Abstract
Objective
The National Healthcare Safety Network classifies breast operations as clean procedures with an expected 1–2% surgical site infection (SSI) incidence. We assessed differences in SSI incidence following mastectomy with and without immediate reconstruction in a large, geographically diverse population.
Design
Retrospective cohort study.
Patients
Commercially-insured women aged 18–64 years with ICD-9-CM procedure or CPT-4 codes for mastectomy from 1/1/2004–12/31/2011.
Methods
Incident SSIs within 180 days after surgery were identified by ICD-9-CM diagnosis codes. The incidence of SSI after mastectomy +/− immediate reconstruction was compared by the chi-square test.
Results
From 2004–2011, 18,696 mastectomy procedures among 18,085 women were identified, with immediate reconstruction in 10,836 (58%) procedures. The 180-day incidence of SSI following mastectomy with or without reconstruction was 8.1% (1,520/18,696). Forty-nine percent of SSIs were identified within 30 days post-mastectomy, 24.5% between 31–60 days, 10.5% between 61–90 days, and 15.7% between 91–180 days. The incidence of SSI was 5.0% (395/7,860) after mastectomy-only, 10.3% (848/8,217) after mastectomy plus implant, 10.7% (207/1,942) after mastectomy plus flap, and 10.3% (70/677) after mastectomy plus flap and implant (p<0.001). The SSI risk was higher after bilateral compared with unilateral mastectomy with (11.4% vs. 9.4%, p=0.001) and without (6.1% vs. 4.7%, p=0.021) immediate reconstruction.
Conclusions
SSI incidence was two-fold higher after mastectomy with immediate reconstruction than after mastectomy alone. Only 49% of SSIs were coded within 30 days after operation. Our results suggest stratification by procedure type will facilitate comparison of SSI rates after breast operations between facilities.
Surgical site infection (SSI) is the most common healthcare-associated infection among hospitalized patients in the United States.1 SSI incidence reported by the National Healthcare Safety Network (NHSN) after breast operations from 2006–2008 was 2.3% for inpatient and 0.6% for outpatient breast operations.2 NHSN recommends surveillance for SSIs for 30 days after surgery or 90 days if an implant is involved, and combines all breast surgical procedures into a single category (e.g., breast-conserving surgery (BCS), reduction mammoplasty, mastectomy, implant and flap reconstruction).3
In contrast to the low incidence reported by NHSN, breast surgery SSI rates reported from individual institutions vary widely. The variation in reported rates depends on the type of breast operation, definitions used for infection, surveillance methods to identify infections, and length of postoperative follow-up.4 Using standardized surveillance for one year after surgery, we previously reported SSI rates for specific breast operations at a single institution ranging from 1.1% after reduction mammoplasty, 4.4% after mastectomy, 6.2% after mastectomy with immediate flap reconstruction, to 12.4–16.5% after mastectomy with immediate implant.5;6 The goal of our study was to determine the incidence of SSI in a large population of women with private health insurance following mastectomy with and without immediate implant or flap reconstruction performed at many different facilities.
MATERIALS AND METHODS
Data Source
We conducted a retrospective cohort study using data from 12 Anthem--affiliated plans in the HealthCore Integrated Research Database (HIRDSM).7 Data include all fully-adjudicated claims submitted from providers, facilities, and outpatient pharmacies linked to health plan enrollment information. Fully insured women with enrollment in a fee-for-service plan with medical coverage of hospital and physician services were eligible for inclusion in the cohort. We excluded women coded for end-stage renal disease, organ transplant, or HIV positive status due to unique risk factors for infection. Medical claims were restricted to paid claims. The database contained up to 5 International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes per claim from 2004–2008 and up to 12 diagnosis codes per claim from 2009–2011. Inpatient hospitals included up to 5 ICD-9-CM procedure codes per claim (8 in 2009–2011), while provider and ambulatory facility claims used CPT-4 (Current Procedural Terminology, 4th edition) codes.
