Summary
Despite three decades of intensive research efforts, the development of an effective prophylactic vaccine against HIV remains an unrealized goal in the global campaign to contain the HIV/AIDS pandemic. Recent characterization of novel epitopes for inducing broadly neutralizing antibodies (BnAbs) has fueled research in the design and synthesis of new, well-defined antigenic constructs for the development of HIV envelope-directed vaccines. The present review will cover previous and recent efforts toward the design of synthetic vaccines based on the HIV viral envelope (Env) glycoproteins, with special emphasis on examples from our own laboratories. The biological evaluation of some of the most representative vaccine candidates, in terms of their antigenicity and immunogenicity, will also be discussed to illustrate the current state-of-the-art toward the development of fully synthetic HIV vaccines.
Keywords: AIDS, HIV vaccine design, broadly neutralizing antibody (BnAb), gp120, synthetic antigen, carbohydrates, glycopeptides, glycosylation
Introduction
More than 60 million people worldwide have been infected by human immunodeficiency virus (HIV) since its discovery approximately 30 years ago as the cause of AIDS; over 25 million have died from the disease [1]. In 2012, an estimated 35.3 million people globally were living with HIV, including 2.3 million newly infected individuals, and the number of AIDS deaths in that year totaled 1.6 million [2]. Therefore, the development of a safe and effective prophylactic vaccine, ideally with elicitation of both T-cell mediated immunity and a broadly neutralizing antibody (BnAb) response, is of paramount importance. While HIV-specific cytotoxic T-lymphocytes (CTL) can recognize and kill infected cells, classic CD8+ CTL with recognition of HIV antigens in the context of MHC Class I molecules are not sufficient on their own to prevent HIV infection and vaccines designed to elicit CD8+ T cell-mediated immune responses have provided no protection in efficacy trials [3]. However, atypical CD8 CTLs induced by HIV antigens in a cytomegalovirus vector recognize simian immunodeficiency virus (SIV) antigens in the context of MHC class II, and have eliminated infection in the setting of acute SIV infection in rhesus macaques [4].
In contrast, passive immunization experiments in animal models have demonstrated that BnAbs can provide protection against viral challenge when present in sufficient plasma levels [5,6]. However, elicitation of such protective BnAbs by active immunization has, so far, not been possible with any vaccine candidate in clinical trials. For example, the candidate vaccine AIDSVAX, a genetically engineered version of HIV’s surface protein gp120, raised only weak neutralizing antibodies and showed no protection in humans in a phase III clinical trial [7]. The RV144 HIV trial, involving more than 16,000 healthy individuals in Thailand, used a gp120-based ALVAC prime, AIDSVAX B/E boost HIV vaccine regime, and resulted in an estimated 31% protective efficacy in HIV transmission [8,9]. However, antibodies capable of neutralizing transmitted/founder viruses were not produced [10], and the protection induced was neither sufficiently robust for deployment, nor of sufficient durability for sustained vaccine efficacy. Thus, the development of a successful HIV vaccine has, thus far, remained elusive. In the ideal case, vaccines capable of inducing the production of BnAbs as well as cellular immune responses in a synergistic manner represent the optimal approach. On this basis, new HIV vaccine design strategies should aim at incorporating both virus neutralizing and T-cell determinants, to create synthetic polyepitope immunogens that include B- and T-cell epitopes for the stimulation of BnAbs along with cytotoxic and T-helper cell responses [11].
The main scientific challenges for the successful development of an HIV-1 vaccine have been attributed, in part, to the wide variety of defense mechanisms by which the virus is able to evade the host immune system, including a high mutational rate of its genome, the large degree of glycosylation of the viral surface proteins and the considerable genetic diversity amongst HIV strains globally [12]. In addition, all BnAbs have unusual traits of antibodies that are limited by immune tolerance mechanisms [13], and some BnAbs have been shown to be deleted in bone marrow due to autoreactivity with host antigens [14]. Vaccine formulations utilized to date have been unable to induce potent and sustained immune responses with effective levels of neutralization to prevent the onset of HIV infection. Thus, eliciting antibodies capable of broadly neutralizing HIV-1 strains (BnAbs) remains a high priority in designing an HIV vaccine [15], especially after the recent failure of the DNA prime, recombinant (r) adenovirus type 5 (Ad5) HIV vaccine in human efficacy trials [16].
BnAbs have been isolated from HIV-1 chronically-infected subjects [17] and are directed to five general HIV-1 envelope (Env) glycoprotein targets [18,19]: the gp41 membrane proximal external region (MPER), the CD4 binding site (CD4bs) on gp120, the gp120 variable loop 1/2 (V1V2), the gp120 variable loop 3 (V3), and a conformational combined gp41-gp120 set of epitopes [20,21,22,23]. The HIV-1 envelope spike, critical for viral infectivity, consists of a trimer of the glycoproteins gp41 and gp120 [24]. It undergoes rapid evolution in each individual patient, resulting in sequence heterogeneity among individual isolates of HIV-1 [25,26]. Moreover, the extensive glycosylation of these envelope glycoproteins can mask underlying protein domains, forming a “glycan shield” that renders neutralization-sensitive polypeptide epitopes inaccessible to recognition by the immune system [27]. As a result, the surface glycans of HIV envelope proteins have become interesting targets for the development of synthetic HIV vaccines based on carbohydrates and glycopeptides. This idea is supported by several facts: (1) some of the HIV-1 glycans are highly conserved, (2) their location on the outer side of the gp120 envelope renders them accessible to the immune system, and (3) glycan-dependent BnAbs have been identified from HIV-1 infected patients [28,29,30].
This review highlights past and present design and synthesis strategies toward the gp120 carbohydrate shield of the HIV-1 virus, undertaken with the goal of gaining access to chemically defined constructs for antigenicity and immunogenicity studies. Importantly, while a synthetic epitope mimic may show high binding affinity for the antibody (antigenicity), this does not necessarily imply that the corresponding immunogen will generate the desired antibody response in vivo (immunogenicity). We report herein previous attempts and recent progress on the design and synthesis of BnAb-inducing, glycan-dependent epitopes toward the development of an effective HIV vaccine. These epitope definitions have been informed by the knowledge provided by the consecutive discovery of potent BnAbs from immune-resistant HIV-infected individuals, and subsequent identification of the structures to which these antibodies bind.
Principles and roles of HIV-1 glycan architecture. Implication of glycan-dependent epitopes for broadly neutralizing antibodies
HIV-1 consists of two envelope glycoproteins, the exterior gp120 and the inner, transmembrane gp41, both of them heavily N-glycosylated. The surface glycoprotein gp120 is characterized by a highly heterogeneous glycosylation pattern, which is dominated by oligomannose sugars [31]. Several groups have studied the composition of the glycan shield of recombinant and virus-derived gp120, and clear differences in the glycosylation profile have been observed [32,33,34,35,31]. Analysis of recombinantly-generated HIV-1SF2 gp120 expressed in mammalian Chinese hamster ovary (CHO) cells showed the presence of both high-mannose and complex-type carbohydrates, which are proposed to exist in distinct clusters on the protein surface [32]. Desaire and co-workers compared recombinant transmitted/founder and chronic HIV envelope glycosylation (gp120/gp41) expressed in human embryonic kidney containing T antigen (293T) cell lines [33], and have recently characterized the glycosylation pattern of recombinant gp120 derived from a transmitted/founder virus expressed in CHO and 293T cells [34]. The results revealed distinct glycosylation profiles between recombinant founder and chronic Envs as well as important diversity of high-mannose, hybrid and complex-type glycans for founder Envs, which had more high-mannose content than chronic Envs [33]. In addition, CHO and 293T cell-derived founder recombinant gp120s were found to display very similar glycosylation pattern with a few subtle distinctions [34]. Distinguishing the host-cell specific variations in glycosylation is crucial in the design and characterization of the heterogeneity of envelope-based immunogens.
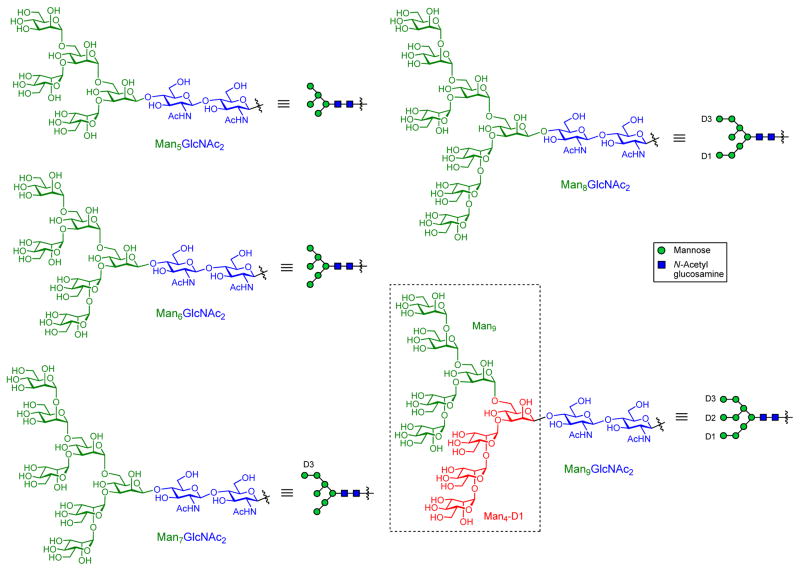
In stark contrast to recombinant gp120, which bears both oligomannose and complex-type N-glycans, virion-associated gp120 from primary isolates of HIV-1 and from simian immunodeficiency virus (SIV) present almost exclusively high-mannose glycans ranging from Man5GlcNAc2 to Man9GlcNAc2 [31,35]. Although individual HIV-1 glycans are similar to those of the host, the extensive high-mannose clustering on gp120 (and gp41) is a hallmark of transmembrane Env on virions [31]. Thus, these oligomannose structures within the glycan shield have been viewed as primary targets for HIV vaccine design.
