.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morata T. Chemical exposure as a risk factor for hearing loss. J Occup Environ Med. 2003;45:676–682. doi: 10.1097/01.jom.0000071507.96740.70. [DOI] [PubMed] [Google Scholar]
- 5.EU-OSHA European Agency for Safety and Health at Work. Combined exposure to noise and ototoxic substances. 2009:3–60. [Google Scholar]
- 6.Saunders J, Dear S, Schneider M. The anatomical consequences of acoustic injury: a review and tutorial. J Acoust Soc Am. 1985;78:833–860. doi: 10.1121/1.392915. [DOI] [PubMed] [Google Scholar]
- 7.Nordman AS, Bohne B, Harding G. Histopathological differences between temporary and permanent threshold shift. Hear Res. 2000;139:13–30. doi: 10.1016/s0378-5955(99)00163-x. [DOI] [PubMed] [Google Scholar]
- 8.Ruel J, Wang J, Rebillard G, Eybalin M, Pujol R, Puel J. Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear Res. 2007;227:19–27. doi: 10.1016/j.heares.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Gatley M, Kelly G, Turnbull I. A case of organic solvent exposure and temporal lobe demyelination. J Soc Occup Med. 1991;41:83–85. doi: 10.1093/occmed/41.2.83. [DOI] [PubMed] [Google Scholar]
- 10.Abbate C, Giorgianni C, Munao F, Brecciaroli R. Neurotoxicity induced by exposure to toluene. Int Arch Occup Environ Health. 1993;64:389–392. doi: 10.1007/BF00517943. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg M. The central nervous system and exposure to toluene: a risk characterization. Environ Res. 1997;72:1–7. doi: 10.1006/enrs.1996.3686. [DOI] [PubMed] [Google Scholar]
- 12.Fuente A, McPherson B. Central auditory processing effects induced by solvent exposure. Int J Occup Med Environ Health. 2007;20:271–279. doi: 10.2478/v10001-007-0030-4. [DOI] [PubMed] [Google Scholar]
- 13.Soler-Martín C, Diez-Padrisa N, Boadas-Vaello P, Llorens J. Behavioral disturbances and hair cell loss in the inner ear following nitrile exposure in mice, guinea pigs, and frogs. Toxicol Sci. 2007;96:123–132. doi: 10.1093/toxsci/kfl186. [DOI] [PubMed] [Google Scholar]
- 14.Tran Ba Huy P, Meulemans A, Wassef M, Manuel C, Sterkers O, Amiel C. Gentamicin persistence in rat endolymph and perilymph after a two-day constant infusion. Antimicrob Agents Chemother. 1983;23:344–346. doi: 10.1128/aac.23.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govaerts P, Claes J, Van De Heyning P, Jorens P, Marquet J, De Broe M. Aminoglycoside-induced ototoxicity. Toxicol Lett. 1990;52:227–251. doi: 10.1016/0378-4274(90)90033-i. [DOI] [PubMed] [Google Scholar]
- 16.Hashino E, Shero M, Salvi R. Lysosomal targeting and accumulation of aminoglycoside in sensory hair cells. Brain Res. 1997;777:75–85. doi: 10.1016/s0006-8993(97)00977-3. [DOI] [PubMed] [Google Scholar]
- 17.Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000;5:3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- 18.Dulon D, Hiel H, Aurousseau C, Erre JP, Aran JM. Pharmacokinetics of gentamicin in the sensory hair cells of the organ of Corti: rapid uptake and long term persistence. C R Acad Sci III. 1993;316:682–687. [PubMed] [Google Scholar]
- 19.Aran JM, Erre JP, Da Costa DL, Debbarh I, Dulon D. Acute and chronic effects of aminoglycosides on cochlear hair cells. Ann N Y Acad Sci. 1999;884:60–68. doi: 10.1111/j.1749-6632.1999.tb08636.x. [DOI] [PubMed] [Google Scholar]
- 20.Brown R, Penny J, Henley C, et al. Ototoxic drugs and noise. Ciba Found Symp. 1981;85:151–171. doi: 10.1002/9780470720677.ch9. [DOI] [PubMed] [Google Scholar]
- 21.Rybak L, Ramkumar V. Ototoxicity. Int Soc Nephrol. 2007;72:931–935. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- 22.McDonald M, Harrison RV, Wake M, Bliss B, McDonald R. Ototoxicity of carboplatin: comparing animal and clinical models at the hospital for sick children. J Otolaryngol. 1994;23:151–159. [PubMed] [Google Scholar]
- 23.Fausti S, Schechter M, Rappaport B, Frey R, Mass R. Early detection of cisplatin ototoxicity: selected case reports. Cancer. 1998;53:224–231. doi: 10.1002/1097-0142(19840115)53:2<224::aid-cncr2820530207>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Montaguti M, Brandolini C, Ferri GG, Hatzopoulos S, Prete A, Pession A. Cisplatin and carboplatin-induced ototoxicity in children: clinical aspects and perspectives for prevention. Acta Otorhinolaryngol Ital. 2002;22:14–18. [PubMed] [Google Scholar]
- 25.Hamers F, Wijbenga J, Wolters F, Klis S, Sluyter S, Smoorenburg G. Cisplatin ototoxicity involves organ of Corti, stria vascularis and spiral ganglion: modulation by alphaMSH and ORG 2766. Audiol Neurootol. 2003;8:305–315. doi: 10.1159/000073515. [DOI] [PubMed] [Google Scholar]
- 26.Van der Hulst RJAM, Dreschler W, Urbanus N. High-frequency audiometry in prospective clinical research of ototoxicity due to platinum derivates. Ann Otol Rhinol Laryngol. 1988;97:133–137. doi: 10.1177/000348948809700208. [DOI] [PubMed] [Google Scholar]