Patient Population
We identified mastectomy operations with at least one day follow-up after operation among women aged 18–64 years from 1/1/2004–12/31/2011 using ICD-9-CM and/or CPT-4 procedure codes from inpatient and outpatient facility and provider claims (Appendix 1). Because of coding inaccuracy and the limited clinical detail in claims data, we implemented steps to increase the likelihood that the procedures we included were truly mastectomies, as described below. We allowed a maximum of two mastectomies per woman during the study period. We excluded claims that contained CPT-4, HCPCS, or UB-04 revenue codes truncated to 4 digits and populated in the fields reserved for ICD-9-CM procedure codes and claims in which a mastectomy procedure code was present only on one line on a single claim with no other claims on the same date, as described previously.8
In 1,300 (6.7%) operations, CPT-4 or ICD-9-CM procedures codes for BCS were present during the same hospital admission or within 3 days of mastectomy. Since concurrent BCS and mastectomy is unlikely and the incidence of SSI after BCS is lower than after mastectomy, we created an algorithm to determine the most likely procedure. We included any of the following information as evidence that mastectomy was performed: procedure code for reconstruction (Appendix 1), CPT-4 pathology code 88309 (modified radical mastectomy), prophylactic removal of the breast (V50.41), mastectomy coded by both facility and surgeon, BCS and mastectomy on opposite breasts per CPT-4 modifier codes, BCS coded only by an assistant surgeon, or diagnosis of acquired absence of the breast in the year following surgery (V45.71). We excluded procedures more consistent with BCS, including surgeon coding only for BCS (mastectomy-only coded by assistant surgeon or facility), and other diagnoses and procedures consistent with BCS but not mastectomy (Appendix 2).9
Establishing the Surgery Date
Mastectomy dates within 7 days were collapsed into a single surgery date because of potential date inaccuracy, particularly on provider claims.10 When there was more than one date within 7 days coded for mastectomy, we incorporated supplemental evidence from unique provider claims for reconstruction, anesthesia, and pathology to determine the most likely surgery date. We excluded facility- and provider-only mastectomy claims that lacked additional evidence for operation, including anesthesia, pathology, or a surgery revenue code (Appendix 1).9
Classification of Procedures
We classified the mastectomy as unilateral or bilateral based on ICD-9-CM procedure and CPT-4 codes, billed units, and CPT-4 modifier codes (Appendix 1). If there was discrepancy between the provider and facility, we considered the procedure to be bilateral unless there was only a single billed unit for pathology. We defined immediate reconstruction based on procedure codes for tissue expander/breast implant and/or flap reconstruction within 7 days of mastectomy (Appendix 1). We prioritized the provider classification of the flap in the case of discrepant facility and provider information. We did not use facility CPT-4 codes to classify flap reconstruction since flap procedures are not performed in ambulatory surgery. Similarly, we did not classify flap reconstruction if it was only coded by a facility with length of stay <2 days using ambiguous procedure codes (ICD-9-CM 85.7, 85.70, or 85.79), due to likely misclassification (e.g., local tissue rearrangement for wound closure coded as a flap).
Indication for Mastectomy
We used ICD-9-CM diagnosis codes to identify carcinoma in situ (CIS), locally invasive, regionally invasive, and metastatic breast cancer, as described previously.9 We classified the indication for mastectomy at the time of the first operation ranked hierarchically: metastatic, regional, local breast cancer, CIS, and benign/prophylactic mastectomy. Mastectomy was considered prophylactic if an ICD-9-CM diagnosis code for prophylactic breast removal, family history (V16.3), or genetic susceptibility to breast neoplasm (V84.01) was coded within 7 days of operation. Because the majority of second or contralateral mastectomies are prophylactic,11;12 we ranked prophylactic codes highest for subsequent operations. If no prophylactic diagnoses were coded within 7 days of the second operation, we used the cancer stage hierarchy above.
Identification and Timing of Surgical Site Infection
SSIs first coded from 2 to 180 days after surgery were identified using ICD-9-CM diagnosis codes from inpatient and outpatient facilities and provider claims (Appendix 3). Coding of Staphylococcus aureus within 7 days of one or more of the following was considered consistent with an SSI: procedure code for incision/drainage, diagnosis of a non-infectious wound complication, or cellulitis. In accordance with the NHSN definition,3 a diagnosis code for cellulitis on the same claim as a procedure code for incision/drainage or on the day of implant removal without insertion was classified as SSI. We previously validated these diagnosis codes in breast surgery patients within 180 days of surgery using microbiology and clinical data based on the NHSN definition for SSI.13
We excluded claims with laboratory CPT-4 codes (88104–88399) since these diagnosis codes may have indicated diagnostic workup. Because ICD-9-CM diagnosis code 611.0 could indicate either breast infection or inflammatory breast cancer, we did not use it as evidence for SSI if it was also coded in the month before mastectomy. Since our goal was to identify infections attributable to surgery, we excluded cellulitis codes after the start of radiotherapy.
SSI onset was defined according to the timing and location of diagnosis. For SSI newly coded by an inpatient facility during the original operative admission, we assigned the date of SSI to the discharge date if the difference between the discharge and admission date was ≥2 days. For SSI diagnosed during a subsequent inpatient admission, SSI onset was assumed to be the hospital readmission date. For SSI diagnosed initially by a provider or in an outpatient setting, the onset date was defined as the first service date.
