Abstract
Background and Purpose
Non-small cell lung cancer (NSCLC) tumours are mostly heterogeneous. We hypothesized that areas within the tumour with a high pre-radiation 18F-deoxyglucose (FDG) uptake, could identify residual metabolic-active areas, ultimately enabling selective-boosting of tumour sub-volumes.
Material and Methods
Fifty-five patients with inoperable stage I-III NSCLC treated with chemo-radiation or with radiotherapy alone were included. For each patient one pre-radiotherapy and one post-radiotherapy FDG-PET-CT scans was available. Twenty-two patients showing persistent FDG-uptake in the primary tumour after radiotherapy were analyzed. Overlap-fractions (OF) were calculated between standardized uptake value (SUV) threshold-based auto-delineations on the pre- and post-radiotherapy scan.
Results
Patients with residual metabolic-active areas within the tumour had a significantly worse survival compared to individuals with a complete metabolic response (p=0.002). The residual metabolic-active areas within the tumour largely corresponded (OF>70%) with the 50%SUV high FDG-uptake area of the pre-radiotherapy scan. The hotspot within the residual area (90%SUV) was completely within the GTV (OF=100%), and had a high overlap with the pre-radiotherapy 50%SUV threshold (OF>84%).
Conclusions
The location of residual metabolic-active areas within the primary tumour after therapy corresponded with the original high FDG-uptake areas pre-radiotherapy. Therefore, a single pre-treatment FDG-PET-CT scan allows for the identification of residual metabolic-active areas.
Keywords: Intra-tumour heterogeneity, Residual metabolic-activity, Radio-resistance, Non-small cell lung cancer, PET-CT
INTRODUCTION
Lung cancer still remains one of the most frequent and lethal solid tumours [1]. Although the prognosis has improved, in locally advanced non-small cell lung cancer (NSCLC), the 5-year survival is 20 % at best [2–7]. As the improved long-term survival with concurrent chemo-radiation compared to sequential chemotherapy and radiotherapy is due to improved local tumour control, strategies to increase local tumour control are warranted [6–8]. Local tumour failure is indeed still observed in about 70 % of patients [5–7].
An important strategy to improve local tumour control is to escalate the radiation dose, because a higher dose has been shown to yield a higher local control rate [9–11]. However, radiation dose increase is limited by the toxicity of radiation to normal tissues such as the lungs and the spinal cord. Although accelerated, high-dose schedules based on normal tissue constraints have shown promising results [12–14], innovative strategies are needed to be able to deliver doses up to 120 Gy, which are needed to obtain local tumour control rates over 90 % [15]. A possibility to achieve this goal would be to take advantage of intra-tumour heterogeneity. It has become increasingly clear, that a tumour is heterogeneous for varying characteristics, possibly also for radio-resistance [16, 17]. Indeed, molecular imaging studies showed significant differences in perfusion, hypoxia, cell density and proliferation within the tumour [18–22]. As the tumour is thus probably composed of areas with different radio-resistance, a strategy to deliver a non-uniform dose-distribution seems logical. It appears to be a way to design volumetric maps of radio-resistance using molecular imaging and to redistribute the radiation dose according to this. More resistant areas within the tumour could thus receive higher doses whilst reducing the dose to more susceptible zones with the same normal tissue exposure [18].
18F-deoxyglucose (FDG) is a commonly used marker in oncology for the assessment of glucose metabolism [23]. FDG uptake in the primary tumour before treatment is prognostic for survival in patients with NSCLC, both treated with surgery and radiotherapy [24–28]. The metabolic response of patients after radiotherapy or chemo-radiation is correlated with survival [29]. In a pre-clinical investigation an increase of radiation dose showed a higher local control for tumours with higher FDG uptake compared to tumours with lower FDG uptake [17]. Therefore, we hypothesized that the high FDG uptake locations before treatment could prossibly identify more radioresistant areas within the tumour. If true, an increase survival could be expected from radiation dose redistribution according to FDG uptake pre-radiotherapy. In an earlier study, we demonstrated that for NSCLC, the high-uptake areas of FDG within the tumour remained stable during a course of fractionated radiotherapy for NSCLC [30], being a prerequisite for selective radiation boosting of these zones. In the present study, we investigated whether the high FDG uptake area within the primary tumour before treatment indeed identifies the location of the residual metabolic-active areas after treatment.