- 27.Gratton M, Salvi R, Kamen B, Saunders S. Interaction of cisplatin and noise on the peripheral auditory system. Hear Res. 1990;50:211–223. doi: 10.1016/0378-5955(90)90046-r. [DOI] [PubMed] [Google Scholar]
- 28.Laurell G. Combined effects of noise and cisplatin: short- and long-term follow-up. Ann Otol Rhinol Laryngol. 1992;101:969–976. doi: 10.1177/000348949210101202. [DOI] [PubMed] [Google Scholar]
- 29.Forge A. A tubulo-cisternal endoplasmic reticulum system in the potassium transporting marginal cells of the stria vascularis and effects of the ototoxic diuretic ethacrynic acid. Cell Tissue Res. 1982;226:375–387. doi: 10.1007/BF00218367. [DOI] [PubMed] [Google Scholar]
- 30.Ding D, McFadden SL, Woo JM, Salvi RJ. Ethacrynic acid rapidly and selectively abolishes blood flow in vessels supplying the lateral wall of the cochlea. Hear Res. 2002;173:1–9. doi: 10.1016/s0378-5955(02)00585-3. [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Rodríguez R, García Lorenzo J, Bellido Peti J, Palou Redorta J, Gómez Ruiz JJ, Villavicencio Mavrich H. Loop diuretics and ototoxicity. Actas Urol Esp. 2007;31:1189–1192. doi: 10.1016/s0210-4806(07)73785-3. [DOI] [PubMed] [Google Scholar]
- 32.Walker EM, Fazekas-May MA, Bowen WB. Nephrotoxic and ototoxic agents. Clin Lab Med. 1990;10:323–354. [PubMed] [Google Scholar]
- 33.Komune S, Snow JB. Potentiating effects of cisplatin and ethacrynic acid in ototoxicity. Arch Otolaryngol. 1981;107:594–597. doi: 10.1001/archotol.1981.00790460006003. [DOI] [PubMed] [Google Scholar]
- 34.Whitworth CA, Hudson TE, Rybak LP. The effect of combined administration of cadmium and furosemide on auditory function in the rat. Hear Res. 1999;129:61–70. doi: 10.1016/s0378-5955(98)00222-6. [DOI] [PubMed] [Google Scholar]
- 35.McCabe P, Dey D. The effect of aspirin upon auditory sensitivity. Ann Otol Rhinol Laryngol. 1965;74:312–325. doi: 10.1177/000348946507400203. [DOI] [PubMed] [Google Scholar]
- 36.Myers EN, Bernstein JM. Salicylate ototoxicity. Arch Otolaryngol. 1965;82:483–493. doi: 10.1001/archotol.1965.00760010485006. [DOI] [PubMed] [Google Scholar]
- 37.Stypulkowski PH. Mechanisms of salicylate ototoxicity. Hear Res. 1990;46:113–146. doi: 10.1016/0378-5955(90)90144-e. [DOI] [PubMed] [Google Scholar]
- 38.Young LL, Wilson KA. Effects of acetylsalicylate acid on speech discrimination. Audiology. 1982;21:342–349. doi: 10.3109/00206098209072749. [DOI] [PubMed] [Google Scholar]
- 39.Johnson A. The ototoxic effect of toluene and the influence of noise, acetyl salicylic acid, or genotype. A study in rats and mice. Scand Audiol. 1993;39:1–40. [PubMed] [Google Scholar]
- 40.Austin CC, Wang D, Ecobichon DJ, Dussault G. Characterization of volatile organic compounds in smoke at municipal structural fires. J Toxicol Environ Health. 2001;63:437–458. doi: 10.1080/152873901300343470. [DOI] [PubMed] [Google Scholar]
- 41.Bolstad-Johnson DM, Burgess JL, Crutchfield CD, Storment S, Gerkin R, Wilson JR. Characterization of firefighter exposures during fire overhaul. Am Ind Hyg Assoc J. 2000;61:636–641. doi: 10.1080/15298660008984572. [DOI] [PubMed] [Google Scholar]
- 42.Fabian T, Borgerson JL, Kerber SI, et al. Firefighter Exposure to Smoke Particulates. IL: Underwriters Laboratories Inc; 2010. [Google Scholar]
- 43.Fent KW, Evans DE. Assessing the risk to firefighters from chemical vapors and gases during vehicle fire suppression. J Environ Monit. 2011;13(3):536–543. doi: 10.1039/c0em00591f. [DOI] [PubMed] [Google Scholar]
- 44.Jankovic J, Jones W, Burkhart J, Noonan G. Environmental study of firefighters. Ann Occup Hyg. 1991;35:581–602. doi: 10.1093/annhyg/35.6.581. [DOI] [PubMed] [Google Scholar]
- 45.Al-Malki AL. Serum heavy metals and hemoglobin related compounds in Saudi Arabia firefighters. J Occup Med Toxicol. 2009;4:18. doi: 10.1186/1745-6673-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golden AL, Markowitz SB, Landrigan PJ. The risk of cancer in firefighters. Occup Med. 1995;10:803–820. [PubMed] [Google Scholar]
- 47.Meek M, Chan P. Toluene: evaluation of risks to human health from environmental exposure in Canada. Environ Carcin Eco Rev. 1994;12:507–515. [Google Scholar]
- 48.Calabrese G, Martini A, Sessa G, et al. Otoneurological study in workers exposed to styrene in the fiberglass industry. Int Arch Occup Environ Health. 1996;68:219–223. doi: 10.1007/BF00381431. [DOI] [PubMed] [Google Scholar]
- 49.Lazar R, Ho S, Melen O, Daghestani A. Multifocal central nervous system damage caused by toluene abuse. Neurology. 1983;33:1337–1340. doi: 10.1212/wnl.33.10.1337. [DOI] [PubMed] [Google Scholar]