The observation period for SSI was through 180 days after surgery, with earlier censoring for the end of insurance enrollment, subsequent mastectomy, implant, flap, or nipple reconstruction. We censored one day after the subsequent surgery since an SSI coded within one day after surgery was considered preexisting and attributable to the previous surgery. Non-breast specific SSI codes (e.g., 998.59) were not classified as SSI if they were first coded after a subsequent non-breast NHSN operation.
ICD-9-CM diagnosis codes for SSI or cellulitis coded from 30 days before to 1 day after mastectomy were considered preexisting infection. For operations with preexisting infection, we required a minimum 30-day gap after mastectomy with no coding of SSI or cellulitis to identify an incident SSI.
Indicators Consistent with Infection
Incision/drainage, implant removal or exchange, and outpatient antibiotic prescription claims after mastectomy and within 14 days of a claim coded for SSI (before censoring) were identified (Appendix 3). Antibiotic prescriptions within two days of mastectomy or mastectomy hospital discharge were considered prophylactic and excluded.
Statistical Analysis
The incidence of SSI within 180 days after mastectomy with and without immediate reconstruction was compared using a chi-square test. A Kruskal-Wallis test was used for continuous variables. All data management and statistical analyses were performed using SAS v9.3 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 19,422 mastectomy operations were initially identified from 2004–2011. The number of procedures was reduced to 18,696 among 18,085 women after excluding procedures with no supporting evidence for operation (n=208), subsequent mastectomy operations following a bilateral mastectomy (n=8), and dually coded operations that were more likely BCS (n=510). Immediate implant or flap reconstruction was performed in 58% of operations (Tables 1 and 2). Women with reconstruction were younger, more likely to have bilateral mastectomy, and more likely to have the mastectomy performed during an inpatient hospitalization. The majority of women had local breast cancer, but a larger proportion of the mastectomy-only population had regional or metastatic breast cancer (27.3% vs. 17.0%, p<0.001). Women with prophylactic mastectomy were more likely to have immediate reconstruction than women with other indications for mastectomy (78.8%, (565/717) vs. 57.1% (10,271/17,979), p<0.001).
TABLE 1.
Characteristics of Mastectomy Operations in 18,085 Patients
| Characteristic | Mastectomy-only n (%) | Mastectomy With Immediate Reconstruction n (%) | Pa |
|---|---|---|---|
| Total procedures | 7,860 | 10,836 | |
| Age, median (range) | 54 (18–64) | 49 (18–64) | <0.001 |
| Bilateral mastectomy | 1,739 (22.1) | 5,529 (51.0) | <0.001 |
| Indication for mastectomy | <0.001 | ||
| Metastatic cancer | 300 (3.8) | 152 (1.4) | |
| Regional cancer | 1,849 (23.5) | 1,686 (15.6) | |
| Local breast cancer | 4,873 (62.0) | 6,681 (61.7) | |
| Ductal carcinomain situ | 617 (7.9) | 1,703 (15.7) | |
| Prophylactic | 152 (1.9) | 565 (5.2) | |
| Benign/other | 69 (0.9) | 49 (0.5) | |
| Inpatient operation | 5,523 (70.3) | 9,625 (88.8) | <0.001 |
P per chi-square test for categorical variables and Kruskal-Wallis test for continuous variables.
TABLE 2.
Incidence of Surgical Site Infection (SSI) For Mastectomy With and Without Immediate Reconstruction and Unilateral versus Bilateral Operations
| Operative Category | Total n (%)a | SSI within Surgery Category n (%) | Relative Risk (95% Confidence Interval) | Pb |
|---|---|---|---|---|
| Mastectomy-only | 7,860 (42.0%) | 395 (5.0%) | 1.00 | <0.001 |
| Mastectomy plus implant | 8,217 (44.0%) | 848 (10.3%) | 2.05 (1.83, 2.30) | |
| Mastectomy plus flap | 1,942 (10.4%) | 207 (10.7%) | 2.12 (1.81, 2.49) | |
| Mastectomy plus flap and implant | 677 (3.6%) | 70 (10.3%) | 2.06 (1.62, 2.62) | |
|
| ||||
| Unilateral versus bilateral procedures | ||||
|
| ||||
| Unilateral mastectomy- only | 6,121 (32.7%) | 289 (4.7%) | 1.00 | 0.021 |
| Bilateral mastectomy- only | 1,739 (9.3%) | 106 (6.1%) | 1.29 (1.04, 1.60) | |
| Unilateral mastectomy plus reconstruction | 5,307 (28.4%) | 497 (9.4%) | 1.00 | 0.001 |
| Bilateral mastectomy plus reconstruction | 5,529 (29.6%) | 628 (11.4%) | 1.21 (1.08, 1.36) | |
Number and percentage of procedures compared to the total number of mastectomy procedures performed (N=18,696).