MATERIAL AND METHODS
Patient Characteristics
Fifty-five patients (16 women and 39 men) with inoperable non-small cell lung cancer (NSCLC), UICC stage I-III, treated with radical radiotherapy (RT) alone (11 patients) or with sequential chemo-radiotherapy (44 patients) were studied as part of two phase II trials (NCT00573040, NCT00572325). No concurrent chemo-radiotherapy was given. The sequential chemo-radiotherapy schedule consisted of 3 cycles of carboplatin or cisplatin and gemcitabine before the start of RT. Patients were included from January 2005 until February 2007. Mean age was 65.4 years (range: 44–83 years). The Medical Ethics Committee according to the Dutch law approved the trial. All patients gave written informed consent before entering the studies.
Two FDG-PET-CT scans were available for each patient. The first scan was performed on average 12.8 days (range: 1–56 days) before start of RT while the second scan was performed 86.1 days (range: 49–184 days) after the end of RT. For the patients receiving sequential chemo-radiotherapy was the pre-RT scan performed after the chemotherapy. No treatment was given to any of the patients between the end of RT and the post-RT scan.
Radiotherapy Simulation
Patients were simulated in radiotherapy position on a dedicated PET-CT-simulator with both arms above the head. For the FDG-PET-CT scans a Siemens Biograph (SOMATOM Sensation-16 with an ECAT ACCEL PET scanner) was used.
An intravenous injection of (weight * 4 + 20) MBq FDG (Tyco Health Care, Amsterdam, The Netherlands) was followed by 10 ml physiologic saline. After a 45 minutes uptake period, during which the patient was encouraged to rest, PET and CT images were acquired. A spiral CT (3 mm slice thickness) with intravenous contrast was performed covering the complete thoracic region.
Radiotherapy Planning
Radiotherapy planning was performed on a XiO (Computerized Medical Systems, St Louis, Missouri) treatment planning system, based on a convolution algorithm using inhomogeneity corrections. The Gross Tumour Volume (GTV) and the Planning Target Volume (PTV) were defined for all patients, based on PET-CT data [13]. The Clinical Target Volume (CTV) was defined as the GTV with a 5 mm margin incorporating microscopic disease. Subsequently, this CTV was expanded with 1 cm to draw the PTV to incorporate the internal respiratory motion and setup errors. Contouring of the lungs was carried out automatically by the treatment planning system. The volume of both lungs excluding the GTV was used for the calculation of the mean lung dose (MLD). The spinal cord was drawn throughout the whole CT scan, considered to be at the inner margin of the bony spinal canal. A 3D conformal treatment plan was calculated on the PTV for all patients according to ICRU 50 guidelines [31]. Dosimetric values were calculated on the basis of dose-volume histograms and dose distributions on each axial CT plan.
For each patient, the radiation dose was escalated to an individualized maximal total tumour dose, applying an MLD of 19 Gy while respecting a maximum spinal cord dose of 54 Gy [14]. The maximal total tumour dose allowed was 79.2 Gy. There were no esophageal dose constraints. Radiotherapy was delivered twice a day, 5 days per week, with a minimum of 8 hours between the two fractions. In all patients, individualized patient dosimetry using electronic portal imaging devices was performed [32].
Image Analysis
The pre- and post-RT scans were analyzed and delineated using the Siemens TrueD system (Version VC-30, Siemens A.G., Darmstad, Germany). Descriptively, a schematic representation of this methodology is shown in Figure 1. The location and volume of the FDG uptake areas pre-RT were quantified within the primary tumour using the threshold 34, 40, 50, 60 and 70% of the maximal SUV (SUVmax). Residual metabolic areas were defined as FDG uptake higher than in the aortic arch (SUV>SUVaorta) [29]. Within the residual FDG-positive areas on the post-RT scan, the high FDG uptake areas were defined using the thresholds 70, 80, and 90% SUVmax. Also the fixed thresholds, SUV 2.5 and SUV 5.0, of the residual disease were delineated on the post-RT scan (not illustrated). Using an automatic rigid registration algorithm based on mutual information of the CT scans, the images of pre-RT scan were fused to the post-RT scan on the Siemens TrueD system. If the automatic registration showed a large deformation between the two CT scans, the images were manually registered on the surrounding anatomy of the tumour, e.g. the bony anatomy or great vessels. The contour delineations on the post-RT scan were then transformed to the pre-RT scan using the derived registration matrices. The contour delineations were exported from TrueD as DICOM-RT structure sets. Using MATLAB 7.1 SP3 (The MathWorks Inc, Natick, MA, USA) the overlap fractions (OF) and volumes of these FDG based delineations were calculated. The overlap fraction was defined as the volume of overlap divided by the smallest volume [30]. See figure 1 for a schematic representation of this calculation. By using this method it is possible to assess which threshold on the pre-RT scan matches the residual disease on the post-RT scan.
Figure 1.