- 50.Möller C, Ödkvist L, Larsby B, Tham R, Ladin T, Bergholtz L. Otoneurological findings in workers exposed to styrene. Scand J Work Environ Health. 1990;16:189–194. doi: 10.5271/sjweh.1795. [DOI] [PubMed] [Google Scholar]
- 51.Pryor GT, Dickinson J, Howd RA, Rebert CS. Transient cognitive deficits and high-frequency hearing loss in weanling rats exposed to toluene. Neurobehav Toxicol Teratol. 1983;5:53–57. [PubMed] [Google Scholar]
- 52.Pryor GT, Rebert CS, Howd RA. Hearing loss in rats caused by inhalation of mixed xylenes and styrene. J Appl Toxicol. 1987;7:55–61. doi: 10.1002/jat.2550070110. [DOI] [PubMed] [Google Scholar]
- 53.Crofton K, Janssen R, Prazma J, Pulver S, Barone S. The ototoxicity of 3,3′-iminodipropionitrile: functional and morphological evidence of cochlear damage. Hear Res. 1994;80:129–140. doi: 10.1016/0378-5955(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 54.Campo P, Lataye R, Loquet G, Bonnet P. Styrene-induced hearing loss: a membrane insult. Hear Res. 2001;154:170–180. doi: 10.1016/s0378-5955(01)00218-0. [DOI] [PubMed] [Google Scholar]
- 55.Cappaert N, Klis S, Muijser H, de Groot J, Kulig B, Smoorenburg G. The ototoxic effects of ethyl benzene in rats. Hear Res. 1999;137:91–102. doi: 10.1016/s0378-5955(99)00141-0. [DOI] [PubMed] [Google Scholar]
- 56.Mattsson J, Gorzinski S, Albee R, Zimmer M. Evoked potential changes from 13 weeks of simulated toluene abuse in rats. Pharmacol Behav. 1990;36:683–689. doi: 10.1016/0091-3057(90)90274-l. [DOI] [PubMed] [Google Scholar]
- 57.Gagnaire F, Langlais C. Relative ototoxicity of 21 aromatic solvents. Arch Toxicol. 2005;79:349–354. doi: 10.1007/s00204-004-0636-2. [DOI] [PubMed] [Google Scholar]
- 58.Johnson A, Canlon B. Progressive hair cell loss induced by toluene exposure. Hear Res. 1994;75:201–208. doi: 10.1016/0378-5955(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 59.Campo P, Lataye R, Cossec B, Placidi V. Toluene-induced hearing loss: a mid-frequency location of the cochlear lesions. Neurotoxicol Teratol. 1997;19:129–140. doi: 10.1016/s0892-0362(96)00214-0. [DOI] [PubMed] [Google Scholar]
- 60.Campo P, Lataye R, Bonnet P. No interaction between noise and toluene on cochlea in the guinea pig. Acta Acoust. 1993;1:35–42. [Google Scholar]
- 61.Cappaert N, Klis S, Muijser H, Kulig B, Ravensberg L, Smoorenburg G. Differential susceptibility of rats and guinea pigs to the ototoxic effects of ethyl benzene. Neurotoxicol Teratol. 2002;24:503–510. doi: 10.1016/s0892-0362(02)00208-8. [DOI] [PubMed] [Google Scholar]
- 62.Kishi R, Harabuchi I, Ikeda T, Yokota H, Miyake H. Neurobehavioural effects and pharmacokinetics of toluene in rats and their relevance to man. Br J Ind Med. 1988;45:396–408. doi: 10.1136/oem.45.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen G, Chi L, Kostyniak P, Henderson D. Styrene induced alterations in biomarkers of exposure and effects in the cochlea: mechanisms of hearing loss. Toxicol Sci. 2007;98:167–177. doi: 10.1093/toxsci/kfm078. [DOI] [PubMed] [Google Scholar]
- 64.Corey D, Hudspeth A. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979;281:675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- 65.Spicer S, Smythe N, Schulte B. Ultrastructure indicative of ion transport in tectal, Deiters and tunnel cells: differences between gerbil and chinchilla basal and apical cochlea. Anat Rec. 2003;271:342–359. doi: 10.1002/ar.a.10041. [DOI] [PubMed] [Google Scholar]
- 66.Yamakura T, Borghese C, Harris R. A transmembrane site determines sensitivity of neuronal nicotinic acetylcholine receptors to general anesthetics. J Biol Chem. 2000;275:40879–40886. doi: 10.1074/jbc.M005771200. [DOI] [PubMed] [Google Scholar]
- 67.MacIver M. Abused inhalants enhance GABA-mediated synaptic inhibition. Neuropsychopharmacol. 2009;34:2296–2304. doi: 10.1038/npp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campo P, Venet T, Thomas A, et al. Inhaled toluene can modulate the effects of anesthetics on the middle-ear acoustic reflex. Neurotoxicol Teratol. 2013;35:1–6. doi: 10.1016/j.ntt.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Cruz S, Mirshahi T, Thomas B, Balster R, Woodward J. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;286:334–340. [PubMed] [Google Scholar]