Fully Synthetic Oligosaccharide Clusters as HIV Carbohydrate Antigens Based on the 2G12 Broadly Neutralizing Antibody Epitope
2G12 Epitope Definition
The idea of utilizing gp120 carbohydrates as antigens for eliciting broadly neutralizing immune responses gained true recognition with the discovery of the 2G12 BnAb [36]. This antibody, isolated from an HIV-1 infected patient, was shown to efficiently neutralize a wide range of HIV isolates in vitro and in vivo by passive immunization [36,37]. Extensive studies on the binding epitope of 2G12 concluded that this antibody recognizes high-mannose N-glycans at Asn295, Asn332, and Asn392 forming a unique oligomannose cluster on gp120, and that a terminal Manα1-2Man disaccharide motif is involved in the binding [38,39]. Moreover, additional mutational studies indicated that the peptide domains of gp120 are apparently not directly involved in the binding of 2G12, but serve only as a rigid scaffold onto which the corresponding N-glycans are closely held to form the cluster [38,39]. Subsequently, the crystal structure of 2G12 revealed a unique domain-exchanged dimeric structure [40]; the variable regions of the heavy chains (VH) swap over to form an extended binding surface that includes a novel VH/VH′ interface in addition to two conventional VH/VL combining sites. This novel domain-swapped architecture creates an extended multivalent binding surface to accommodate an oligomannose cluster where at least two oligosaccharides bind to spatially adjacent pockets on the surface of the antibody, greatly enhancing the carbohydrate-antibody affinity to the nanomolar range. This is an elegant, unique binding solution to the problem of generally reduced affinities of antibodies to carbohydrate antigens. The potential therapeutic value of the 2G12 epitope as an attractive HIV-1 vaccine target has been highlighted by the finding that 2G12 can protect macaques against simian-human immunodeficiency virus (SHIV) challenge even at low serum neutralizing titers [37]. From a vaccine design viewpoint, the dense cluster of high-mannose sugars contributed by the N-glycans on gp120 (Asn295, Asn332, Asn392) was initially considered to create novel epitopes that could be recognized as foreign by the immune system, thus constituting the ultimate target of the domain-exchanged 2G12. This valuable structural information revealed a potential vaccine strategy targeting these conserved oligomannose clusters as antigens to be probed using 2G12 as an initial recognition template. While it is currently known that 2G12 contacts only the outermost mannose residues of the interacting glycans centered around the Asn332 site [28], at the outset, the precise structure of the high-mannose domains (Man5 to Man9) had not yet been defined (Figure 1), and it was unclear which of the oligomannose subunits were critical for recognition.
Figure 1.
Typical structures of HIV-1 high-mannose type oligosaccharides.
The work with 2G12 was the starting point for the design of glycan mimics of gp120 epitopes. Wang and coworkers prepared three high-mannose oligosaccharides (Man9GlcNAc, Man6GlcNAc and Man5GlcNAc) and analyzed their binding affinity to 2G12 [41]. Competitive enzyme-linked immunosorbent assays (ELISA) against immobilized gp120 indicated that Man9GlcNAc is the preferred subunit on gp120 for 2G12 recognition, providing direct evidence that the terminal Manα1,2Man moiety is essential for binding. In another study, Wong et al. synthesized a number of differently-sized, Manα1,2Man-containing oligomannose structures with variation at the D1, D2 and D3 arms of Man9, and studied their ability to inhibit the interaction between 2G12 and gp120 in an ELISA experiment [42]. Interestingly, comparison between the trimannose Man3 and tetramannose Man4 suggested that addition of an extra α1,2-linked mannose at the D1 arm could greatly increase the binding efficiency to levels comparable to those of the native Man9GlcNAc2 glycan. These results were consistent with the X-ray structural study [40], which highlighted the central importance of the D1 arm in antibody binding, and revealed that this arm in Man4 could serve as an effective minimal building block en route to multivalent constructs that mimic the 2G12 epitope. Independently, the Seeberger group evaluated the binding of a series of synthetic oligomannose derivatives (from Man3 to Man9) to 2G12 in a microarray setting, and also confirmed the structural requirement of a Manα1-2Man linkage for recognition by 2G12 [43]. More precise characterization of the fine carbohydrate-binding specificity of 2G12 involved X-ray crystal structure studies on the complexes of 2G12 with four different synthetic oligomannoses (Man4, Man5, Man7 and Man8) [44]. The crystallographic information, combined with solution-binding analysis, revealed that 2G12 is capable of binding the Manα1–2Man at the termini of both the D1 and D3 arms of the Man9GlcNAc2 moiety, providing more flexibility for the multivalent interactions required for high affinity binding. This work further confirmed that 2G12 is highly specific for terminal Manα1–2Man, but in the context of an extended oligosaccharide structure.
The following sections present design and synthesis approaches that have provided access to chemically-defined oligomannose constructs for antigenicity and immunogenicity studies. We reiterate that an observed binding affinity between synthetic epitope mimic and antibody (antigenicity) may not necessarily translate to the desired in vivo antibody response (immunogenicity).
Multivalent High-mannose Glycan Clusters as 2G12-based Epitope Mimics for Binding and Immunization Studies
Danishefsky Constructs
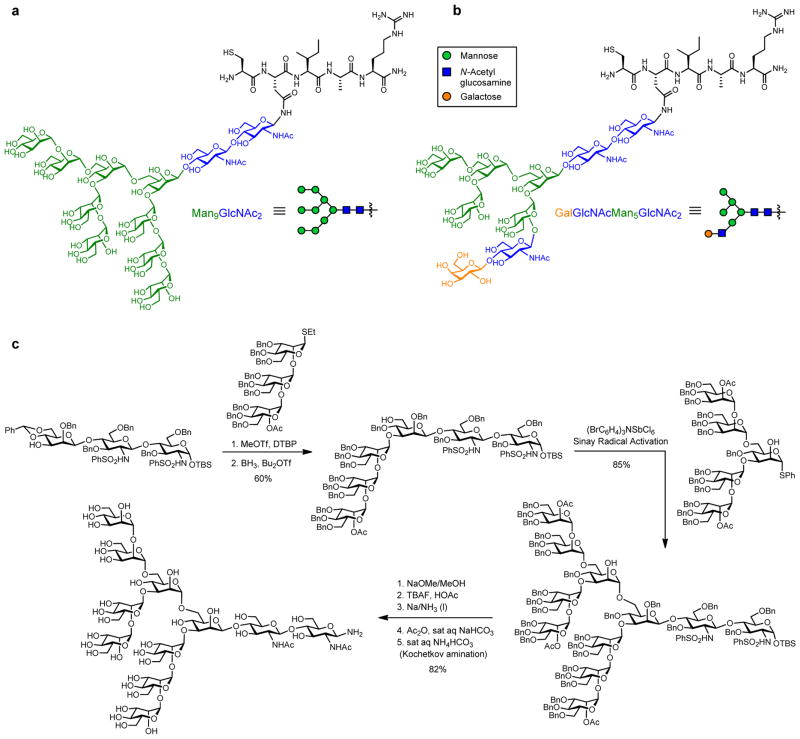
Collectively, the valuable results of these chemical and structural studies formed the basis for the design of immunogens to elicit 2G12-like neutralizing antibodies. Bearing all these considerations in mind, we set out, more than ten years ago to develop fully synthetic constructs mimicking the 2G12 carbohydrate epitope as potential antigen candidates for HIV vaccine development. Our first efforts in this regard involved the total synthesis of high-mannose and hybrid-type glycans and their incorporation into gp120 N-linked glycopeptides (Figure 2a,b) [45,46]. En route to the high-mannose gp120 fragment, two synthetic strategies involving linear (“the layer approach”) and convergent (“the block approach”, shown in Figure 2c) carbohydrate assembly were utilized for the efficient preparation of the fully protected Man9GlcNAc2 oligosaccharide. A similar method to the convergent block approach was used in the synthesis of the hybrid-type glycan. Global deprotection consisting of deacetylation, desilylation, and Birch reduction provided the free sugars, whose reducing ends were then aminated following the Kochetkov procedure [47]. The corresponding glycosylamines were coupled to Cys-containing gp120 peptide fragments via Lansbury aspartylation [48], to give, following deprotection, the homogeneous gp120 N-glycopeptides carrying high-mannose and hybrid-type glycans (Figure 2a,b). The successful syntheses of these large gp120 fragments constituted an important achievement from a synthetic chemistry viewpoint and also enabled us to probe these structures as mimics of the epitope of BnAb 2G12. Surface plasmon resonance (SPR) binding analyses indicated that the constructs bearing the hybrid-type glycan did not bind to 2G12. Interestingly, while the compound carrying a single high-mannose N-glycan bound to 2G12 only weakly, the dimeric form of this glycopeptide (through a disulfide bond) exhibited significantly increased binding [49]. These results revealed that hybrid-type N-glycans are not recognized by 2G12, and demonstrated the existence of a clustering effect for the bivalent high-mannose glycopeptide antigen, which is in agreement with the cocrystal structure of the 2G12/high-mannose sugar complex.
Figure 2.
N-glycopeptide fragments derived from gp120331–335 bearing (a) high-mannose and (b) hybrid-type glycans. (c) Total synthesis of Man9GlcNAc2 glycan using the convergent “block approach”.