Schematic representation of the overlap-fractions (OF) quantification of FDG-uptake pre-radiotherapy with residual metabolic-active areas post-radiotherapy. Within the pre-radiotherapy GTV (defined with the knowledge of the FDG-PET-CT scan), the FDG-PET uptake areas were quantified (34–70%SUVmax). Within the residual areas, the high FDG-uptake areas (70–90%SUVmax) were quantified. As an example, the OF of the 50%SUVmax FDG high-uptake zone pre-radiotherapy with the residue was illustrated (OF about 70%).
Statistical Analysis
All data are expressed as mean ± 95% confidence intervals (95% CI). Statistical differences between the parameters were evaluated in SPSS (Version 15.0 for Windows, Chicago, IL), using the Mann-Whitney U test. Differences were considered to be significant when the p-value was less than 0.05. A power calculation was performed to assess the power of the patient group for the metabolic-active areas located at the FDG hotspot vs. at random [33]. The overall survival of the patients and the 95% CI were calculated using the Kaplan-Meier method. Differences between the groups were assessed using the log-rank test. The Cox regression method was used to estimate the hazard ratio.
RESULTS
Patient Characteristics
To assess the location of residual metabolic active areas compared with initial FDG uptake in the primary tumour, two 18Fluorodeoxyglucose (FDG) PET-CT scans were analyzed for all patients, one before radiotherapy (pre-RT) and one after radiotherapy (post-RT). Of all 55 patients, 28 showed metabolic active areas with residual FDG uptake in the proximity or within the primary tumour on the post-RT scan. The other 27 patients had a complete metabolic response, showing no residual metabolic activity in the primary tumour. Both patients with and without residual areas showed heterogeneous FDG uptake patterns within the macroscopic tumour before treatment, where the high uptake area was not necessarily in the center of the tumour. The maximum FDG uptake on the pre-RT scan for patients with residual areas was significantly higher than for patients with a complete metabolic response (SUVmax= 9.6 [95%CI: 8.0–11.2] and 7.1 [95%CI: 5.6–8.7] respectively; p = 0.029). Also the GTV volume was significantly higher for patients showing residual areas (with residual areas: 117.3 cm3 [95%CI: 70.3–164.3 cm3], without residual areas: 54.7 cm3 [95%CI: 38.7–70.6 cm3]; p = 0.003). The total tumour dose was not different (p = 0.547) from the patients without residual disease (65±8.6 Gy), compared to the patients with residual disease (64.3±10.9 Gy). Also the overall treatment time was similar for the patients without residual disease (26.5±5.8 days) compared to the patients with residual disease (24.8±4.7 days) (p = 0.326). Concordant with literature [29], with a median follow-up of 29.9 months, patients with residual areas had a worse survival than individuals with a complete metabolic response (12.2 months vs. median survival not reached; hazard ratio for death: 2.94 [95%CI: 1.44–5.99], p = 0.002; Figure 2).
Figure 2.
Kaplan-Meier estimates of overall survival of all 55 patients with residual metabolic active areas (n=28) and without residual areas (n=27) on the post-radiotherapy FDG-PET-CT scan. The hazard ratio for death for patients with residual areas compared to individuals without was 2.94 (95% confidence interval: 1.44 to 5.99; p=0.002 by the log-rank test, two-sided).
Twenty-two out of the 28 patients remained for further analysis. Three patients were excluded because the residue was not clearly distinguishable from the surrounding tissue due to FDG avid inflammation, pneumonitis or to the vicinity of the heart. In addition two patients were excluded due to large deformation of anatomical structures between the pre- and post-RT scans, because no reliable registration could be performed (determined by two independent observers). One patient with progressive disease was excluded because the residual disease was larger than the primary tumour, excluding reliable calculation of overlap fractions. The characteristics of these patients are shown in Table 1.
Table 1.
Patient characteristics of the 22 evaluated patients with residual metabolic active areas after treatment.