- 70.Beckstead M, Weiner J, Eger E, Gong D, Mihic S. Glycine and δ-aminobutyric acid receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- 71.Woodward JJ, Nowak M, Davies DL. Effects of the abused solvent toluene on recombinant P2X receptors expressed in HEK293 cells. Brain Res Mol Brain Res. 2004;125:86–95. doi: 10.1016/j.molbrainres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Lopreato G, Phelan R, Borghese C, Beckstead M, Mihic M. Inhaled drugs of abuse enhance serotonin-3 receptor function. Drug Alcohol Depend. 2003;70:11–15. doi: 10.1016/s0376-8716(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 73.Bale A, Meacham A, Benignus V, Bushnell P, Shafer T. Volatile organic compounds inhibit human and rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Toxicol Appl Pharmacol. 2005;205:77–88. doi: 10.1016/j.taap.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 74.Lataye R, Maguin K, Campo P. Increase in cochlear microphonic potential after toluene administration. Hear Res. 2007;230:34–42. doi: 10.1016/j.heares.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Maguin K, Campo P, Parietti-Winkler C. Toluene can perturb the neuronal voltage-dependent Ca2+ channels involved in the middle-ear reflex. Toxicol Sci. 2009;107:473–481. doi: 10.1093/toxsci/kfn242. [DOI] [PubMed] [Google Scholar]
- 76.Venet T, Rumeau C, Campo P, Rieger B, Thomas A, Cour C. Neuronal circuits involved in the middle-ear acoustic reflex. Toxicol Sci. 2011;119:146–155. doi: 10.1093/toxsci/kfq312. [DOI] [PubMed] [Google Scholar]
- 77.Johnson A, Juntunen L, Nylén P, Borg E, Höglund G. Effect of interaction between noise and toluene on auditory function in the rat. Acta Otolaryngol. 1988;105:56–63. doi: 10.3109/00016488809119446. [DOI] [PubMed] [Google Scholar]
- 78.Lataye R, Campo P. Combined effects of a simultaneous exposure to noise and toluene on hearing function. Neurotoxicol Teratol. 1997;19:373–382. doi: 10.1016/s0892-0362(97)00049-4. [DOI] [PubMed] [Google Scholar]
- 79.Lataye R, Campo P, Loquet G, Morel G. Combined effects of noise and styrene on hearing: comparison between active and sedentary rats. Noise Health. 2005;7:49–64. doi: 10.4103/1463-1741.31633. [DOI] [PubMed] [Google Scholar]
- 80.Mäkitie AA, Pirvola U, Pyykö I, Sakakibara H, Riihimäki V, Ylikoski J. The ototoxic interaction of styrene and noise. Hear Res. 2003;179:9–20. doi: 10.1016/s0378-5955(03)00066-2. [DOI] [PubMed] [Google Scholar]
- 81.Cappaert N, Klis S, Muijser H, Kulig B, Smoorenburg G. Simultaneous exposure to ethylbenzene and noise: synergistic effects on outer hair cells. Hear Res. 2001;162:67–79. doi: 10.1016/s0378-5955(01)00373-2. [DOI] [PubMed] [Google Scholar]
- 82.Campo P, Maguin K, Lataye R. Effects of aromatic solvents on acoustic reflexes mediated by central auditory pathways. Toxicol Sci. 2007;99:582–590. doi: 10.1093/toxsci/kfm180. [DOI] [PubMed] [Google Scholar]
- 83.Chang S, Shih T, Chou T, Chen C, Chang H, Sung F. Hearing loss in workers exposed to carbon disulfide and noise. Environ Health Perspect. 2003;111:1620–1624. doi: 10.1289/ehp.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang S, Chen C, Lien C, Sung F. Hearing loss in workers exposed to toluene and noise. Environ Health Perspect. 2006;114:1283–1286. doi: 10.1289/ehp.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson A, Morata T, Lindblad AC, et al. Audiological findings in workers exposed to styrene alone or in concert with noise. Noise Health. 2006;8:45–57. doi: 10.4103/1463-1741.32467. [DOI] [PubMed] [Google Scholar]
- 86.Morata T. Study of the effects of simultaneous exposure to noise and carbon disulfide on workers hearing. Scand Audiol. 1989;18:53–58. doi: 10.3109/01050398909070723. [DOI] [PubMed] [Google Scholar]
- 87.Morata T, Dunn D, Kretschmer L, Lemasters G, Keith R. Effects of occupational exposure to organic solvents and noise on hearing. Scand J Work Environ Health. 1993;19:245–254. doi: 10.5271/sjweh.1477. [DOI] [PubMed] [Google Scholar]
- 88.Morata T, Johnson A, Nylen P, et al. Audiometric findings in workers exposed to low levels of styrene and noise. J Occup Environ Med. 2002;44:806–814. doi: 10.1097/00043764-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 89.Prasher D, Al-Hajjaj H, Aylott S, Aksentijevic A. Effect of exposure to a mixture of solvents and noise on hearing and balance in aircraft maintenance workers. Noise Health. 2005;7:31–39. doi: 10.4103/1463-1741.31876. [DOI] [PubMed] [Google Scholar]
- 90.Sliwinska-Kowalska M, Zamyslowska-Szmytke E, Szymczak W, et al. Ototoxic effects of occupational exposure to styrene and co-exposure to styrene and noise. J Occup Environ Med. 2003;45:15–24. doi: 10.1097/00043764-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 91.Sliwinska-Kowalska M, Zamyslowska-Szmytke E, Szymczak W, et al. Exacerbation of noise-induced hearing loss by co-exposure to workplace chemicals. Environ Toxicol Pharmacol. 2005;19:547–553. doi: 10.1016/j.etap.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 92.Schäper M, Seeber A, van Thriel C. The effects of toluene plus noise on hearing thresholds: an evaluation based on repeated measurements in the German printing industry. Int J Occup Med Environ Health. 2008;21:191–200. doi: 10.2478/v10001-008-0030-z. [DOI] [PubMed] [Google Scholar]