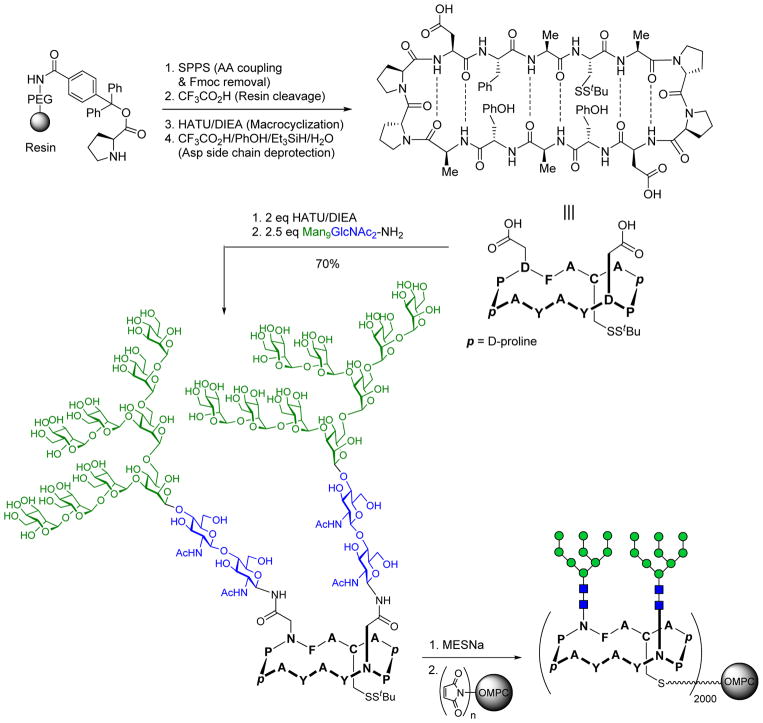
These studies placed us in a favorable position to apply the above information about the interaction between 2G12 and its gp120 epitope in a vaccine approach. In our previous experiments [49], the gp120 peptide alone had exhibited no affinity for 2G12. This fact, together with other systematic studies suggesting that the peptide portions do not directly contribute to the binding [38,39] point to a primary structural role of the protein backbone in presenting the glycans in the appropriate orientation. On this basis, we rationalized that an unnatural peptide would enable greater design flexibility than one relying on the natural sequence, and we focused our efforts on the development of fully synthetic immunogens wherein the glycans would be presented in a clustered arrangement emulating the 2G12 epitope on gp120. The unnatural peptide was designed to afford variability in the number of carbohydrate chains that can be incorporated, as well as in the distances between glycans, approximating those defined from the crystal structure. Thus, we synthesized a modular cyclic peptide scaffold bearing aspartic acid residues, to which the previously prepared high-mannose (Man9GlcNAc2) glycosyl amine was attached via Lansbury aspartylation in a maximally convergent manner (Figure 3) [50]. Glycopeptides having from zero to three glycans were prepared and then probed for their ability to bind 2G12 by SPR. Interestingly, the non- and monoglycosylated constructs showed no measurable response, and only the bivalent and trivalent glycopeptides exhibited strong binding to the antibody. This result confirmed the importance of multivalent antigen presentation in recognition by 2G12, and suggested homology of the bi- and trivalent target structures to the natural epitope on gp120, despite being completely unrelated to the native gp120 peptide sequence. Having confirmed the ability of our synthetic glycopeptides to serve as antigenic 2G12 epitope mimics, we next sought to attach the selected constructs to the purified outer membrane protein complex (OMPC) through a single cysteine residue contained in the cyclic peptide scaffold (Figure 3). OMPC, derived from Neisseria meningitidis, is a macromolecular lipoprotein complex with potent adjuvant activity which serves as an immunostimulatory carrier for poorly immunogenic peptide and carbohydrate antigens. In the event, OMPC was derivatized with maleimide units on its surface and the activated carrier was reacted with the previous bivalent glycopeptide bearing a free cysteine via thiol-maleimide coupling to give the OMPC-conjugated construct with incorporation of ~2000 copies of the cyclic glycopeptide.
Figure 3.
Synthesis of scaffolded, bivalent high mannose (Man9GlcNAc2) cluster as a 2G12 epitope-based immunogen.
Subsequent ELISA analysis confirmed recognition of the conjugate by 2G12. To test the efficacy of this construct as an immunogen capable of inducing a 2G12-like BnAb response, the immunogenicity of the glycoconjugate was evaluated in animal vaccination studies. By using a differential immunoassay, induction of high levels of carbohydrate-specific antibodies was observed in two animal species, guinea pigs and rhesus macaques [51]. However, a significant “2G12-like” response was not mounted as these antibodies did not specifically recognize recombinant HIV gp160 (the biosynthetic precursor of gp120 and gp41). Unfortunately, the immune sera from both animal models also failed to neutralize a panel of viral isolates. These results indicate that presentation of Man9GlcNAc2 on this particular constrained cyclic scaffold is not sufficient to induce an immune response that recognizes the native 2G12 epitope, presumably due to recognition of irrelevant conformations of the flexible glycan chains. Overall, these studies suggests that although incorporation of these high-mannose clustered glycans provides good binding to 2G12, the synthetic mimotopes do not realistically represent the antibody epitope and, therefore, are insufficient to function as an effective immunogen. Nonetheless, this work pointed a way toward future efforts to improve immunogen design, and signaled the prospect that a successful strategy will likely need to comprise a design whereby the optimal oligosaccharide conformation can be fixed in a preferred orientation – for instance, by cross-linking oligomannose chains at positions supported by crystallographic data.
Wang Constructs
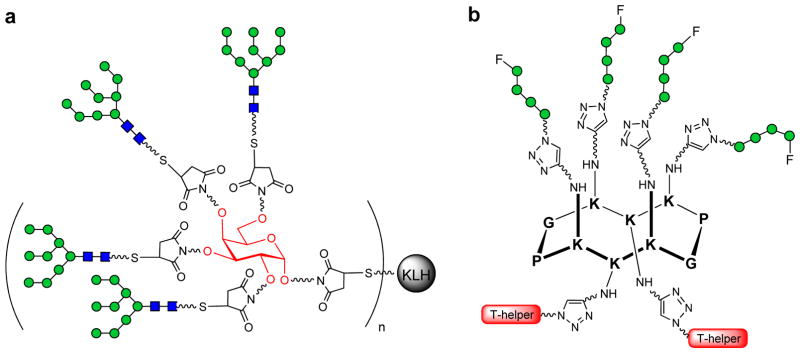
Wang et al. have also attempted to mimic the putative epitope of 2G12 with the design and synthesis of several oligomannose clusters based on a galactoside moiety (displaying between 2 and 4 Man9GlcNAc2 units) (Figure 4a) [41], and cholic acid (bearing three Man9GlcNAc2 glycans) [52] as cyclic scaffolds. In ELISA assays, the affinity for 2G12 of the tetravalent Man9 galactoside and the trivalent Man9 cholic acid cluster was 70-fold and 46-fold higher, respectively, than that of Man9GlcNAc2Asn, demonstrating a clear glycan clustering effect in 2G12 binding. Overall, these studies point to a number of factors that are important for tight binding to 2G12, namely: the spatial orientation of the oligomannose sugars, the length of the spacers, and the rigidity/configuration of the scaffold. To probe the ability of these 2G12 epitope mimics to generate antibody responses, and given the poor immunogenicity of the HIV-1 carbohydrates themselves, the Wang group conjugated the galactoside-based, tetravalent Man9GlcNAc2 cluster to keyhole limpet hemocyanin (KLH, a strong immune-stimulating carrier protein with abundant T cell-helper epitopes), affording the functional immunogen [53]. Rabbit immunization studies with the oligomannose-containing glycoconjugates revealed that only modest titers of carbohydrate-specific antibodies were induced, with most of the antibody responses directed against the linker. The anti-sera showed only weak cross-reactivity against gp120 and did not exhibit HIV-1 neutralizing activity. Possible solutions to improve the immunogenicity of this construct may include the use of appropriate, non-immunogenic linkers, powerful immunoadjuvants, or modified immunization protocols.
Figure 4.
Immunogen design based on template-assembled oligomannose clusters as epitopes mimics for 2G12 antibody. (a) Galactose-based tetravalent Man9GlcNAc2 cluster conjugated to KLH (b) Cyclic peptide scaffold bearing clusters of the D1 Man4 oligosaccharide (its fluorinated derivative) and T-helper epitopes.
The much lower affinity of these synthetic oligosaccharide clusters for 2G12 (only in the micromolar range) in comparison to that of HIV-1 gp120 (at the nanomolar level), together with the failure of the corresponding glycoconjugate immunogens to elicit high levels of anti-HIV-1 carbohydrate antibodies, led Wang and coworkers to synthesize a different cyclic peptide template onto which four Man4 oligosaccharides (corresponding to the D1 arm of the entire Man9GlcNAc2) were attached, forming a novel oligomannose cluster [54]. In addition, in an attempt to increase the immunogenicity of the oligomannose cluster, a new variant of the previous structure containing 4 units of the selectively fluorinated D1 Man4 glycan on the peptide scaffold, was also synthesized (Figure 4b), With the same objective, on the opposite face of this novel template, two T-helper peptides were introduced for T-cell activation purposes, affording the synthetic vaccine candidate. Binding studies with these template-assembled glycan clusters showed enhanced affinity for 2G12 in comparison to both the oligosaccharide subunit alone, and the cluster bearing only four individual mannose residues at each position. However, while the fluorinated derivative also exhibited apparent affinity for 2G12, it was lower than that observed for the natural D1 arm cluster.