| Patient | Age | Gender | Stage | TTD (Gy) | OTT (days) | Time pre-scan to start RT (days) | Time end RT to post scan (days) | RT or sequential CT-RT | Response | GTV volume (cm3) | SUVmax Pre-RT | SUVmax Post-RT | SUV aorta Post-RT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | M | T3N2M0 | 55.9 | 22.0 | 9 | 96 | seq CT-RT | SMD | 152.7 | 6.68 | 5.77 | 3.53 |
| 2 | 72 | M | T2N3M0 | 72.0 | 28.0 | 7 | 64 | seq CT-RT | PMR | 72.4 | 8.80 | 3.50 | 2.03 |
| 3 | 54 | M | T2N3M0 | 79.2 | 30.0 | 7 | 49 | seq CT-RT | PMR | 77.0 | 10.31 | 5.35 | 3.25 |
| 4 | 64 | M | T4N0M0 | 79.2 | 20.0 | 12 | 76 | seq CT-RT | PMR | 29.0 | 5.50 | 3.60 | 2.90 |
| 5 | 61 | F | T2N2M0 | 46.8 | 17.0 | 8 | 89 | seq CT-RT | PMR | 272.2 | 7.70 | 4.10 | 2.54 |
| 6 | 72 | M | T3N2M1 | 70.0 | 27.0 | 28 | 122 | seq CT-RT | PMR | 86.8 | 15.65 | 5.78 | 2.45 |
| 7 | 83 | M | T2N0M0 | 79.2 | 32.0 | 8 | 102 | RT | PMR | 122.3 | 10.61 | 7.39 | 3.26 |
| 8 | 79 | M | T2N2M0 | 72.0 | 28.0 | 12 | 92 | seq CT-RT | PMR | 55.4 | 10.40 | 6.70 | 1.84 |
| 9 | 83 | M | T2N1M0 | 59.4 | 22.0 | 8 | 106 | RT | SMD | 87.2 | 9.63 | 11.94 | 2.96 |
| 10 | 58 | F | T3N2M0 | 54.4 | 20.0 | 14 | 77 | seq CT-RT | SMD | 103.8 | 18.69 | 17.37 | 3.67 |
| 11 | 63 | M | T2N0M0 | 54.1 | 21.0 | 56 | 88 | RT | PMR | 26.4 | 8.41 | 4.14 | 2.78 |
| 12 | 44 | F | T2N2M0 | 75.6 | 30.0 | 8 | 112 | seq CT-RT | SMD | 20.0 | 4.50 | 4.20 | 3.40 |
| 13 | 77 | M | T4N0M0 | 54.0 | 25.0 | 12 | 99 | seq CT-RT | PMR | 125.0 | 19.70 | 3.60 | 2.50 |
| 14 | 63 | F | T2N2M0 | 57.6 | 21.0 | 13 | 76 | seq CT-RT | SMD | 130.6 | 11.80 | 13.90 | 2.65 |
| 15 | 80 | M | T2N1M0 | 54.0 | 22.0 | 41 | 70 | RT | SMD | 138.8 | 8.54 | 7.43 | 2.85 |
| 16 | 79 | F | T4N0M0 | 57.6 | 24.0 | 5 | 69 | seq CT-RT | PMR | 19.0 | 8.50 | 5.80 | 2.95 |
| 17 | 69 | M | T2N2M0 | 63.0 | 25.0 | 9 | 86 | seq CT-RT | SMD | 38.6 | 11.91 | 11.91 | 3.65 |
| 18 | 53 | M | T2N2M0 | 54.0 | 22.0 | 8 | 82 | seq CT-RT | PMR | 128.0 | 15.90 | 8.90 | 2.88 |
| 19 | 69 | M | T1N2M0 | 54.0 | 18.0 | 10 | 131 | seq CT-RT | PMR | 48.3 | 16.02 | 7.60 | 3.10 |
| 20 | 56 | M | T4N2M0 | 79.2 | 30.0 | 6 | 83 | seq CT-RT | PMR | 102.2 | 8.40 | 3.90 | 1.75 |
| 21 | 73 | M | T4N2M0 | 79.2 | 32.0 | 7 | 66 | seq CT-RT | PMR | 148.0 | 4.30 | 3.30 | 2.90 |
| 22 | 50 | F | T4N2M0 | 56.9 | 21.0 | 13 | 71 | seq CT-RT | PMR | 120.5 | 9.20 | 6.10 | 3.65 |
|
| |||||||||||||
| Average: 66.2 | 64.0 | 24.4 | 13.7 | 86.6 | 95.6 | 10.5 | 6.9 | 2.9 | |||||
| Std: 11.5 | 11.0 | 4.6 | 12.4 | 19.9 | 58.8 | 4.3 | 3.8 | 0.6 | |||||
| min: 44 | 46.8 | 17.0 | 5 | 49.0 | 19.0 | 4.3 | 3.3 | 1.8 | |||||
| max: 83 | 79.2 | 32.0 | 56 | 131.0 | 272.2 | 19.7 | 17.4 | 3.7 | |||||
RT: radiotherapy; M: male; F: female; TTD: total tumour dose; OTT: overall treatment time of radiotherapy; post scan: FDG-PET-CT scan after the end of radiotherapy; RT: radiotherapy alone; seq CT-RT: sequential chemotherapy and radiotherapy; Response (EORTC criteria): SMD: stable metabolic disease PMR: partial metabolic response; GTV: gross tumour volume of the primary tumour.