- 93.Johnson AC, Morata TC. Occupational exposure to chemicals and hearing impairment. The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals. Nordic Expert Group. Gothenburg. Arbete och Hälsa. 2010;44:177. < http://hdl.handle.net/2077/23240>. [Google Scholar]
- 94.Albee R, Spencer P, Johnson K, et al. Lack of trigeminal nerve toxicity in rats exposed to trichloroethylene vapor for 13 weeks. Int J Toxicol. 2006;25:531–540. doi: 10.1080/10915810600972678. [DOI] [PubMed] [Google Scholar]
- 95.Crofton K, Zhao X. Mid-frequency hearing loss in rats following inhalation exposure to TCE: evidence from reflex modification audiometry. Neurotoxicol Teratol. 1993;15:413–423. doi: 10.1016/0892-0362(93)90059-w. [DOI] [PubMed] [Google Scholar]
- 96.Fechter L, Liu Y, Herr D, Crofton K. Trichlorethylene ototoxicity: evidence for a cochlear origin. Toxicol Sci. 1998;42:28–35. doi: 10.1006/toxs.1997.2413. [DOI] [PubMed] [Google Scholar]
- 97.Muijser H, Lammers J, Kullig B. Effects of exposure to trichloroethylene and noise on hearing in rats. Noise Health. 2000;2:57–66. [PubMed] [Google Scholar]
- 98.Vyskocil A, Leroux T, Truchon G, et al. Ototoxicity of trichloroethylene in concentrations relevant for the working environment. Hum Exp Toxicol. 2008;27:195–200. doi: 10.1177/0960327108090267. [DOI] [PubMed] [Google Scholar]
- 99.Johnson A, Nylén P. Effects of industrial solvents on hearing. Occup Med. 1995;10:623–640. [PubMed] [Google Scholar]
- 100.Hirata M, Ogawa Y, Okayama A, Goto S. Changes in auditory brainstem response in rats chronically exposed to carbon disulfide. Arch Toxicol. 1992;66:334–338. doi: 10.1007/BF01973628. [DOI] [PubMed] [Google Scholar]
- 101.Hirata M, Ogawa Y, Okayama A, Goto S. A cross-sectional study on the brainstem auditory evoked potential among workers exposed to carbon disulfide. Int Arch Occup Environ Health. 1992;64:321–324. doi: 10.1007/BF00379540. [DOI] [PubMed] [Google Scholar]
- 102.Howd R, Rebert C, Dickinson J, Pryor G. A comparison of the rates of development of functional hexane neuropathy in weanling and young adult rats. Neurobehav Toxicol Teratol. 1983;6:63–68. [PubMed] [Google Scholar]
- 103.Rebert C, Becker E. Effects of inhaled carbon disulfide on sensory-evoked potentials of Long-Evans rats. Neurobehav Toxicol Teratol. 1986;8:533–541. [PubMed] [Google Scholar]
- 104.Vyskocil A, Leroux T, Truchon G, Gendron M, El Majidi N, Viau C. Occupational ototoxicity of n-hexane. Hum Exp Toxicol. 2008;27:471–476. doi: 10.1177/0960327108093719. [DOI] [PubMed] [Google Scholar]
- 105.Balbuena E, Llorens J. Comparison of cis- and trans-crotononitrile effects in rat reveals specificity in the neurotoxic properties of nitrile isomers. Toxicol Appl Pharmacol. 2003;187:89–100. doi: 10.1016/s0041-008x(02)00039-x. [DOI] [PubMed] [Google Scholar]
- 106.Fechter L, Klis S, Shirwany N, Moore T, Rao D. Acrylonitrile produces transient cochlear function loss and potentiates permanent noise-induced hearing loss. Toxicol Sci. 2003;75:117–123. doi: 10.1093/toxsci/kfg169. [DOI] [PubMed] [Google Scholar]
- 107.Pouyatos B, Gearhart C, Fechter D. Acrylonitrile potentiates hearing loss and cochlear damage induced by moderate noise exposure in rats. Toxicol Appl Pharmacol. 2005;204:46–56. doi: 10.1016/j.taap.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 108.Fechter L, Thorne P, Nutall A. Effects of carbon monoxide hypoxia on cochlear bloodflow and electrophysiological potentials. Hear Res. 1987;27:37–45. doi: 10.1016/0378-5955(87)90024-4. [DOI] [PubMed] [Google Scholar]
- 109.Liu Y, Fechter L. MK-801 protects against carbon monoxide induced hearing loss. Toxicol Appl Pharmacol. 1995;132:196–202. doi: 10.1006/taap.1995.1099. [DOI] [PubMed] [Google Scholar]
- 110.Tawackoli W, Chen G, Fechter L. Disruption of cochlear potentials by chemical asphyxiants. Cyanide and carbon monoxide. Neurotoxicol Teratol. 2001;23:157–165. doi: 10.1016/s0892-0362(01)00135-0. [DOI] [PubMed] [Google Scholar]; Van Heijst A, Maes R, Mtanda A, Chuwa L, Rwiza H, Moshi N. Chronic cyanide poisoning in relation to blindness and tropical neuropathy. Clin Toxicol. 1994;32:549–556. doi: 10.3109/15563659409011059. [DOI] [PubMed] [Google Scholar]
- 111.Kanthasamy A, Borowitz J, Pavlakovic G, Isom G. Dopaminergic neurotoxicity of cyanide: neurochemical, histological and behavioural characterization. Toxicol Appl Pharmacol. 1994;126:156–163. doi: 10.1006/taap.1994.1102. [DOI] [PubMed] [Google Scholar]
- 112.Chen G, Fechter L. Potentiation of octave-band noise induced auditory impairment by carbon monoxide. Hear Res. 1999;132:149–159. doi: 10.1016/s0378-5955(99)00044-1. [DOI] [PubMed] [Google Scholar]
- 113.Chen G, McWilliams M, Fechter L. Intermittent noise-induced hearing loss and the influence of carbon monoxide. Hear Res. 1999;138:181–191. doi: 10.1016/s0378-5955(99)00157-4. [DOI] [PubMed] [Google Scholar]