Additional Constructs
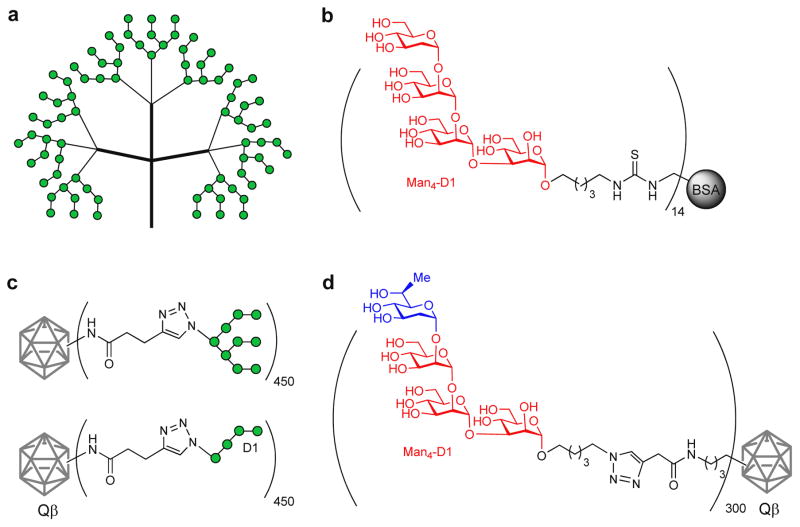
To further optimize the design of oligomannose clusters as effective 2G12 epitope mimics, Wong et al. designed and synthesized polyvalent glycan clusters based on oligomannose dendrimers bearing multiple copies (3, 9 and 27) of the D1 arm tetrasaccharide (Man4) or the entire high-mannose sugar (Man9) lacking the internal GlcNAc units (Figure 5a) [55]. Overall, these dendrimers showed increased binding affinities for 2G12; most notably the nine-valent Man9 derivative inhibited the interaction between gp120 and 2G12 in the nanomolar range. These high affinity levels, which are similar to the native gp120, suggest that these glyco-dendrimers can effectively mimic the dense high-mannose clusters on the 2G12 epitope, and point to their potential use as immunogens for HIV vaccine development. It should be noted that the great affinity obtained is likely due to the elevated density of oligomannose presentation resulting from the high multivalency of these glycodendrimers.
Figure 5.
(a) Dendrimer-based multivalent Man9 cluster as 2G12-epitope mimic. (b) D1-arm tetramannose conjugated to BSA for immunization studies. (c) Qβ glycoconjugates displaying oligomannose structures (Man9 and Man4) as 2G12 epitope-associated immunogens. (d) Virus-like particle (Qβ) conjugate containing a non-self D1-arm tetrasaccharide mimic as 2G12 epitope-based immunogen.
In addition to the previously described attempts [51,53], several other immunization studies have been carried out with synthetic immunogens. Burton et al. developed several BSA-glycoconjugates displaying a variable number of copies of the synthetic D1 arm tetrasaccharide (Man4), and showed that increased multivalent presentation of the Man4 on the BSA scaffold led to higher binding affinity of 2G12 for Man4, though only up to a certain point (~10 copies) (Figure 5c). Rabbit immunization with a Man4-BSA conjugate, (BSA-Man4)14, elicited anti-mannose antibodies directed to the Man4 structure; however, these antibodies did not recognize the natural high-mannose glycans within gp120. This finding suggests that synthetic oligomannoses may possess different disposition and accessibility relative to natural versions; this disparity might be addressed through modulation of the density and conformational flexibility of the glycans, perhaps by presenting them in a branched and more densely clustered display, or controlling the rigidity of the linker [56].
In a further study, Finn, Burton and co-workers explored the use of virus-like particles, such as Qβ, as scaffolds to present oligomannose clusters in a more controlled and dense display, in the hope of better mimicking the clustering arrangement on gp120 (Figure 5d) [57]. The Qβ glycoconjugates displaying Man4 and Man9 structures interacted with 2G12 with nanomolar affinity, whereas the Qβ-Man8 derivative showed weak binding. Interestingly, a mixed combination of Qβ-Man8/Man9 resulted in the most effective glycoconjugate antigen with the highest affinity for 2G12. Immunogenicity studies in rabbits with the Man4 and Man9-containing Qβ glycoconjugates showed high levels of mannose-specific antibodies that especially recognized each particular structure. However, these antibodies were not able to cross-react with native gp120, and did not show any neutralization activity. Since these glycoconjugates lacked the inner GlcNAc2 subunit, this immunological discrimination may be due to conformational differences in the presentation of these truncated, artificially-linked oligomannoses compared to the native N-linked high-mannose glycans. Thus, it is conceivable that these two GlcNAc2 residues play a role in defining the optimal orientation of the N-glycan in the construct. This concept is supported by the finding of Doms and coworkers whereby immunization with a yeast mutant expressing the Man8GlcNAc2 glycan in its native cluster form was able to induce carbohydrate-specific antibodies that were able to recognize monomeric gp120 and to efficiently neutralize HIV-1 virions expressing only high-mannose N-glycans, although wild-type HIV was not neutralized [58].
In an elegantly designed strategy aimed at improving the immunogenicity of synthetic sugar mimics of the 2G12 epitope, Doores et al. synthesized a series of unnatural, mannose-derived monosaccharides that were incorporated into the D1 arm of synthetic oligomannoses, creating monovalent non-self glycans that bound with high affinity to 2G12 (Figure 5b) [59]. A unique non-self sugar mimic (a C-6 methylated Man4 glycan) was then conjugated to virus-like particle bacteriophage Qβ, and its immunogenicity was studied in rabbits. Whereas higher titers of mannose-specific antibodies cross-reactive with the natural D1 arm tetrasaccharide were generated in comparison to the self Qβ glycoconjugate, these antibodies did not bind to this glycan motif on gp120, and lacked HIV-neutralizing activity. These results may be explained by the unique presentation mode of the sugar on the Qβ glycoconjugate, but they open the door to further investigations into alternative clustered presentations of the nonself glycan in an attempt to better recreate the structural features of the carbohydrate shield of gp120.
Despite showing high binding affinities to 2G12, and in some cases being able to generate mannose-directed antibody responses, none of the above synthetic constructs has proven to be an effective immunogen, capable of inducing 2G12-like antibodies that bind gp120 and/or neutralize HIV-neutralizing antibodies. While various factors could account for this fact, the most basic is that none of the evaluated constructs properly recapitulated the spacial conformation of oligomannose residues in native gp120. It is also conceivable that, given the unusual domain-exchanged structure of 2G12, this type of antibody may, by nature, be hard to induce. Another possible explanation is that these synthetic glycan antigen mimics may be processed and presented to the immune system in a different fashion in comparison to the native ones. Overall, these studies strongly suggest that, in order to increase the immunogenicity of the 2G12 epitope mimics, further refinements towards more relevant epitope presentation of these mimics – for instance, on different carriers or scaffolds – may be key for directing the immune system to produce HIV-reactive neutralizing antibodies; inclusion of special adjuvants may even prove essential as well. Similarly, a better understanding of how the immune system recognizes natural high-mannose glycans on gp120 compared to the recognition of oligomannosides on synthetic glycoconjugates is key to designing a successful carbohydrate-based HIV vaccine. Finally, the precursors of glycan-reactive BnAbs may be rare due to reduction in B cell precursor pool size because of tolerance mechanisms recognizing HIV glycans as self, thus disfavoring glycan-reactive B cell development [13].
Fully Synthetic V1/V2 HIV Glycopeptide Antigens Based on the PG9, PG16 and CH01–CH04 Broadly Neutralizing Antibody Epitope
PG9, PG16 and CH01–CH04 Epitope Definition
The recent discoveries of new, highly potent human BnAbs represent a significant step forward in the design of new targets for HIV vaccine development [18,19]. The BnAbs PG9 and PG16, recently isolated from an HIV-infected African individual, have been found to neutralize ~75% of the circulating HIV-1 isolates and show higher potency than 2G12 in neutralization of virus [60]. Initial epitope mapping indicated that PG9 and PG16, and the clonal lineage of CH01–CH04 recognize a glycan-dependent region within the first and second variable loops (V1/V2) of gp120, distinct from that of 2G12 [61,62]. Subsequent crystal structure studies of the complex between PG9 and a scaffolded V1V2 domain revealed that the antibody makes contacts with two high-mannose glycans at Asn160 and Asn156 and a contiguous V1V2 peptide β-strand [63]. Of the two oligomannose chains, the entire Man5GlcNAc2 carbohydrate was evident in the former site, whereas only four mannose residues are visible for the Asn156 glycan. Importantly, this recognition site involves both the carbohydrate and peptide domains within the V1V2 region, in contrast to the binding site of 2G12, which apparently does not include the peptide backbone. This valuable structural information has provided an excellent framework for immunogen design based on the PG9 and PG16 epitope. However, past work has largely relied on recombinant gp120 acquired as heterogeneous mixtures of glycoforms, which seriously complicates the precise correlation between glycan composition and immunoactivity.