Overlap fractions between FDG-PET-CT scans pre- and post-radiotherapy
In Figure 3, representative images are shown of three typical patients with a large homogeneous tumour (Patient 1), a large heterogeneous tumour (Patient 2) and a small tumour (Patient 3). The location of the residual areas (SUV>SUVaorta) on the post-RT scan and the high FDG uptake areas (50% SUVmax) on the pre-RT scan are shown. The residual areas are transposed to the pre-RT scan, to show the overlap with 50% SUVmax high uptake area pre-RT. Visual evaluation shows that the location of the residual areas largely corresponds with the high FDG uptake areas pre-RT.
Figure 3.
Representative FDG-PET-CT images of 3 patients pre- and post-radiotherapy. The green lines indicate the 50%SUVmax FDG high uptake area pre-radiotherapy. The blue lines indicate the residual metabolic active areas post-radiotherapy, also transposed on the pre-radiotherapy scan. Visual evaluation shows a large correspondence between the residual areas post-radiotherapy with the high FDG uptake areas pre-radiotherapy.
Volumes of the FDG based thresholds
The volumes of the FDG based thresholds of the tumour pre- and post-RT are shown in Figure 4. The high FDG uptake areas (50–70% SUVmax) within the tumour on the pre-RT scan were small compared to the GTV volume. The 50% SUVmax encompassed 39.0% [95%CI: 31.9–46.0%] of the original GTV, whereas this was 24.9% [95%CI: 20.4–29.4%] for the 60% SUVmax, and 13.7% [95%CI: 11.0–16.3%] for the 70% SUVmax threshold (Fig. 4A).
Figure 4.
Volumes of the SUV thresholds of the tumour pre-radiotherapy (A) and post-radiotherapy (B). All volumes (A and B) are relative to the pre-radiotherapy gross tumour volume (GTV). The data are expressed as mean ± 95% confidence intervals (error-bars). Note that the volume of residual metabolic active areas was on average 22% of the GTV volume.
The volume of the residual metabolic active areas on the post-RT scan was 21.7% [95%CI: 15.1–28.3%] of the pre-RT GTV volume (Fig. 4B). The relative volumes of the high uptake areas within the residual areas were very small: 7.8% [95%CI: 4.4–11.2%] for the 70% SUVmax, 2.5% [95%CI: 1.5–3.5%] for the 80% SUVmax, and 0.8% [95%CI: 0.4–1.3%] for the 90% SUVmax threshold. Also the absolute thresholds of the residual areas had a relative small volume: 31.5% [95%CI: 22.4–40.6%] and 7.0% [95%CI: 3.0–11.0%] for SUV2.5 and SUV5 respectively.
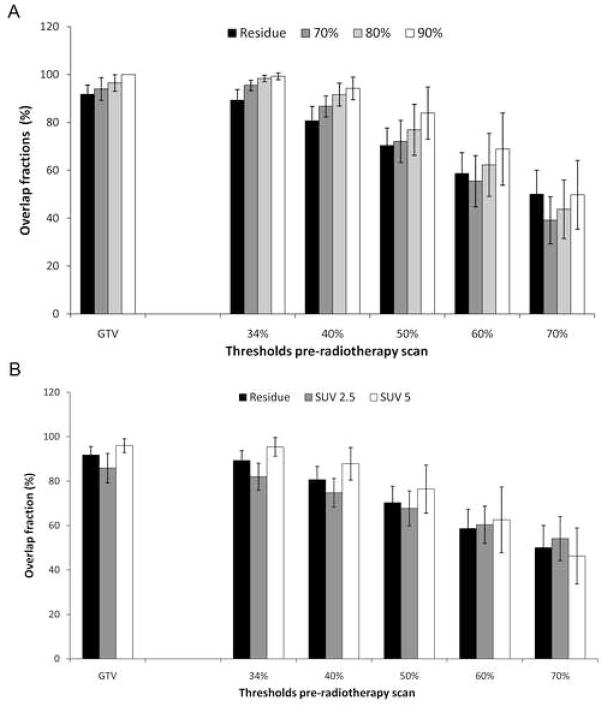
Overlap Fractions
Figure 5 depicts the overlap fractions of the FDG uptake within the primary tumour pre-RT with the post-RT thresholds. The residual areas were mainly located within the original GTV (OF = 91.8%[95%CI: 87.9–95.6%]) (Fig. 5A). The 70% and 80% SUVmax high-uptake areas within the residual areas were also mainly located within the GTV (OF = 94.0% [95%CI: 89.2–98.7%] and 96.5% [95%CI: 93.1–100.0%] respectively). Whereas, the 90% SUVmax high-uptake areas were all completely located within the GTV (OF = 100%). Comparing the pre-RT FDG uptake with the residual areas, the 34% threshold pre-RT had a large overlap fraction with the residual areas (89.3% [95%CI: 84.8–93.8%]) and with the high-uptake areas within the residue. Moreover, the same is true for the pre-RT 50% SUVmax high FDG uptake area (OF = 70.4% [95%CI: 63.0–77.7%]). This pre-RT 50% SUVmax area also largely corresponded with the 70–90% SUVmax high-uptake areas within the residue. The 22 analyzable patients resulted in a power of over 0.9 (with alpha = 0.01, H0: 30% of patients with residue randomly located at FDG hotspot vs H1: 70% in case of non-random location of residue).