P per chi-square test.
The 180-day incidence of SSI following mastectomy with and without reconstruction was 8.1% (1,520/18,696). The incidence of SSI was 5.0% after mastectomy-only, 10.3% after mastectomy plus implant, 10.7% after mastectomy plus flap, and 10.3% after mastectomy plus flap and implant (p<0.001, Table 2). Among mastectomies with and without reconstruction, the SSI risk was significantly higher after bilateral compared with unilateral procedures (p=0.001 and p=0.021 respectively, Table 2). The incidence of SSI was similar after prophylactic mastectomy with reconstruction compared to mastectomy with reconstruction for other indications (10.1%, (57/565) vs. 10.4% (1,068/10,271), p=0.814). The incidence of SSI was higher for inpatient versus outpatient procedures for mastectomy-only (5.4% [301/5,523] versus 4.0% [94/2,337], p=0.008), but was similar for inpatient and outpatient procedures with immediate reconstruction (10.4% [1,004/9,625] versus 10.0% [121/1,211], p=0.637).
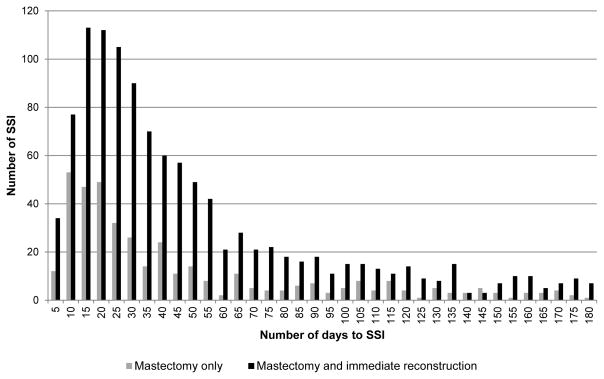
The time to presentation with SSI varied depending on whether mastectomy-only or mastectomy plus immediate reconstruction was performed (median time to onset 26 vs. 33 days; p=0.006). Among mastectomy-only, SSIs were first coded within 30 days post-mastectomy in 55.4% (219/395) of procedures. Eighteen percent of SSIs had onset between 31–60 days, 9.4% between 61–90 days, and 16.7% of SSIs were first coded between 91–180 days following mastectomy-only (Figure 1). Among mastectomy with reconstruction, SSIs had onset within 30 days in 47.2% (531/1,125) of procedures. Twenty-seven percent of SSIs had onset between 31–60 days, 10.9% between 61–90 days, and 15.3% of SSIs were first coded between 91–180 days following mastectomy with reconstruction. The percentages of SSIs within 30 days post-mastectomy with implant and with flap reconstruction were the same (47.3% and 46.4%, respectively).
FIGURE 1.
Days to surgical site infection (SSI) following mastectomy with and without immediate reconstruction (n=1,520 SSI).
Within 60 days post-mastectomy, the majority of the infections characterized as SSI (930/1,116, 83.3%) were coded with a standard SSI code(s) (e.g., 683, 998.5x, 996.69). The remaining SSIs with onset ≤60 days were coded for cellulitis, Staphylococcal infection, or breast abscess plus incision and drainage or implant removal/exchange (n=85, 7.6%) or breast abscess alone (n=101, 9.1%). The percentage of SSIs with onset > 60 days coded with standard SSI codes was lower (254/404, 62.9%), and the percentages coded for cellulitis/staphylococcal infection with a wound care procedure (n=63, 15.6%) and breast abscess alone (n=87, 21.5%) were higher than SSIs with onset earlier after surgery (p<0.001).
Among women with prescription drug coverage (87% of women with SSI), SSI coding was present on a single claim for 36% (475/1,317). Of SSI coded on only one claim, 86% (410/475) had additional evidence supporting the SSI diagnosis, including 328 (69%) with an outpatient antibiotic prescription claim and 258 (54%) with an incision/drainage or implant removal/exchange within 14 days of the SSI claim.