V1V2 N-glycopeptide Antigens as Epitope Mimics for Binding Studies
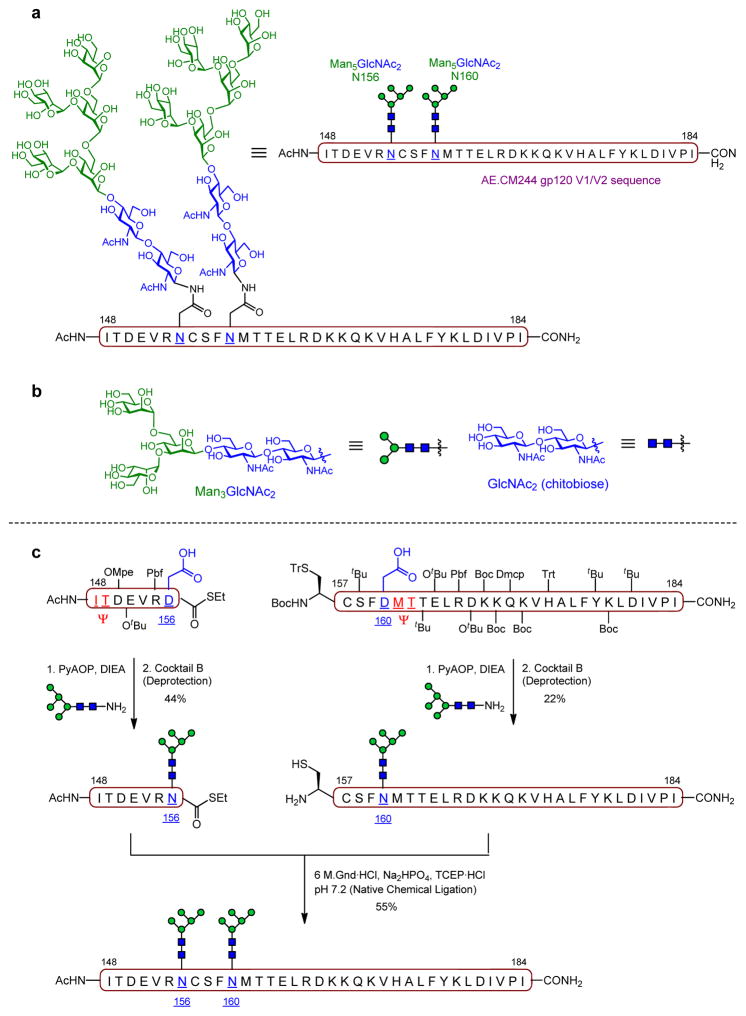
On this basis, a series of gp120 V1V2 N-glycopeptides derived from the CRF 01AE A244 HIV-1 strain and bearing well-defined N-glycans (at Asn160 and Asn156) was designed and chemically synthesized, as potential epitope mimics of the PG9 antibody (Figure 6) [64]. The peptide sequence comprised 35 amino acids corresponding to positions 148–184 of gp120, the main region of the envelope glycoprotein primarily recognized by PG9. Regarding the carbohydrate domain, we decided to target Man5GlcNAc2, based on the previous structural analyses. Thus, our primary target was the glycopeptide bearing Man5GlcNAc2 glycans at the two N-glycosylation sites, which encompasses the important components of the binding epitope of PG9 (Figure 6a). In addition, we pursued other simplified glycopeptides with Man3GlcNAc2 and chitobiose (GlcNAc2) units to probe the importance of the outer mannose residues for recognition (Figure 6b).
Figure 6.
(a) Chemical structure of the primary gp120 V1V2 glycopeptide target bearing two closely spaced Man5GlcNAc2 N-linked glycans (Asn160 and Asn156) as epitope mimic of PG9 BnAb. (b) Stucture of simplified N-linked glycans (Man3GlcNAc2 and GlcNAc2) also incorporated into the V1V2 peptide backbone to gain access to additional simpler glycoforms. (c) Chemical synthesis of Man5GlcNAc2-bearing V1V2 glycopeptide via Native Chemical Ligation (pseudoproline dipeptide used to prevent aspartamide formation shown in red).
Chemical Synthesis
The chemical synthesis of these homogeneous constructs as single glycoforms was very challenging due to the close spacing of large carbohydrate domains within the peptide backbone. As described earlier, we followed a convergent chemical assembly strategy, wherein the N-glycans were coupled, as unprotected glycosyl amines via Lansbury aspartylation reaction, to the free aspartic acid residues of a protected peptide backbone bearing pseudoproline functionalities (Figure 6c) [65]. Our synthetic efforts started with the preparation of the required oligosaccharides (Man5GlcNAc2 and Man3GlcNAc2) employing carefully optimized carbohydrate chemistry. For the construction of the target glycopeptides, we pursued a two-fragment approach whereby shorter peptide segments were individually glycosylated, and then coupled together in unprotected form via Native Chemical Ligation (NCL) (Figure 6c). Despite the considerable challenge imposed by the presence of the bulky glycan at the C-terminal, thioester-bearing residue (Asn156) in one of the coupling partners, and the close proximity of the two sterically demanding carbohydrates around the ligation site, this strategy was successfully executed and enabled practical access to the target compounds in good yields.
Binding Studies
To evaluate the ability of our synthetic V1V2 N-glycopeptides to recapitulate the PG9 BnAb epitope, SPR analysis was performed to assess the binding of these constructs to PG9. Notably, the Man5GlcNAc2 glycopeptide and the simplified Man3GlcNAc2-bearing variant both showed considerable affinity for PG9 (KD = 311 and 119 nM, respectively), whereas the chitobiose-containing construct did not bind to the antibody, pointing to an important role of the outer mannose residues for recognition. Furthermore, samples of non-glycosylated peptide (“aglycone”), protein-free Man5GlcNAc2 and Man3GlcNAc2 oligosaccharides, and mixtures of “aglycone” and glycan failed to show detectable binding, demonstrating that both peptide and carbohydrate domains are essential for recognition by PG9 and that covalent linkage between these domains is required. It is conceivable that the high binding affinity observed with these constructs may well have its origin in the multivalent interaction of PG9 with both the peptide β-strand and the Asn-linked glycans. Likewise, N-glycosylation of the peptide backbone could also induce a favorable conformational change on the resulting glycopeptide such that the peptide and/or sugar residues adopt an optimal orientation for antibody recognition. Further, as demonstrated previously [49], we rationalized that the presence of the free cysteine residue in the peptide sequence (Cys157) would likely exert also an important conformational effect on the glycopeptide structures and, consequently, on their interactions with the antibody. To explore this possibility, we examined several different oxidation protocols for dimer formation, namely spontaneous air oxidation in phosphate buffer, iodine treatment and DMSO co-solubilization. Under air oxidation conditions, variable binding results were obtained on a batch-to-batch basis. Iodine oxidation gave oligomers and aggregates of the glycopeptides that only showed weak binding to PG9. Fortunately, solubilization in 20% DMSO/phosphate buffer gave Man3 and Man5 glycopeptides that were completely oxidized to the disulfide-linked dimers, as assessed by SDS/PAGE analysis and size-exclusion chromatography [66]. Importantly, the DMSO-treated glycopeptides bound avidly to PG9, and also to CH01 (another glycan-dependent BnAb tested in these experiments, which is known to bind well to the V1V2 β-strand epitope in the A244 strain) [62]. In addition, the binding to a strain-specific, non-broadly neutralizing V2 monoclonal antibody (mAb CH58) [67] was minimal. Analysis of the biophysical properties of the synthetic glycopeptides by circular dichroism (CD) showed that the DMSO-oxidized, disulfide-linked dimeric constructs adopted a more ordered, β-sheet secondary structure in solution, which is likely to be play a role in the selective binding of the V1V2 BnAbs. To probe if the active structure responsible for binding was in fact the oxidized glycopeptide dimer, we converted the cysteine amino acid (Cys157) into an alanine residue by chemoselective desulfurization of the parent Man3GlcNAc2-glycopeptide [68]. The resulting V1V2 mutated (C157A) construct presented, mainly, a random-coil conformation by CD; binding of the PG9 and CH01 BnAbs was totally abrogated, suggesting that both dimerization and β-sheet secondary structure of the glycopeptides are important for recognition. From the antigenicity studies, it was concluded that only the β-sheet structured, disulfide-linked V1V2 glycopeptide dimers bearing high-mannose glycans (Asn160 and Asn156) were able to selectively bind to both BnAbs (PG9 and CH01) with strong affinities, in the nanomolar range (~30–40 nM). It has been proposed that a rational approach for inducing BnAbs should also target the unmutated common ancestors (UCA), which are predicted to be the receptors of the BnAb naive B-cell precursors, and intermediate antibodies of BnAb lineages [69]. Importantly, the Man5 and Man3 glycopeptides bound not only to the mature BnAbs but also to their UCAs, which is a key feature of an optimal immunogen. Prior to this work, few constructs derived from the HIV-1 envelope had been found to bind to the CH01 lineage UCA [70] and none has been reported to bind to the PG9 UCA. In particular, the Man5GlcNAc2 glycopeptide showed higher affinity than the Man3 construct to the UCA of the subdominant BnAbs (PG9 and CH01). In addition, the Man5-derivatized glycopeptide exhibited higher-affinity binding to these PG9 and CH01 UCAs than to the UCA of the strain-specific vaccine-induced CH58 mAb.
In conclusion, while PG9 binds preferentially (though not exclusively [67]) to trimeric gp140s, the high nanomolar-affinity binding of these fully synthetic, medium-size, homogeneous glycopeptides to both mature V1V2 BnAbs and, importantly, to their UCAs as well compares favorably with the antigenicity of the monomeric [60] and trimeric [71] native envelope structures, indicating their ability to mimic conformations of the V1V2 gp120 epitopes. Key to this outcome are the structural features imparted by the dimerization in promoting and stabilizing the optimal, desired topology of the glycopeptides, either by appropriately mimicking the quaternary features of the PG9 epitope via quaternary structure-level interactions, or by affording a more orderly presentation of the glycans and/or the peptide moiety. Further, while these Man5 and Man3-containing constructs bound poorly to the dominant, strain-specific V2 mAb CH58, they showed high, preferential reactivity toward the V1V2 BnAbs, which makes them particularly attractive immunogens for the induction of subdominant, unfavored BnAb responses. In addition, as these V1V2 glycopeptides also contain T-helper epitopes in their peptidic sequence [72], this important work represents an encouraging step forward to the development of experimental vaccine immunogens to be evaluated in animal models for their ability to produce BnAbs directed to the gp120 V1V2 epitope.