Figure 5.
Overlap-fractions (OF) of the pre-radiotherapy with the post-radiotherapy relative (A) and absolute (B) SUVmax thresholds are shown. OF s of the post-radiotherapy residual-areas are indicated with the black-bars. The other bars indicate the OF with the high FDG-uptake areas within the residue (A) and absolute thresholds (B). The data are expressed as mean ± 95% confidence-intervals.
The extent of the GTV corresponded largely with the absolute SUV2.5 (OF = 85.9% [95%CI: 79.2–92.5%]) and the SUV5 (OF = 96.0% [95%CI: 92.9–99.1%]) threshold on the post-RT scan (Fig. 5B). Also the 34% threshold pre-RT largely corresponded with the SUV2.5 and SUV5 thresholds post-RT (OF = 82.0% [95%CI: 75.9–88.1%] and 95.4% [95%CI: 91.2–97.9%] respectively). The 50% pre-RT threshold had an OF of 67.8% [95%CI: 59.9–75.6%] with the SUV2.5 threshold and an OF of 76.4% [95%CI: 65.6–87.3%] with the SUV5 threshold.
DISCUSSION
There is a growing interest in radiation oncology to selectively target radio-resistant areas with a high probability of persisting tumour cells after treatment within the tumour [18, 34, 35]. Indeed, by selectively boosting radio-resistant areas whilst decreasing the dose to more susceptible zones, local tumour control rates could increase without increased side effects. The availability of molecular imaging techniques that enable visualization and quantification of areas with different characteristics within the tumour makes this a feasible strategy. FDG as a PET tracer is of particular interest in this respect because first, its maximal uptake in the tumour is prognostic for survival of patients with NSCLC, both treated with surgery or radiotherapy [24–28]. Second, our group has previously shown that the FDG uptake patterns within the tumour remain stable throughout a radiotherapy course [30], and third FDG is widely available.
We hypothesized that areas of high FDG uptake within the tumour before treatment would allow identification of residual metabolic-areas after therapy. Moreover, in most tumours, a heterogeneous FDG uptake pattern before treatment is observed, where the high FDG uptake areas were often not in the centre of the tumour. This opens the potential prospect for clinically relevant radiation dose redistribution within the tumour. However, at first it has to be established whether resistant areas within the tumour can be defined on the basis of pre-treatment imaging. As a first step, we investigated the patterns of residual metabolic areas (based on FDG uptake) within the primary tumour after high-dose radiotherapy, mostly preceded by chemotherapy. In 28 out of 55 patients, FDG persisted in the areas with a high uptake before radiotherapy. The residual areas were located almost completely (OF > 91%) within the GTV. The residual FDG positive areas largely corresponded (OF > 70%) with the 50% SUV high uptake area of the pre-RT scan. The average volume of this 50% SUV threshold was 39% of the original GTV volume. The volume of the residual FDG-positive areas was on average 22% of the GTV volume. The hotspot within the residual area (90% SUV) was completely within the GTV (OF=100%), and had a high overlap with the pre-radiotherapy 50% SUV threshold (OF>84%). On basis of these findings, we conclude that residual metabolic active areas within the tumour can be identified using a single pre-RT FDG-PET-CT scan. A quantitative voxel-based analysis between the metabolic state of a tumour voxel after treatment and pre-treatment parameters, such as dose and GTV volume, was analyzed by Petit et al. (ref other paper back-to-back submission).