- 114.Fechter L, Young J, Carlisle L. Potentiation of noise induced threshold shifts and hair cell loss by carbon monoxide. Hear Res. 1988;34:39–47. doi: 10.1016/0378-5955(88)90049-4. [DOI] [PubMed] [Google Scholar]
- 115.Fechter L, Chen G, Rao D, Larabee J. Predicting exposure conditions that facilitate the potentiation of noise-induced hearing loss by carbon monoxide. Toxicol Sci. 2000;58:315–323. doi: 10.1093/toxsci/58.2.315. [DOI] [PubMed] [Google Scholar]
- 116.Fechter L, Chen G, Johnson D. Potentiation of noise-induced hearing loss by low concentrations of hydrogen cyanide in rats. Toxicol Sci. 2002;66:131–138. doi: 10.1093/toxsci/66.1.131. [DOI] [PubMed] [Google Scholar]
- 117.Young J, Upchurch M, Kaufman M, Fechter L. Carbon monoxide exposure potentiates high-frequency auditory threshold shifts induced by noise. Hear Res. 1987;26:37–43. doi: 10.1016/0378-5955(87)90034-7. [DOI] [PubMed] [Google Scholar]
- 118.Lacerda A, Leroux T, Gagn JP. The combined effect of noise and carbon monoxide on hearing thresholds of exposed workers. J Acoust Soc Am. 2005;117:2481. [Google Scholar]
- 119.Starck J, Toppila E, Pyckkö I. Smoking as a risk factor in sensory neural hearing loss among workers exposed to occupational noise. Acta Otolaryngol (Stockh) 1999;119:302–305. doi: 10.1080/00016489950181288. [DOI] [PubMed] [Google Scholar]
- 120.Karlsmose B, Lauritzen T, Engberg M, Parving A. A five-year longitudinal study of hearing in a Danish rural population aged 31–50 years. Br J Audiol. 2000;34:47–55. doi: 10.3109/03005364000000117. [DOI] [PubMed] [Google Scholar]
- 121.Barone J, Peters J, Garabrant D, Bernstein L, Krebsbach R. Smoking as a risk factor in noise-induced hearing loss. J Occup Med. 1987;29:741–745. [PubMed] [Google Scholar]
- 122.Cruickshanks K, Klein R, Klein B, Wiley T, Nondahl D, Tweed T. Cigarette smoking and hearing loss, the epidemiology of hearing loss study. J Am Med Assoc. 1998;279:1715–1719. doi: 10.1001/jama.279.21.1715. [DOI] [PubMed] [Google Scholar]
- 123.Mizoue T, Miyamoto T, Shimizu T. Combined effect of smoking and occupational exposure to noise on hearing loss in steel factory workers. Occup Environ Med. 2003;60:56–59. doi: 10.1136/oem.60.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nomura K, Nakao M, Yano E. Hearing loss associated with smoking and occupational noise exposure in a Japanese metal working company. Int Arch Occup Environ Health. 2005;78:178–184. doi: 10.1007/s00420-005-0604-z. [DOI] [PubMed] [Google Scholar]
- 125.Uchida Y, Nakashima T, Ando F, Niino N, Shimokata H. Is there a relevant effect of noise and smoking on hearing? A population-based aging study. Int J Audiol. 2005;44:86–91. doi: 10.1080/14992020500031256. [DOI] [PubMed] [Google Scholar]
- 126.Stanbury M, Rafferty A, Rosenman K. Prevalence of hearing loss and work-related noise-induced hearing loss in Michigan. J Occup Environ Med. 2008;50:72–79. doi: 10.1097/JOM.0b013e31815b568c. [DOI] [PubMed] [Google Scholar]
- 127.Wild DC, Brewster MJ, Banerjee AR. Noise-induced hearing loss is exacerbated by long-term smoking. Clin Otolaryngol. 2005;30:517–520. doi: 10.1111/j.1749-4486.2005.01105.x. [DOI] [PubMed] [Google Scholar]
- 128.Araki S, Murata K, Yokoyama K, Uchida E. Auditory event-related potential in relation to peripheral nerve conduction in workers exposed to lead, zinc, and copper: effects of lead on cognitive function. Am J Ind Med. 1992;21:539–547. doi: 10.1002/ajim.4700210409. [DOI] [PubMed] [Google Scholar]
- 129.Bleecker M, Ford D, Lindgren K, Scheetz K, Tiburzi M. Association of chronic and current measures of lead exposure with different components of brainstem auditory evoked potentials. Neurotox. 2003;24:625–631. doi: 10.1016/s0161-813x(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 130.Counter S, Buchanan L. Neuro-ototoxicity in Andean adults with chronic lead and noise exposure. J Occup Environ Med. 2002;44:30–38. doi: 10.1097/00043764-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 131.Discalzi G, Capellaro F, Bottalo L, Fabro D, Mocellini A. Auditory brainstem evoked potentials (BAEPs) in lead-exposed workers. Neurotoxicology. 1992;13:207–210. [PubMed] [Google Scholar]
- 132.Forst LS, Freels S, Persky V. Occupational lead exposure and hearing loss. J Occup Environ Med. 1997;39:658–660. doi: 10.1097/00043764-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 133.Wu T, Shen C, Lai J, et al. Effects of lead and noise exposure on hearing ability. Arch Environ Health. 2000;55:109–114. doi: 10.1080/00039890009603396. [DOI] [PubMed] [Google Scholar]
- 134.Hwang YH, Chiang HY, Yen-Jean MC, Wang JD. The association between low levels of lead in blood and occupational noise-induced hearing loss in steel workers. Sci Total Environ. 2009;408:43–49. doi: 10.1016/j.scitotenv.2009.09.016. http://dx.doi.org/10.1016/j.scitotenv.2009.09.016. [Epub 2009 Oct 9] [DOI] [PubMed] [Google Scholar]