V1V2 N-glycopeptide Antigens as PG9-and PG16-based Epitope Mimics for Glycan Specificity Characterization and Binding Studies
In another independent study, Wang and coworkers designed and synthesized, by a chemoenzymatic method, a library of different homogeneous V1V2 cyclic glycopeptides consisting of 24 amino acids (154–177) derived from distinct HIV-1 strains (CAP45 and ZM109) [73]. Mutations at Lys155 and Phe176 to Cys allowed for cyclization of the constructs via disulfide-bond formation to stabilize the β-hairpin present in the crystal structure. Specific high-mannose and/or complex-type glycans were installed chemoenzymatically at the two predetermined N-glycosylation sites (Asn160 and Asn156/Asn173), and the well-defined glycopeptides were probed for binding with PG9 and PG16 antigen-binding fragments (Fabs) by SPR. While the overall binding affinity of these glycopeptide constructs was in the micromolar range, these studies have provided important insights into the glycan specificity of the PG9 and PG16 BnAbs. Taken together, the results confirmed that a Man5GlcNAc2 at Asn160 is critical for recognition, whereas an additional sialylated complex-type N-glycan at the secondary glycosylation site was found to enhance the binding affinity. This unexpected finding, which was not revealed by the original PG9 structural study [63], is consistent with a recent crystal structure of a scaffolded V1V2 domain/PG16 Fab complex [74] showing that PG16 interacts extensively with a sialylated hybrid N-glycan present at the secondary site (Asn156 or Asn173). A similar result was obtained by Wong and coworkers in a glycan array-based study of the glycan binding specificity of PG9 and PG16, wherein PG16 was observed to bind to complex-type multi-antennary N-oligosaccharides bearing a terminal α-2,6-linked sialic acid unit [75]. Collectively, the characterization of the nature and location of the N-glycan for PG9 and PG16 recognition, together with the reconstitution of the fine epitopes of these BnAbs antibodies enabled by these synthetic glycopeptides provides a valuable framework for HIV-1 vaccine design.
Fully Synthetic V3 HIV Glycopeptide Antigens Based on the PGT128 Broadly Neutralizing Antibody Epitope
PGT128 Epitope Definition
The PGT class of monoclonal antibodies is a new group of glycan-dependent BnAbs isolated from HIV-infected elite neutralizers. Most of these antibodies (PGT121–123 and 125–128) showed exceptional breadth and high potency, being ten- and 100-fold more potent than PG9 and 2G12, respectively [76]. In particular, PGT128 neutralizes over 70% of globally circulating viruses. The recently disclosed crystal structure of PGT128 Fab in complex with a Man9 oligosaccharide and a glycosylated, third variable (V3) loop domain of gp120 revealed that the epitope recognized by PGT128 consists of two conserved high-mannose glycans (at Asn332 and Asn301) and a short β-strand C-terminal peptide fragment of the gp120 V3 region [77]. More recently, structural studies of a number of these novel antibodies have shown that they bind to a high-mannose region centered on the Asn332 site that is very accessible and conserved across HIV-1 isolates, thereby representing a supersite for neutralization [28,29,30]. Considering the breadth and potency of the BnAbs recognizing this epitope, this antigenic region constitutes a very attractive vaccine target if appropriate immunogens can be designed.
V3 N-glycopeptide Antigens for Studying Conformational Effects of Glycosylation
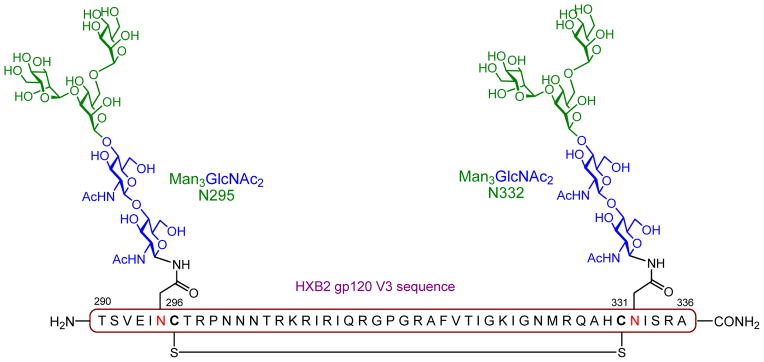
The V3 domain was once termed “the principal neutralization determinant” [78] and early synthetic efforts in the field were focused on the design of N-glycopeptides derived from this region to probe the effects of N-glycosylation on the conformation and proteolytic stability of V3 peptide domains. Wang et al. synthesized a disulfide-linked, cyclic V3 glycopeptide incorporating the N-linked core pentasaccharide (Man3GlcNAc2) at two conserved sites (Asn332 and Asn295) applying their chemoenzymatic approach (Figure 7) [79]. The synthetic glycopeptide showed improved resistance to proteolysis than the naked peptide, suggesting that it could be more stable in vivo. Circular dichroism and Fourier transform-infrared studies showed that N-glycosylation induced changes in the global conformations of the V3 peptide, which might be exploited in the design of HIV glycopeptide immunogens with more favorable conformational epitopes for BnAbs. Current efforts in the field are being focused on the design and synthesis of homogeneous V3 glycopeptides to define the role of specific glycan/peptide structures on PGT BnAb recognition [80]. Provided that some of these rationally designed, synthetic V3 glycopeptides are able to effectively mimic the V3 gp120 epitope in antigenicity studies, it is expected that they will be further evaluated as immunogens in animal models in the near future.
Figure 7.
Chemical structure of the gp120 V3 cyclic glycopeptide target carrying two Man3GlcNAc2 N-linked glycans (Asn295 and Asn332).
Expert commentary
The viral envelope glycoproteins gp120 and gp41 are attractive targets for HIV vaccine development. Extensive efforts have been devoted to the development of a number of carbohydrate- and glycopeptide-based HIV experimental vaccines targeting known epitopes of broadly neutralizing antibodies. First attempts focused on the design and synthesis of template-assembled, high-mannose oligosaccharide clusters as mimics of the 2G12 epitope. Despite encouraging binding data of the antigens, immunogenicity studies with the corresponding vaccine candidates demonstrated the difficulty in generating 2G12-like immune responses. This has been attributed, in part, to the non-optimal presentation mode of the sugars on the glycoconjugates and the unique domain-exchanged structure of 2G12, which might be formed very rarely in response to vaccine challenge. Thus, only limited progress has been made in eliciting gp120 cross-reactive antibodies, and none of these 2G12-based vaccine approaches has been able to show HIV-neutralizing activity.
Nonetheless, the recent isolation of classes of broadly neutralizing antibodies, including PG9, PG16, CH01–CH04, which target V1V2 glycans, and antibodies that target V3-glycans such as PGT 128, as well as the recent understanding of host controls of HIV Env BnAbs [13], have brought some hope for the development of a glycopeptide-based HIV vaccine. Structural elucidation and characterization of the fine specificities of these neutralizing epitopes are enabling the design and chemical synthesis of novel homogeneous glycopeptide antigens that show nanomolar-affinity binding, and can therefore be considered as effective mimics of the native gp120 structures. Finally, design of glycopeptide-based immunogens that target the unmutated ancestor antibodies of BnAbs provides a strategy for overcoming host tolerance controls and for driving otherwise unfavored BnAb B cell lineages [13,81]. These promising strategies provide new directions for the prospect of designing improved immunogens capable of inducing broadly neutralizing responses and thus the development of an effective HIV vaccine.
Five-year view
Following the encouraging data of the RV144 clinical trial in 2009, and the subsequent correlates studies, it is expected that more results from follow-up investigations of this trial will bring more detailed information to guide the design of novel HIV vaccine candidates. In the meanwhile, innovative strategies for immunogen design will be increasingly pursued based on promising results obtained from antigenicity studies between recently identified BnAbs, such as PG9, and several glycopeptide constructs. Evaluation of their immunogenicity in animal models in search of broadly neutralizing responses will hopefully lead to the development of novel vaccine approaches using synthetic glycoconjugates. However, it is important to acknowledge the formidable challenge that such an undertaking represents. In addition, it is also likely within this timescale that access to detailed structural information of the complexes between some of the recently identified BnAbs and these synthetic constructs can be gained, which will further aid in the design of better immunogens. Similarly, immunological results from such Phase I studies could provide important information in the structure and activity correlation that would be extremely valuable to optimize immunogen design. Alternatively, isolation of novel BnAbs from HIV-infected donors and identification of their corresponding epitopes can also be achieved in a five-year time, rendering new relevant targets for vaccine design. Likewise, it is certain that within the next years the power of chemical synthesis in the preparation of additional homogeneous glycopeptides derived from other gp120 regions will continue to play an important role in the characterization and reconstitution of existing and new neutralizing epitopes for HIV vaccine development. Undoubtedly, it is the multidisciplinary combination of efforts within the entire research and clinical community that will be a key enabler in the design and, ultimately, successful development of an effective HIV vaccine.
Key issues.
Broadly neutralizing antibodies (BnAbs) able to neutralize multiple, diverse HIV-1 strains are only produced by ~20% of infected subjects after years of infection, but have not been successfully induced by current vaccines.