DISCUSSION
SSI incidence in this cohort of younger women was two-fold higher after mastectomy with reconstruction compared with mastectomy-only and higher after bilateral compared with unilateral procedures. The SSI incidence of 5.0% after mastectomy-only is consistent with infection rates reported in the last decade from individual U.S. institutions (Table 3).5;14;15 For mastectomy with implant reconstruction, the SSI incidence was 10.3% compared to rates per person ranging from 1.5%–12.7% reported in the surgical literature since 2006 from individual U.S. institutions (Table 3).5;16–24 The SSI incidence in our cohort for mastectomy with flap reconstruction was 10.7%. It is more difficult to compare the SSI rate after immediate flap reconstruction to rates reported from individual institutions since the majority of published studies do not separate infection rates after immediate versus delayed flap reconstruction. SSI rates per person reported after primarily immediate TRAM flap reconstruction in the last decade range from 0.8–6.9%,5;22;25–28 although three of these studies did not specifically describe the observation period for infection,22;25;27 and only our prior study used the NHSN definition for SSI (Table 3).5
TABLE 3.
Methods and Surgical Site Infection (SSI) Incidence Rates by Mastectomy and Type of Reconstruction
| Reference | Time frame for surveillance | Definition of SSI | N patients | SSI n (%) |
|---|---|---|---|---|
| Mastectomy-only | ||||
| Olsen 2008 | 12 months | NHSN | 296 | 13 (4.4) |
| Mortenson 2004 | ND, mean 36 months follow up | ND | 66 | 3 (4.5) |
| Edwards 2014 | Until postoperative evaluation | NHSN, or clinical diagnosis of cellulitis | 425 | 31 (7.3) |
| Mastectomy plus implanta | ||||
| Olsen 2008 | 12 months | NHSN | 121 | 15 (12.4) |
| Cordeiro 2006 | 12 months | ND | 1,176 | 37 (3.1) |
| Sbitany 2009 | ND | Infection requiring implant removal | 100 | 7 (7.0) |
| Reish 2013 | At least 1 year | Erythema suspicious for infection, treated with intravenous antibiotics or implant removal | 1,241 | 94 (7.6) |
| Weichman 2013 | ND | Infection requiring oral antibiotics (minor) or hospital readmission and intravenous antibiotics (major) | 345 | 47 (8.6)b |
| McCullough 2014 | ND, median time to infection 29 days | NHSN | 378 | 48 (12.7) |
| Rundell 2014 | ND, mean follow up 12 months | Infection requiring oral antibiotics (minor) or hospitalization, intravenous antibiotics, or debridement (major) | 203 | 23 (11.3) |
| Crosby 2011 | ND, mean follow up 13 months | Erythema plus intravenous antibiotics | 334c | 20 (6.0) cancer breast 18 (5.4) prophylactic breast |
| Halvorson 2007 | At least 1 year | Infection requiring implant removal | 2,539 | 39 (1.5) |
| Mitchell 2013 | At least 1 year | ND | 103 | 9 (8.7) |
| Mastectomy plus TRAM flapd | ||||
| Olsen 2008 | 12 months | NHSN | 162 | 10 (6.2)e |
| Crosby 2011 | ND, mean follow up 13 months | Erythema plus intravenous antibiotics | 142c | 2 (1.4) cancer breast 3 ( 2.1) prophylactic breast |
| Bristol 2006 | ND | Cellulitis or purulent discharge plus antibiotics | 247 | 17 (6.9) |
| Meretoja 2007 | 5 years | ND | 151 | 5 (3.3) |
| Chun 2010 | At least 11 months, mean follow up 6 years | ND | 105 | 4 (3.8) breast 2 (1.9) donor site |
| Kim 2009 | At least 1 year, mean follow up 41 months | ND | 500 | 4 (0.8) breast 5 (1.0) donor site |
NOTE. ND, not described; NHSN, National Healthcare Safety Network; TRAM, Transverse Rectus Abdominis Myocutaneous.
Sbitany (2009), unclear if all implants were immediate reconstruction.
SSI and incidence is per breast (n=546) rather than per person (n=345).
All procedures were bilateral; SSI and incidence are per side (i.e., cancer side and prophylactic side).
Bristol (2006) and Chun (2010), not all TRAMs were immediate reconstruction.
10 infections at the person level; 6 breast SSI, 7 donor site SSI, 3 women had dual infections.
The NHSN breast surgery SSI rate is much lower than reported in our studies since the NHSN breast category includes simpler procedures with lower SSI rates, such as BCS,29 that can make up a large proportion of breast operations from some hospitals. The higher risk of SSI after mastectomy compared with other breast procedures may be due in part to the larger incision, longer operative time, and potential for large dead space after complete removal of the breast with accumulation of lymphatic or serous fluid. The addition of reconstructive surgery, with or without a foreign body and additional surgical site(s), increases the length of procedure and further increases the risk of SSI.