- 135.Wassick K, Yonovitz A. Methylmercury ototoxicity in mice determined by auditory brainstem responses. Acta Otolaryngol. 1985;99:35–45. doi: 10.3109/00016488509119143. [DOI] [PubMed] [Google Scholar]
- 136.Rice D, Gilbert S. Exposure to methyl mercury from birth to adulthood impairs high-frequency hearing in monkeys. Toxicol Appl Pharmacol. 1992;115:6–10. doi: 10.1016/0041-008x(92)90361-u. [DOI] [PubMed] [Google Scholar]
- 137.Discalzi G, Fabbro D, Meliga F, Mocellini A, Capellaro F. Effects of occupational exposure to mercury and lead on brainstem auditory evoked potentials. Int J Psychophys. 1993;14:21–25. doi: 10.1016/0167-8760(93)90080-9. [DOI] [PubMed] [Google Scholar]
- 138.Chuu J, Hsu C, Lin-Shiau S. Abnormal auditory brainstem responses for mice with mercurial compounds: involvement of excessive nitric oxide. Toxicology. 2001;162:11–22. doi: 10.1016/s0300-483x(01)00348-1. [DOI] [PubMed] [Google Scholar]
- 139.Counter S, Buchanan L, Ortega F, Laurell G. Normal auditory brainstem and cochlear function in extreme pediatric plumbism. J Neurol Sci. 1997;152:85–92. doi: 10.1016/s0022-510x(97)00149-4. [DOI] [PubMed] [Google Scholar]
- 140.Counter S, Vahter M, Laurell G, Buchanan L, Ortega F, Skerfving S. High lead exposure and auditory sensory-neural function in Andean children. Environ Health Perspect. 1997;105:522–526. doi: 10.1289/ehp.97105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fechter L, Carlisle L, Nutall A. Trimethyltin ototoxicity: evidence for a cochlear site of injury. Hear Res. 1986;23:275–282. doi: 10.1016/0378-5955(86)90116-4. [DOI] [PubMed] [Google Scholar]
- 142.Young JS, Fechter L. Trimethyltin exposure produces an unusual form of toxic auditory damage in rats. Toxicol Appl Pharmacol. 1986;82:87–93. doi: 10.1016/0041-008x(86)90441-2. [DOI] [PubMed] [Google Scholar]
- 143.Clerici W, Ross B, Fechter L. Acute ototoxicity of trialkyltins in the guinea pig. Toxicol Appl Pharmacol. 1991;109:547–556. doi: 10.1016/0041-008x(91)90017-9. [DOI] [PubMed] [Google Scholar]
- 144.Fechter L, Carlisle L. Auditory dysfunction and cochlear vascular injury following trimethyltin exposure in the guinea pig. Toxicol Appl Pharmacol. 1990;105:133–143. doi: 10.1016/0041-008x(90)90365-2. [DOI] [PubMed] [Google Scholar]
- 145.Hoeffding V, Fechter L. Trimethyltin disrupts auditory function and cochlear morphology in pigmented rats. Neurotoxicol Teratol. 1991;13:135–145. doi: 10.1016/0892-0362(91)90003-f. [DOI] [PubMed] [Google Scholar]
- 146.Besser R, Kramer G, Thumler R, Bohl J, Gutmann L, Hopf H. Acute trimethyltin limbic-cerebellar syndrome. Neurology. 1987;37:945–950. doi: 10.1212/wnl.37.6.945. [DOI] [PubMed] [Google Scholar]
- 147.Yamasoba T, Goto Y, Komaki H, Mimaki M, Sudo A, Susuki M. Cochlear damage due to germanium-induced mitochondrial dysfunction in guinea pigs. Neurosci Lett. 2006;395:18–22. doi: 10.1016/j.neulet.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 148.Lin C, Chen T, Chen S. Functional changes on ascending auditory pathway in rats caused by germanium dioxide exposure: an electrophysiological study. Toxicology. 2009;256:110–117. doi: 10.1016/j.tox.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 149.Nikolov Z. Hearing reduction caused by manganese and noise. J Fr Otorhinolaryngol Audiophonol Chir Maxillofac. 1974;23:231–234. [PubMed] [Google Scholar]
- 150.Crofton K, Ding D, Padich R, Taylor M, Henderson D. Hearing loss following exposure during development to polychlorinated biphenyls: a cochlear site of action. Hear Res. 2000;144:196–204. doi: 10.1016/s0378-5955(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 151.Lasky RE, Widholm J, Crofton K, Schantz S. Perinatal exposure to Aroclor 1254 impairs distortion product otoacoustic emissions (DPOAEs) in rats. Toxicol Sci. 2002;68:458–464. doi: 10.1093/toxsci/68.2.458. [DOI] [PubMed] [Google Scholar]
- 152.Hadjab S, Maurel D, Cazals Y, Siaud P. Hexachlorobenzene, a dioxin-like compound, disrupts auditory function in rat. Hear Res. 2004;191:125–134. doi: 10.1016/j.heares.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 153.Goldey E, Kehn L, Lau C, Rehnberg G, Crofton K. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- 154.Zoeller RT. Environmental chemicals as thyroid hormone analogues: new studies indicate that thyroid hormone receptors are targets of industrial chemicals. Mol Cell Endocrinol. 2005;242:10–15. doi: 10.1016/j.mce.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 155.Powers B, Widholm J, Lasky R, Schantz S. Auditory deficits in rats exposed to an environmental PCB mixture during development. Toxicol Sci. 2006;89:415–422. doi: 10.1093/toxsci/kfj051. [DOI] [PubMed] [Google Scholar]