It is generally agreed that a key feature of an effective HIV-1 vaccine would be its ability to elicit BnAbs, which have been shown to target conserved HIV-1 envelope regions, such as glycans and glycopeptides fragments on the gp120 glycoprotein.
A number of BnAbs and their respective epitopes on the HIV-1 envelope have been isolated and identified, enabling the design and synthesis of carbohydrate and glycopeptide epitope mimics and their glycoconjugate immunogens for HIV vaccine development.
Attempts to induce 2G12-like broadly neutralizing responses by using synthetic oligomannose clusters as mimics of the 2G12 epitope have been largely unsuccessful.
Current efforts are focused on the characterization and reconstitution of the epitopes of recently identified BnAbs (PG9, PG16 and the PGT class) by designing and synthesizing homogeneous glycopeptides.
Fully synthetic homogeneous glycopeptides corresponding to the V1V2 region of gp120 have been able to effectively mimic the PG9 epitope, showing nanomolar-affinity binding, comparable to native gp120 itself.
These rationally designed, synthetic constructs are being evaluated in animal models for their potential as immunogens to elicit BnAbs, and may be useful targets for the development of HIV-1 vaccines.
Chemical synthesis of additional glycopeptide structures as mimics of other BnAb epitopes provides a powerful means to access homogeneous constructs for future antigenicity and immunogenicity studies. The structural-activity relationships made possible by these synthetic compounds will also enable exploration of the impact of glycosylation in promoting induction of BnAb-like specificities in a vaccination setting. Overall, this information will further direct the design of effective immunogens for HIV vaccine development.
Acknowledgments
We acknowledge our colleagues of the Danishefsky laboratory whose work is presented in this manuscript. We also thank our collaborators S Munir Alam and Joseph Sodroski for their role in the design of the V1V2 glycopeptide.
Footnotes
Financial and competing interests disclosure
BF Haynes and SJ Danishefsky were supported by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Division of AIDS grant for the Center for HIV/AIDS Vaccine Immunology. SJ Danishefsky was also supported by William and Alice Goodwin and the Commonwealth Foundation for Cancer Research. A Fernández-Tejada thanks the European Commission (Marie Curie International Outgoing Fellowship) for funding. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Contributor Information
Alberto Fernández-Tejada, Email: aftejada@cib.csic.es.
Barton F. Haynes, Email: barton.haynes@duke.edu.
Samuel J. Danishefsky, Email: s-danishefsky@ski.mskcc.org.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Gallo C, Montagnier L. The discovery of HIV as the cause of AIDS. N Eng J Med. 2003;349:2283–85. doi: 10.1056/NEJMp038194. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS report on the global AIDS epidemic 2013. Joint United Nations Programme on HIV/AIDS; http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Google Scholar]
- 3.McMichael AJ, Koff WC. Vaccines that stimulate T cell immunity to HIV-1: the next step. Nature Immunol. 2014;15:319–22. doi: 10.1038/ni.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba TW, Liska V, Hofmann-Lehmann R, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian–human immunodeficiency virus infection. Nat Med. 2000;6:200–6. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 6.Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Clinical research. A Setback and an Advance on the AIDS Vaccine Front. Science. 2003;300:28–29. doi: 10.1126/science.300.5616.28a. [DOI] [PubMed] [Google Scholar]
- 8.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 9.Vaccari M, Poonam P, Franchini G. Phase III HIV vaccine trial in Thailand: a step toward a protective vaccine for HIV. Expert Rev Vaccines. 2010;9:997–1005. doi: 10.1586/erv.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. This correlates study of the RV144 trial proposes the hypothesis that V1V2 antibodies mediate vaccine-induced protection against HIV-1 infection. Thus, vaccines designed to elicit higher levels of V1V2 antibodies may have improved efficacy against HIV-1 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karpenko LI, Bazhan SI, Antonets DV, Belyakov IM. Novel approaches in polyepitope T-cell vaccine development against HIV-1. Exp Rev Vaccines. 2014;13:155–73. doi: 10.1586/14760584.2014.861748. [DOI] [PubMed] [Google Scholar]
- 12.Virgin HW, Walker BD. Immunology and the elusive AIDS vaccine. Nature. 2010;464:224–31. doi: 10.1038/nature08898. [DOI] [PubMed] [Google Scholar]
- 13.Haynes BF, Verkoczy L. Host controls of HIV neutralizing antibodies. Science. 2014;344:588–89. doi: 10.1126/science.1254990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verkoczy L, Chen Y, Zhang J, et al. Induction of HIV-1 broad neutralizing antibodies in 2F5 knock-in mice: selection against membrane proximal external region–associated autoreactivity limits T-dependent responses. J Immunol. 2013;191:2538–50. doi: 10.4049/jimmunol.1300971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montefiori DC, Mascola JR. Neutralizing antibodies against HIV-1: can we elicit them with vaccines and how much do we need? Curr Opin HIV AIDS. 2009;4:347–51. doi: 10.1097/COH.0b013e32832f4a4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammer SM, Sobieszczyk ME, Janes H, et al. Efficacy Trial of a DNA/rAd5 HIV-1 Preventive Vaccine. N Engl J Med. 2013;369:2083–92. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33:542–54. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev. 2013;254:225–44. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Kang BH, Pancera M, et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515:138–42. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falkowska E, Le KM, Ramos A, et al. Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity. 2014;40:657–68. doi: 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scharf L, Scheid JF, Lee JH, et al. Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike. Cell Reports. 2014;7:785–95. doi: 10.1016/j.celrep.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blattner C, Lee JH, Sliepen K, et al. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity. 2014;40:669–80. doi: 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–8. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 25.Johnson WE, Desrosiers RC. Viral persistence: HIV’s strategies of immune system evasion. Annu Rev Med. 2002;53:499–518. doi: 10.1146/annurev.med.53.082901.104053. [DOI] [PubMed] [Google Scholar]
- 26.Liao HX, Lynch R, Zhou T, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–76. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 28••.Kong L, Lee JH, Doores KJ, et al. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat Struct Mol Biol. 2013;20:796–803. doi: 10.1038/nsmb.2594. Reports the crystal structure of PGT135 binding a cluster of N-glycans centered on Asn332 and a peptide segment within the V3 domain. A combined structural analysis of other antibodies of the PGT family revealed a supersite of vulnerability around Asn332 for immune recognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sok D, Doores KJ, Briney B, et al. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci Transl Med. 2014;6:236ra63. doi: 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Garces F, Sok D, Kong L, et al. Structural evolution of glycan recognition by a family of potent HIV antibodies. Cell. 2014;159:69–79. doi: 10.1016/j.cell.2014.09.009. This paper describes the co-crystal structure of PGT124 Fab in complex with a mini-V3 loop of gp120 revealing the fine structural specificities of the binding event. It also investigates the structural evolution of the PGT class of BnAbs towards the high-affinity recognition of complex epitopes involving glycan and peptide domains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doores KJ, Bonomelli C, Harvey DJ, et al. Envelope glycans of immunodeficiency virions are entirely oligomannose antigens. Proc Natl Acad Sci USA. 2010;107:13800–13805. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu X, Borchers C, Bienstock RJ, Tomer KB. Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochemistry. 2000;39:11194–204. doi: 10.1021/bi000432m. [DOI] [PubMed] [Google Scholar]
- 33.Go EP, Hewawasam G, Liao HX, et al. Characterization of glycosylation profiles of HIV-1 transmitted/founder envelopes by mass spectrometry. J Virol. 2011;85:8270–84. doi: 10.1128/JVI.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go EP, Liao HX, Alam SM, et al. Characterization of host-cell line specific glycosylation profiles of early transmitted/founder HIV 1 gp120 envelope proteins. J Proteome Res. 2013;12:1223–34. doi: 10.1021/pr300870t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonomelli C, Doores KJ, Dunlop DC, et al. The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade. PLoS ONE. 2011;6:e23521. doi: 10.1371/journal.pone.0023521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trkola A, Purtschner M, Muster T, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–08. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hessell AJ, Rakasz EG, Poignard P, et al. Broadly Neutralizing Human Anti-HIV Antibody 2G12 Is Effective in Protection against Mucosal SHIV Challenge Even at Low Serum Neutralizing Titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders RW, Venturi M, Schiffner L, et al. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol. 2002;76:7293–305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scanlan CN, Pantophlet R, Wormald MR, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1→2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–21. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Calarese DA, Scanlan CN, Zwick MB, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–71. doi: 10.1126/science.1083182. Describes the crystal structure study of 2G12 in complex with Man9GlcNAc2 and characterizes the molecular basis of the recognition revealing an unusual domain-exchanged structure for 2G12. [DOI] [PubMed] [Google Scholar]
- 41.Wang LX, Ni J, Singh S, Li H. Binding of high-mannose-type oligosaccharides and synthetic oligomannose clusters to human antibody 2G12: implications for HIV-1 vaccine design. Chem Biol. 2004;11:127–34. doi: 10.1016/j.chembiol.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Lee HK, Scanlan CN, Huang CY, et al. Reactivity-based one-pot synthesis of oligomannoses: defining antigens recognized by 2G12, a broadly neutralizing anti-HIV-1 antibody. Angew Chem Int Ed. 2004;43:1000–03. doi: 10.1002/anie.200353105. [DOI] [PubMed] [Google Scholar]