Some limitations to our study should be addressed. First, of the 55 included patients, 28 had residual FDG uptake, of which 6 (21 %) had to be excluded because of progressive disease (1 patient), large tumour deformation (2 patients) or because FDG-uptake in the surrounding tissues made accurate delineation of the tumour area impossible (3 patients). Improvements in deformation analysis and better delineation methods are needed to include these patients in the analysis. Second, the 50% of SUVmax threshold value was found to be the most suitable amongst several other threshold levels. This threshold was not only chosen because it yielded good results, but also because it is a simple and reproducible method and the delineation software is available in clinical settings. A lower threshold would result in boosting the entire tumour and not only the most resistant areas, whereas a too high threshold would lead to only a few voxels to be treated, which is difficult to radiate with the equipment used currently. Third, our surrogate endpoint, persistent FDG uptake in the tumour, should not be regarded as equivalent to tumour persistence, nor does metabolic complete response equals cure. However, in agreement with literature [29], the present data show that survival of patients with persistent FDG uptake is significantly worse than those without, underlining its clinical validity. Fourth, for the image registration between the pre and post-scans a regid registration was performed, not incoorperating deformable tissue changes, possibly induced by the delivered therapy. This could be improved by using deformable registration techniques. However, these are difficult to validate and the reproducibility, especially in different institutes, is limited.
We show in clinical data that high FDG areas within the tumour before radiotherapy can identify areas with residual metabolic-activity, which are probably more radio-resistant. Due to the spatial resolution of the FDG-PET imaging, this could only be performed on a macroscopic scale, i.e. voxel level. However, carefully designed future trails should provide the basis to test the assumption if FDG uptake reflects radio-resistance, by boosting high FDG uptake areas. It obviously may be argued that the FDG uptake in the tumour does not reflect a single biological characteristic of the tumour, but is influenced by many pathways that are related to therapy-resistance [27]. Indeed, more specific tracers, such as 18F-misonidazol, may be useful for dose-painting within the tumour as well [36].
In conclusion, our results show that the residual metabolic-active areas within the tumour after radiotherapy of chemo-radiation, is located in the high FDG uptake areas before therapy and can be delineated. This will be the basis for new clinical studies with dose redistributions according to pre-radiotherapy FDG uptake.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Betticher DC, Hsu Schmitz SF, Totsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. British journal of cancer. 2006;94:1099–1106. doi: 10.1038/sj.bjc.6603075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. Journal of the National Cancer Institute. 2007;99:442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 4.Garrido P, Gonzalez-Larriba JL, Insa A, et al. Long-term survival associated with complete resection after induction chemotherapy in stage IIIA (N2) and IIIB (T4N0-1) non small-cell lung cancer patients: the Spanish Lung Cancer Group Trial 9901. J Clin Oncol. 2007;25:4736–4742. doi: 10.1200/JCO.2007.12.0014. [DOI] [PubMed] [Google Scholar]
- 5.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 6.Zatloukal P, Petruzelka L, Zemanova M, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung cancer (Amsterdam, Netherlands) 2004;46:87–98. doi: 10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 8.Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. The New England journal of medicine. 1992;326:524–530. doi: 10.1056/NEJM199202203260805. [DOI] [PubMed] [Google Scholar]
- 9.Bradley J. A review of radiation dose escalation trials for non-small cell lung cancer within the Radiation Therapy Oncology Group. Seminars in oncology. 2005;32:S111–113. doi: 10.1053/j.seminoncol.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Rojas AM, Lyn BE, Wilson EM, et al. Toxicity and outcome of a phase II trial of taxane-based neoadjuvant chemotherapy and 3-dimensional, conformal, accelerated radiotherapy in locally advanced nonsmall cell lung cancer. Cancer. 2006;107:1321–1330. doi: 10.1002/cncr.22123. [DOI] [PubMed] [Google Scholar]
- 11.Hayman JA, Martel MK, Ten Haken RK, et al. Dose escalation in non-small-cell lung cancer using three-dimensional conformal radiation therapy: update of a phase I trial. J Clin Oncol. 2001;19:127–136. doi: 10.1200/JCO.2001.19.1.127. [DOI] [PubMed] [Google Scholar]