- 156.Gilad O, Glorig A. Presbycusis: the aging ear. Ear Hear. 1979;4:195–207. [PubMed] [Google Scholar]
- 157.Working Group on Speech Understanding and Aging, Speech understanding and aging. J Acoust Soc Am. 1988;83:859–895. [PubMed] [Google Scholar]
- 158.Lui XZ, Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- 159.Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- 160.Schaette R, Kempter R. Development of hyperactivity after hearing loss in a computational model of the dorsal cochlear nucleus depends on neuron response type. Hear Res. 2008;240:57–72. doi: 10.1016/j.heares.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 161.Kaltenbach A. Intense sound-induced plasticity in the DCN of rats; evidence for cholinergic receptor upregulation. Hear Res. 2007;226:232–243. doi: 10.1016/j.heares.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 162.Willot JF, Lu S. Noise-induced hearing loss can alter neural coding and increase excitability in the central nervous system. Science. 1982;216:1331–1332. doi: 10.1126/science.7079767. [DOI] [PubMed] [Google Scholar]
- 163.Salvi R, Wang J, Powers N. Rapid functional reorganization in the inferior colliculus and cochlear nucleus after acute cochlear damage. In: Salvi R, Hendersen D, Fiorino F, Colletti V, editors. Auditory System Plasticity and Regeneration. 1996. pp. 275–296. [Google Scholar]
- 164.Prosen C, Moody D, Stebbins W, Smith D. Apical hair cells and hearing. Hear Res. 1990;44:179–194. doi: 10.1016/0378-5955(90)90079-5. [DOI] [PubMed] [Google Scholar]
- 165.Campo P, Venet T, Rumeau C, et al. Impact of noise or styrene exposure on the kinetics of presbycusis. Hear Res. 2011;280:122–132. doi: 10.1016/j.heares.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 166.NIOSH. Criteria for a recommended standard: occupational exposure to noise (revised criteria) Cincinnati, OH: 1998. p. 1998. (NIOSH Publication No. 98-126). [Google Scholar]
- 167.Wallhagen MI, Pettengill E. Hearing impairment: significant but underassessed in primary care settings. J Gerontol Nurs. 2008;34:36–42. doi: 10.3928/00989134-20080201-12. [DOI] [PubMed] [Google Scholar]
- 168.Ödkvist L, Arlinger S, Edling C, Larsby B, Bergholtz L. Audiological and vestibulo-oculomotor findings in workers exposed to solvents and jet fuel. Scand Audiol. 1987;16:75–81. doi: 10.3109/01050398709042159. [DOI] [PubMed] [Google Scholar]
- 169.Ödkvist L, Möller C, Thuomas K. Otoneurologic disturbances caused by solvent pollution. Otolaryngol Head Neck Surg. 1992;06:687–692. doi: 10.1177/019459989210600612. [DOI] [PubMed] [Google Scholar]
- 170.Pollastrini L, Abramo A, Cristalli G, Baretti F, Greco A. Early signs of occupational ototoxicity caused by inhalation of benzene derivative industrial solvents. Acta Otorhinolaryngol Ital. 1994;14:503–512. [PubMed] [Google Scholar]
- 171.Laukli E, Hansen PW. An audiometric test battery for the evaluation of occupational exposure to industrial solvents. Acta Otolaryngol. 1995;115:162–164. doi: 10.3109/00016489509139282. [DOI] [PubMed] [Google Scholar]
- 172.Niklasson M, Arlinger S, Ledin T, et al. Audiological disturbances caused by long-term exposure to industrial solvents. Relation to the diagnosis of toxic encephalopathy. Scand Audiol. 1998;27:131–136. doi: 10.1080/010503998422629. [DOI] [PubMed] [Google Scholar]
- 173.Varney NR, Morrow LA, Pinkston JB, Wu JC. PET scan findings in a patient with a remote history of exposure to organic solvents. Appl Neuropsychol. 1998;5:100–106. doi: 10.1207/s15324826an0502_6. [DOI] [PubMed] [Google Scholar]
- 174.Venet T, Campo P, Eluecque H, Rumeau C, Parietti-Winkler C. EchoScan: a system to objectively assess peripheral hearing disorders. Noise Health. 2012;14(60):253–259. doi: 10.4103/1463-1741.102964. [DOI] [PubMed] [Google Scholar]
- 175.Directive 2003/10/EC. The minimum health and safety requirements regarding the exposure of workers to the risks arising from noise. Off J Eur Com. 2003:L 042/38. [Google Scholar]
- 176.U.S. Army. Occupational ototoxins (ear poisons) and hearing loss. Aberdeen, MD: U.S. Army Center for Health Promotion and Preventive Medicine; 2003. (Fact sheet 51-002-0903). from http://www.nmcphc.med.navy.mil/downloads/occmed/toolbox/occupationalototoxinfactsheet-chppm.pdf. [Google Scholar]
- 177.American College of Occupational and Environmental Medicine (ACOEM) Occupational noise-induced hearing loss: ACOEM Task Force on Occupational Hearing Loss. J Occup Environ Med. 2012;54:106–108. doi: 10.1097/JOM.0b013e318242677d. [DOI] [PubMed] [Google Scholar]
- 178.American Conference of Governmental Industrial Hygienists [ACGIH] Threshold limit values and biological exposure indices. Cincinnati, OH: ACGIH; 2009. [Google Scholar]