In part, the variation in SSI rates reported after mastectomy with immediate reconstruction in the surgical literature may be due to variation in the definition used for infection (Table 3). In one study of SSI associated with implant reconstruction, implant removal was required to define infection,23 while in others intravenous antibiotic therapy22 and/or surgical treatment was required.18 In many reports, the criteria used to define SSI were not stated.16;17;24;26–28 Most studies in the surgical literature do not report infection rates per person but rather per breast, making comparisons difficult.
Recently, a number of investigators have used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database to study 30-day complication rates after breast operations. Nguyen reported an incidence of SSI of 2.5% after mastectomy-only using 2005–2009 data,30 although partial and subcutaneous mastectomies were included which have lower risk of SSI.5 Mioton reported an SSI rate of 3.4% after mastectomy with immediate implant reconstruction from 2006–2010,31 and Costa found that the SSI rate after immediate flap reconstruction was 4.9% using 2005–2009 NSQIP data.32
In our present study, the 180-day incidence of SSI was 10.4% after mastectomy with reconstruction compared with 5.0% after mastectomy-only, similar in magnitude to the difference in SSI rates found previously in a single-institution study.5 If we restrict the observation time to 30 days after surgery, the SSI incidence after mastectomy-only in our current study was 2.8%, very similar to the 2.5% rate reported by Nguyen using NSQIP data. In contrast, the 30-day incidence of SSI we calculated after mastectomy with implant reconstruction is 4.9%, higher than the 3.5% rate reported by Nguyen. In addition, we found that only 47% of SSIs were identified within 30 days after mastectomy with reconstruction; in a previous institutional study, we found that 52% of SSIs had onset within 30 days after mastectomy with implant reconstruction.6 This highlights the importance of continuing surveillance beyond 30 days postoperatively to capture SSIs, particularly for operations involving a foreign body, as recommended by NHSN.3
We found significantly higher incidence of SSI in women with bilateral compared to unilateral mastectomy, both with and without reconstruction. Osman reported higher risk of SSIs after bilateral compared to unilateral mastectomy-only in women with breast cancer using NSQIP data.33 In another study using NSQIP data, Fischer and colleagues found that bilateral surgery was an independent risk factor for surgical complications.34 This is important information to present to women given increasing use of contralateral prophylactic mastectomy in women with unilateral breast cancer and bilateral prophylactic mastectomy in high-risk women.35;36
By definition, using claims data for SSI surveillance involves secondary analysis of data collected for administrative purposes. There is potential for misclassification of diagnoses and likely undercoding of SSIs, particularly minor infections during the 90-day global surgical reimbursement period.37 Thus our calculations for the incidence of SSI after mastectomy are likely underestimates of the true infection rates after these procedures. It is also possible that some SSIs classified as attributable to mastectomy were due to another procedure, particularly infections coded >30 days after mastectomy in the absence of an implant. We minimized this by censoring at the time of subsequent breast and NHSN procedures, but it is possible that an SSI could be attributable to a non-NHSN procedure. Finally, we could not capture the onset of signs/symptoms of SSI in claims data, and were limited to defining the onset of infection based on the first paid claim coded for SSI, resulting in overestimation of the time to onset of infection in some cases.
The SSI incidence after mastectomy in this large, geographically diverse cohort of younger women was 8.1%, much higher than the SSI incidence for breast surgical procedures reported by NHSN. Only half of all SSIs were coded on medical claims within 30 days of surgery. Additional studies with verification of infection meeting NHSN definitions are needed to determine if longer-term SSI surveillance after mastectomy is warranted. Our finding of variation in SSI incidence for mastectomy-only compared to mastectomy with immediate reconstruction suggests that stratification of SSI rates by type of procedure is important.
Supplementary Material
Acknowledgments
Financial support
This work was supported by the National Institutes of Health (NIH) [5R01CA149614 to M.A.O.]. Additional support was provided by the Centers for Disease Control and Prevention (CDC) Epicenters Program (U54CK000162) (VJF, MAO). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the official view of NIH or CDC.
We thank Cherie Hill for database and computer management support.
Footnotes
Potential conflicts of interest
MAO reports consultant work with Merck, Pfizer, and Sanofi Pasteur and grant funding through Pfizer, Cubist Pharmaceuticals, and Sanofi Pasteur for work outside the submitted manuscript. All other authors report no conflicts of interest relevant to this article.