- 12.Belderbos JS, Heemsbergen WD, De Jaeger K, Baas P, Lebesque JV. Final results of a Phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. International journal of radiation oncology, biology, physics. 2006;66:126–134. doi: 10.1016/j.ijrobp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 13.De Ruysscher D, Wanders R, van Haren E, et al. HI-CHART: a phase I/II study on the feasibility of high-dose continuous hyperfractionated accelerated radiotherapy in patients with inoperable non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2008;71:132–138. doi: 10.1016/j.ijrobp.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 14.van Baardwijk A, Bosmans G, Boersma L, et al. Individualized radical radiotherapy of non-small-cell lung cancer based on normal tissue dose constraints: a feasibility study. International journal of radiation oncology, biology, physics. 2008;71:1394–1401. doi: 10.1016/j.ijrobp.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 15.Fowler JF, Tome WA, Fenwick JD, Mehta MP. A challenge to traditional radiation oncology. International journal of radiation oncology, biology, physics. 2004;60:1241–1256. doi: 10.1016/j.ijrobp.2004.07.691. [DOI] [PubMed] [Google Scholar]
- 16.Cooper RA, Carrington BM, Loncaster JA, et al. Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol. 2000;57:53–59. doi: 10.1016/s0167-8140(00)00259-0. [DOI] [PubMed] [Google Scholar]
- 17.Schutze C, Bergmann R, Yaromina A, et al. Effect of increase of radiation dose on local control relates to pre-treatment FDG uptake in FaDu tumours in nude mice. Radiother Oncol. 2007;83:311–315. doi: 10.1016/j.radonc.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Bentzen SM. Theragnostic imaging for radiation oncology: dose-painting by numbers. The lancet oncology. 2005;6:112–117. doi: 10.1016/S1470-2045(05)01737-7. [DOI] [PubMed] [Google Scholar]
- 19.Vanderstraeten B, Duthoy W, De Gersem W, De Neve W, Thierens H. [18F]fluoro-deoxy-glucose positron emission tomography ([18F]FDG-PET) voxel intensity-based intensity-modulated radiation therapy (IMRT) for head and neck cancer. Radiother Oncol. 2006;79:249–258. doi: 10.1016/j.radonc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Tanderup K, Olsen DR, Grau C. Dose painting: art or science? Radiother Oncol. 2006;79:245–248. doi: 10.1016/j.radonc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Piert M, Machulla HJ, Picchio M, et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med. 2005;46:106–113. [PubMed] [Google Scholar]
- 22.Foo SS, Abbott DF, Lawrentschuk N, Scott AM. Functional imaging of intratumoral hypoxia. Mol Imaging Biol. 2004;6:291–305. doi: 10.1016/j.mibio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Phelps ME. Inaugural article: positron emission tomography provides molecular imaging of biological processes. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9226–9233. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borst GR, Belderbos JS, Boellaard R, et al. Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer. 2005;41:1533–1541. doi: 10.1016/j.ejca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Downey RJ, Akhurst T, Gonen M, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255–3260. doi: 10.1200/JCO.2004.11.109. [DOI] [PubMed] [Google Scholar]
- 26.Eschmann SM, Friedel G, Paulsen F, et al. Is standardised (18)F-FDG uptake value an outcome predictor in patients with stage III non-small cell lung cancer? European journal of nuclear medicine and molecular imaging. 2006;33:263–269. doi: 10.1007/s00259-005-1953-2. [DOI] [PubMed] [Google Scholar]
- 27.van Baardwijk A, Dooms C, van Suylen RJ, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007;43:1392–1398. doi: 10.1016/j.ejca.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Vansteenkiste JF, Stroobants SG, Dupont PJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: An analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol. 1999;17:3201–3206. doi: 10.1200/JCO.1999.17.10.3201. [DOI] [PubMed] [Google Scholar]
- 29.Mac Manus MP, Hicks RJ, Matthews JP, Wirth A, Rischin D, Ball DL. Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung cancer (Amsterdam, Netherlands) 2005;49:95–108. doi: 10.1016/j.lungcan.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Aerts HJ, Bosmans G, van Baardwijk AA, et al. Stability of 18F-deoxyglucose uptake locations within tumor during radiotherapy for NSCLC: a prospective study. International journal of radiation oncology, biology, physics. 2008;71:1402–1407. doi: 10.1016/j.ijrobp.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 31.International Commission on Radiation Units and Measurements. Oxford University Press; Oxford, UK: 1993. ICRU Report 50, Prescribing, Recording, and reporting Photon Beam Therapy. [Google Scholar]
- 32.Nijsten SM, Mijnheer BJ, Dekker AL, Lambin P, Minken AW. Routine individualised patient dosimetry using electronic portal imaging devices. Radiother Oncol. 2007;83:65–75. doi: 10.1016/j.radonc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 33.A’Hern RP. Sample size tables for exact single-stage phase II designs. Statistics in medicine. 2001;20:859–866. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 34.Bentzen SM. Dose painting and theragnostic imaging: towards the prescription, planning and delivery of biologically targeted dose distributions in external beam radiation oncology. Cancer treatment and research. 2008;139:41–62. [PubMed] [Google Scholar]
- 35.Sovik A, Malinen E, Skogmo HK, Bentzen SM, Bruland OS, Olsen DR. Radiotherapy adapted to spatial and temporal variability in tumor hypoxia. International journal of radiation oncology, biology, physics. 2007;68:1496–1504. doi: 10.1016/j.ijrobp.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 36.Thorwarth D, Eschmann SM, Paulsen F, Alber M. Hypoxia dose painting by numbers: a planning study. International journal of radiation oncology, biology, physics. 2007;68:291–300. doi: 10.1016/j.ijrobp.2006.11.061. [DOI] [PubMed] [Google Scholar]