- 43.Adams EW, Ratner DM, Bokesch HR, et al. Oligosaccharide and glycoprotein microarrays as tools in HIV glycobiology; glycan-dependent gp120/protein interactions. Chem Biol. 2004;11:875–81. doi: 10.1016/j.chembiol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Calarese DA, Lee HK, Huang CY, et al. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci USA. 2005;102:13372–77. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandal M, Dudkin VY, Geng X, Danishefsky SJ. In pursuit of carbohydrate-based HIV vaccines, part 1: the total synthesis of hybrid-type gp120 fragments. Angew Chem Int Ed. 2004;43:2557–61. doi: 10.1002/anie.200353625. [DOI] [PubMed] [Google Scholar]
- 46.Geng X, Dudkin VY, Mandal M, Danishefsky SJ. In pursuit of carbohydrate-based HIV vaccines, part 2: the total synthesis of high-mannose-type gp120 fragments–evaluation of strategies directed to maximal convergence. Angew Chem Int Ed. 2004;43:2562–65. doi: 10.1002/anie.200353626. [DOI] [PubMed] [Google Scholar]
- 47.Likhosherstov LM, Novikova OS, Derevitskaja VA, Kochetkov NK. A new simple synthesis of amino sugar β-D-glycosylamines. Carbohydr Res. 1986;146:C1–C6. [Google Scholar]
- 48.Cohen-Anisfeld ST, Lansbury PT., Jr A practical, convergent method for glycopeptide synthesis. J Am Chem Soc. 1993;115:10531–37. [Google Scholar]
- 49•.Dudkin V, Orlova M, Geng X, et al. Toward fully synthetic carbohydrate-based HIV antigen design: on the critical role of bivalency. J Am Chem Soc. 2004;126:9560–9562. doi: 10.1021/ja047720g. Demonstrates that hybrid-type N-glycans are not recognized by 2G12, and that a bivalent glycopeptide dimer bearing high-mannose glycans binds to 2G12 with higher affinity than the corresponding monomer, demonstrating the existence of a clustering effect for binding to 2G12. [DOI] [PubMed] [Google Scholar]
- 50•.Krauss IJ, Joyce JG, Finnefrock AC, et al. Fully synthetic carbohydrate HIV antigens designed on the logic of the 2G12 antibody. J Am Chem Soc. 2007;129:11042–44. doi: 10.1021/ja074804r. Describes the synthesis and antigenicity studies of a rationally designed, scaffolded high-mannose cluster that serves as a 2G12 epitope mimic. [DOI] [PubMed] [Google Scholar]
- 51.Joyce JG, Krauss IJ, Song HC, et al. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proc Natl Acad Sci USA. 2008;105:15684–89. doi: 10.1073/pnas.0807837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, Wang LX. Design and synthesis of a template-assembled oligomannose cluster as an epitope mimic for human HIV-neutralizing antibody 2G12. Org Biomol Chem. 2004;2:483–8. doi: 10.1039/b314565d. [DOI] [PubMed] [Google Scholar]
- 53.Ni J, Song H, Wang Y, Stamatos NM, Wang LX. Toward a carbohydrate-based HIV-1 vaccine: synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjug Chem. 2006;17:493–500. doi: 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Li H, Zou G, Wang LX. Novel template-assembled oligosaccharide clusters as epitope mimics for HIV-neutralizing antibody 2G12. Design, synthesis, and antibody binding study. Org Biomol Chem. 2007;5:1529–40. doi: 10.1039/b702961f. [DOI] [PubMed] [Google Scholar]
- 55.Wang SK, Liang PH, Astronomo RD, et al. Targeting the carbohydrates on HIV-1: Interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. Proc Natl Acad Sci USA. 2008;105:3690–95. doi: 10.1073/pnas.0712326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Astronomo RD, Lee HK, Scanlan CN, et al. A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J Virol. 2008;82:6359–68. doi: 10.1128/JVI.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Astronomo RD, Kaltgrad E, Udit AK, et al. Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chem Biol. 2010;17:357–70. doi: 10.1016/j.chembiol.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agrawal-Gamse C, Luallen RJ, Liu B, et al. Yeast-elicited cross-reactive antibodies to HIV Env glycans efficiently neutralize virions expressing exclusively high-mannose N-linked glycans. J Virol. 2011;85:470–80. doi: 10.1128/JVI.01349-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Doores KJ, Fulton Z, Hong V, et al. A nonself sugar mimic of the HIV glycan shield shows enhanced antigenicity. Proc Natl Acad Sci USA. 2010;107:17107–12. doi: 10.1073/pnas.1002717107. Reports the design, synthesis and immunization studies of an unnatural sugar mimic of the 2G12 epitope with increased binding affinity and immunogenicity than the self glycan structure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Walker LM, Phogat SK, Chan-Hui P-Y, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–89. doi: 10.1126/science.1178746. Describes the isolation of PG9 and PG16 broadly neutralizing antibodies and the discovery that they target glycan-dependent epitopes on conserved regions of the variable loops of gp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J Virol. 2010;84:10510–21. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonsignori M, Hwang K-K, Chen X, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.McLellan JS, Pancera M, Carrico C, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–43. doi: 10.1038/nature10696. This paper provides the first crystal structure of PG9 in complex with a scaffolded V1V2 domain, and reveals a new paradigm of antibody-antigen recognition whereby PG9 binds a conserved glycopeptide epitope consisting of two glycans and a strand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Aussedat B, Vohra Y, Park PK, et al. Chemical synthesis of highly congested gp120 V1V2 N-glycopeptide antigens for potential HIV-1-directed vaccines. J Am Chem Soc. 2013;135:13113–20. doi: 10.1021/ja405990z. Reports a convergent chemical synthesis of V1V2 N-glycopeptides bearing closely spaced high-mannose glycans, that were able to bind PG9 with surprisingly high affinities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang P, Aussedat B, Vohra Y, Danishefsky SJ. An advance in the chemical synthesis of homogeneous N-linked glycopolypeptides by convergent aspartylation. Angew Chem Int Ed. 2012;51:11571–75. doi: 10.1002/anie.201205038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Alam SM, Dennison SM, Aussedat B, et al. Recognition of synthetic glycopeptides by HIV-1 broadly neutralizing antibodies and their unmutated ancestors. Proc Natl Acad Sci USA. 2013;110:18214–19. doi: 10.1073/pnas.1317855110. This paper characterizes the antigenic properties of synthetic homogeneous V1V2 glycopeptides capable of binding to V1V2 BnAbs and to their unmutated common ancestors with nanomolar affinity, while binding minimally to strain-specific vaccine-induced mAb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao HX, Bonsignori M, Alam SM, et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–86. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wan Q, Danishefsky SJ. Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew Chem Int Ed. 2007;46:9248–52. doi: 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]
- 69.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol. 2012;30:423–33. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adam SM, Liao HX, Tomaras GD, et al. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J Virol. 2013;87:1554–68. doi: 10.1128/JVI.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Julien JP, Lee JH, Cupo A, et al. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc Natl Acad Sci USA. 2013;110:4351–56. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Souza MS, Ratto-Kim S, Chuenarom W, et al. The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol. 2012;188:5166–76. doi: 10.4049/jimmunol.1102756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Amin MN, McLellan JS, Huang W, et al. Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies. Nat Chem Biol. 2013;9:521–526. doi: 10.1038/nchembio.1288. This article describes the chemoenzymatic synthesis and binding studies of homogeneous V1V2 glycopeptides and defines the glycan specificities of PG9 and PG16 antibodies, providing valuable information for HIV vaccine design. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Pancera M, Shahzad-Ul-Hussan S, Doria-Rose NA, et al. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1–V2-directed antibody PG16. Nat Struct Mol Biol. 2013;20:804–13. doi: 10.1038/nsmb.2600. A report of the crystal structure of antibody PG16 in complex with a sialylated V1V2 domain reveals an enhanced recognition of complex-type N-linked glycans at the second glycosylation site, suggesting that there may be a greater promiscuity with regard to glycan recognition at this site by PG16 and PG9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shivatare SS, Chang SH, Tsai TI, et al. Efficient convergent synthesis of bi-, tri-, and tetra-antennary complex type N-glycans and their HIV-1 antigenicity. J Am Chem Soc. 2013;135:15382–91. doi: 10.1021/ja409097c. [DOI] [PubMed] [Google Scholar]
- 76••.Walker LM, Huber M, Doores KJ, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–70. doi: 10.1038/nature10373. This article describes the identification of a new group of glycan-dependent broadly neutralizing antibodies corresponding to the PGT class that neutralize HIV-1 with exceptional breadth and potency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77••.Pejchal R, Doores KJ, Walker LM, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–103. doi: 10.1126/science.1213256. The crystal structure of PGT128 in complex with a glycosylated gp120 domain is reported, revealing that the antibody recognizes two conserved N-glycans and a short peptide segment of the gp120 V3 loop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zolla-Pazner S. Identifying epitopes of HIV-1 that induce protective antibodies. Nat Rev Immunol. 2004;4:199–210. doi: 10.1038/nri1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li H, Li B, Song H, et al. Chemoenzymatic synthesis of HIV-1 V3 glycopeptides carrying two N-glycans and effects of glycosylation on the peptide domain. J Org Chem. 2005;70:9990–96. doi: 10.1021/jo051729z. [DOI] [PubMed] [Google Scholar]
- 80.Orwenyo J, Amin MN, Lomino JV, Wang LX. Synthesis of homogeneous HIV-1 V3 glycopeptides for characterizing the glycan specificity of glycan-dependent HIV-neutralizing antibodies. CARB-64. Abstracts of Papers, 247th National Meeting of the American Chemical Society; Dallas, TX. March 16–20. [Google Scholar]
- 81.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol. 2012;30:423–33. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]