Reference List
- 1.Anderson DJ, Pyatt DG, Weber DJ, Rutala WA. Statewide costs of health care-associated infections: estimates for acute care hospitals in North Carolina. Am J Infect Control. 2013;41:764–768. doi: 10.1016/j.ajic.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) Procedure-Associated (PA) Module: Surgical Site Infection (SSI) event. Centers for Disease Control and Prevention; 2013. [Accessed November 14, 2013]. http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf. [Google Scholar]
- 4.Hall JC, Hall JL. The measurement of wound infection after breast surgery. Breast J. 2004;10:412–415. doi: 10.1111/j.1075-122X.2004.21401.x. [DOI] [PubMed] [Google Scholar]
- 5.Olsen MA, Chu-Ongsakul S, Brandt KE, Dietz JR, Mayfield J, Fraser VJ. Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg. 2008;143:53–60. doi: 10.1001/archsurg.2007.11. [DOI] [PubMed] [Google Scholar]
- 6.Lankiewicz JD, Yokoe DS, Olsen MA, et al. Beyond 30 days: does limiting the duration of surgical site infection follow-up limit detection? Infect Control Hosp Epidemiol. 2012;33:202–204. doi: 10.1086/663715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren DK, Nickel KB, Wallace AE, Mines D, Fraser VJ, Olsen MA. Can additional information be obtained from claims data to support surgical site infection diagnosis codes? Infect Control Hosp Epidemiol. 2014;35(Suppl 3):S124–S132. doi: 10.1086/677830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickel KB, Wallace AE, Warren DK, Mines D, Olsen MA. Using Claims Data to Perform Surveillance for Surgical Site Infection: The Devil is in the Details. In: Battles JB, Cleeman JI, Kahn KK, Weinberg DA, editors. Advances in the Prevention and Control of HAIs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. Publication No. 14-0003. [Google Scholar]
- 9.Olsen MA, Nickel KB, Margenthaler JA, et al. Increased Risk of Surgical Site Infection Among Breast-Conserving Surgery Re-excisions. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, King C, deGara C, White J, Winget M. Validation of colorectal cancer surgery data from administrative data sources. BMC Med Res Methodol. 2012;12:97. doi: 10.1186/1471-2288-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56:1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 12.Nichols HB, Berrington de GA, Lacey JV, Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29:1564–1569. doi: 10.1200/JCO.2010.32.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen MA, Fraser VJ. Use of diagnosis codes and/or wound culture results for surveillance of surgical site infection after mastectomy and breast reconstruction. Infect Control Hosp Epidemiol. 2010;31:544–547. doi: 10.1086/652155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortenson MM, Schneider PD, Khatri VP, et al. Immediate breast reconstruction after mastectomy increases wound complications: however, initiation of adjuvant chemotherapy is not delayed. Arch Surg. 2004;139:988–991. doi: 10.1001/archsurg.139.9.988. [DOI] [PubMed] [Google Scholar]
- 15.Edwards BL, Stukenborg GJ, Brenin DR, Schroen AT. Use of prophylactic postoperative antibiotics during surgical drain presence following mastectomy. Ann Surg Oncol. 2014;21:3249–3255. doi: 10.1245/s10434-014-3960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications Plast Reconstr Surg. 2006;118:825–831. doi: 10.1097/01.prs.0000232362.82402.e8. [DOI] [PubMed] [Google Scholar]
- 17.Sbitany H, Sandeen SN, Amalfi AN, Davenport MS, Langstein HN. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735–1740. doi: 10.1097/PRS.0b013e3181bf803d. [DOI] [PubMed] [Google Scholar]
- 18.Reish RG, Damjanovic B, Austen WG, Jr, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. 2013;131:1223–1230. doi: 10.1097/PRS.0b013e31828bd377. [DOI] [PubMed] [Google Scholar]
- 19.Weichman KE, Wilson SC, Saadeh PB, et al. Sterile "ready-to-use" AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;132:725–736. doi: 10.1097/PRS.0b013e31829fe35b. [DOI] [PubMed] [Google Scholar]
- 20.McCullough MC, Chu CK, Duggal CS, Losken A, Carlson GW. Antibiotic Prophylaxis and Resistance in Surgical Site Infection After Immediate Tissue Expander Reconstruction of the Breast. Ann Plast Surg. 2014 doi: 10.1097/SAP.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 21.Rundell VL, Beck RT, Wang CE, et al. Complication prevalence following use of tutoplast-derived human acellular dermal matrix in prosthetic breast reconstruction: A retrospective review of 203 patients. J Plast Reconstr Aesthet Surg. 2014;67:1345–1351. doi: 10.1016/j.bjps.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Crosby MA, Garvey PB, Selber JC, et al. Reconstructive outcomes in patients undergoing contralateral prophylactic mastectomy. Plast Reconstr Surg. 2011;128:1025–1033. doi: 10.1097/PRS.0b013e31822b6682. [DOI] [PubMed] [Google Scholar]
- 23.Halvorson EG, Disa JJ, Mehrara BJ, Burkey BA, Pusic AL, Cordeiro PG. Outcome following removal of infected tissue expanders in breast reconstruction: a 10-year experience. Ann Plast Surg. 2007;59:131–136. doi: 10.1097/01.sap.0000252716.73356.68. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell RE. Porcine acellular dermis-assisted breast reconstruction: influence of adjuvant radiotherapy on complications and outcomes. Plast Reconstr Surg Glob Open. 2013;1:e77. doi: 10.1097/GOX.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bristol SG, Lennox PA, Clugston PA. A comparison of ipsilateral pedicled TRAM flap with and without previous irradiation. Ann Plast Surg. 2006;56:589–592. doi: 10.1097/01.sap.0000205057.23543.48. [DOI] [PubMed] [Google Scholar]
- 26.Meretoja TJ, Rasia S, von Smitten KA, Asko-Seljavaara SL, Kuokkanen HO, Jahkola TA. Late results of skin-sparing mastectomy followed by immediate breast reconstruction. Br J Surg. 2007;94:1220–1225. doi: 10.1002/bjs.5815. [DOI] [PubMed] [Google Scholar]
- 27.Chun YS, Sinha I, Turko A, Lipsitz S, Pribaz JJ. Outcomes and patient satisfaction following breast reconstruction with bilateral pedicled TRAM flaps in 105 consecutive patients. Plast Reconstr Surg. 2010;125:1–9. doi: 10.1097/PRS.0b013e3181c2a620. [DOI] [PubMed] [Google Scholar]
- 28.Kim EK, Eom JS, Ahn SH, Son BH, Lee TJ. Evolution of the pedicled TRAM flap: a prospective study of 500 consecutive cases by a single surgeon in Asian patients. Ann Plast Surg. 2009;63:378–382. doi: 10.1097/SAP.0b013e3181951708. [DOI] [PubMed] [Google Scholar]
- 29.de Blacam C, Ogunleye AA, Momoh AO, et al. High body mass index and smoking predict morbidity in breast cancer surgery: a multivariate analysis of 26,988 patients from the national surgical quality improvement program database. Ann Surg. 2012;255:551–555. doi: 10.1097/SLA.0b013e318246c294. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TJ, Costa MA, Vidar EN, et al. Effect of immediate reconstruction on postmastectomy surgical site infection. Ann Surg. 2012;256:326–333. doi: 10.1097/SLA.0b013e3182602bb7. [DOI] [PubMed] [Google Scholar]
- 31.Mioton LM, Smetona JT, Hanwright PJ, et al. Comparing thirty-day outcomes in prosthetic and autologous breast reconstruction: a multivariate analysis of 13,082 patients? J Plast Reconstr Aesthet Surg. 2013;66:917–925. doi: 10.1016/j.bjps.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Costa MA, Rommer E, Peric M, et al. Incidence of surgical-site infection is not affected by method of immediate breast reconstruction. Plast Reconstr Surg. 2013;132:20e–29e. doi: 10.1097/PRS.0b013e318290f87e. [DOI] [PubMed] [Google Scholar]
- 33.Osman F, Saleh F, Jackson TD, Corrigan MA, Cil T. Increased Postoperative Complications in Bilateral Mastectomy Patients Compared to Unilateral Mastectomy: An Analysis of the NSQIP Database. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-3116-1. [DOI] [PubMed] [Google Scholar]
- 34.Fischer JP, Tuggle CT, Au A, Kovach SJ. A 30-day risk assessment of mastectomy alone compared to immediate breast reconstruction (IBR) J Plast Surg Hand Surg. 2014;48:209–215. doi: 10.3109/2000656X.2013.865633. [DOI] [PubMed] [Google Scholar]
- 35.Cemal Y, Albornoz CR, Disa JJ, et al. A paradigm shift in U.S. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg. 2013;131:320e–326e. doi: 10.1097/PRS.0b013e31827cf576. [DOI] [PubMed] [Google Scholar]
- 36.Pesce CE, Liederbach E, Czechura T, Winchester DJ, Yao K. Changing surgical trends in young patients with early stage breast cancer, 2003 to 2010: a report from the National Cancer Data Base. J Am Coll Surg. 2014;219:19–28. doi: 10.1016/j.jamcollsurg.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Medicare & Medicaid Services. Global Surgery Fact Sheet. Centers for Medicare & Medicaid Services; 2013. [Accessed November 14, 2013]. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/GloballSurgery-ICN907166.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.