Abstract
Leukocyte transmigration into the central nervous system promotes multiple sclerosis pathogenesis, yet ambiguity remains regarding the mechanisms controlling the migration of distinct immune cell subsets. Using in vitro, ex vivo and postmortem human materials, we identified a significant upregulation of junctional adhesion molecule‐like expression at the blood–brain barrier, monocytes, and CD8 T cells of multiple sclerosis patients. We also detected junctional adhesion molecule‐like+ trans‐migratory cups when monocytes/CD8 T cells adhered to the blood–brain barrier, however, their migratory capacity was significantly compromised when junctional adhesion molecule‐like was blocked. These findings highlight a novel role for junctional adhesion molecule‐like in leukocyte transmigration and its potential as a promising therapeutic target.
Introduction
Multiple sclerosis (MS) is an immune‐mediated disorder of the central nervous system (CNS) characterized by multifocal areas of leukocyte infiltration, demyelination, and axonal damage. Demyelination in MS plaques is typically associated with accumulation of leukocytes migrating from the periphery via the CNS barriers.1 The vasculature associated with the blood–brain barrier (BBB) is formed by specialized endothelial cells (ECs) maintaining unique morphological and metabolic properties including their intrinsic immunoquiescent state.1, 2 In MS, this delicate microenvironment is perturbed by peripheral and central inflammation that leads to endothelial activation and leukocyte transmigration. The latter is characterized by the sequential activation and interaction of molecular effectors expressed by ECs, including selectins, chemokines, cells adhesion molecules (CAMs), and their counter ligands expressed by immune cells.1 Additional CAMs involved in this process are the junctional adhesion molecule (JAM) family (JAM‐A to C), which are type I transmembrane proteins differentially expressed at the junctions of ECs, epithelial cells, and on various leukocytes.3 A more recently identified member of this family, JAM like (JAML), is known to mediate the transmigration of neutrophils and monocytes by interacting with coxsackie‐adenovirus receptor (CAR) expressed by epithelia.4 JAML is also expressed by endothelium where it homodimerizes in cis, although homophilic trans interactions have been reported in areas of cell–cell contact.5 To establish whether JAML influences the recruitment of specific subsets of pathogenic cells into the CNS and could serve as a therapeutic target to dampen CNS inflammation, we sought to determine JAML expression on the BBB and on immune cells, and its plausible role in the process of leukocyte migration.
Material and Methods
Primary cultures of BBB‐ECs
Human adult CNS tissue was obtained from patients undergoing surgery for intractable epilepsy. Informed consent and ethic approval were given prior to surgery (HD04.046). Primary cultures of BBB‐ECs were established as previously described.6, 7
RNA isolation and quantitative PCR
Human BBB‐ECs were cultured to confluency and then treated for 18 h with TNF and IFN‐gamma, cells were trypsinized and RNA was isolated as described before.7, 8 RNA was reverse‐transcribed using Life Technologies(Grand Island, NY) high‐capacity cDNA reverse transcription kit following manufacturer's recommendations. For quantification of JAML (AMICA1), cDNA samples were prepared in triplicate with Life Technologies Power SYBR® Green PCR Master Mix and the primers FWD: 5′‐GGTGATCATTGTGGGAATTGT‐3′ REV: 5′‐TCGTGTTCTTCACCAAGACTGT‐3′. qPCR was run on Applied Biosystems StepOnePlus™ System (Waltham, MA). Results were normalized to GAPDH controls (primers: FWD: 5′‐GCAAATTCCATGGCACCGT‐3′ – REV: 5′‐TCGCCCCACTTGATTTTGG‐3′) and quantified using the associated StepOnePlus™ software.
Protein isolation and western blot analysis
Protein from resting and inflamed human BBB‐ECs was isolated as previously described.6 20 μg of proteins were run on Bio‐Rad 4–20% Mini‐PROTEAN® TGX™ gels (Hercules, CA) under reducing conditions. Proteins were transferred to nitrocellulose membranes (Bio‐Rad) and blocked for 1 h at room temperature (RT) in Odyssey® Blocking Buffer (TBS) (Li‐Cor). Rabbit anti‐JAML (Abcam) and Mouse anti‐β‐actin (Sigma) were diluted in blocking buffer and incubated overnight at 4°C. Membranes were washed three times in TBS‐Tween 20 (0.1%). Fluorescent IRDye® secondary antibodies (Li‐Cor) were diluted in blocking buffer (1:10,000) and added to the membrane for 1 h at RT. Membranes were washed three times in TBS‐T and visualized using the Odyssey Infrared Imaging System (LI‐COR, Inc ‐ Lincoln, ME). Quantifications were performed using Image Studio™ Software (LI‐COR, Inc ‐ Lincoln, ME) according to the company's recommendations. All results were normalized to β‐actin protein levels.
Patients
Blood was drawn/obtained from MS patients and healthy donors after written informed consent. MS patients had relapsing‐remitting MS (RRMS) and at the time of blood/CSF (cerebrospinal fluid) draw had not received steroids or disease‐modifying therapies. RRMS patients consisted of 10 women and five men with a mean age of 37.5 years of age (range 22–52 years) and healthy controls were five women and three men with a mean age of 35.1 years of age (range 21–54 years).
Flow cytometry analysis
MS patients were classified as previously described.8 peripheral blood mononuclear cells (PBMCs) were isolated, stained, and analyzed as previously reported.7 PBMCs were phenotyped using antibodies (10 mg/mL) specific for human JAML‐FITC (R&D systems), CD3‐APC, CD4‐PE‐Cy7, CD8‐PB, CD14‐PE, and CD19‐Alexa700 or corresponding isotype controls (all from BD Biosciences) (East Rutherford, NJ). Cells were acquired on a BD LSRII cytometer (BD Bioscience) and analyzed using the BD FACSDiva software (BD Biosciences ‐ East Rutherford, NJ)).
Immunostainings
Primary cultures of BBB‐ECs were grown to confluency, inflamed, and stained as previously described.8 In brief, BBB‐ECs (n = 3) were stained with anti‐JAML antibody (R&D systems, 1/50), followed by donkey anti goat‐Alexa 488 (Jackson ImmunoResearch‐West grove, PA). Immunohistofluorescent stainings in postmortem brain sections from MS patients (n = 5) were performed according to institutional guidelines (CRCHUM, SL05.022, SL05.023, and BH07.001).8 Postmortem frozen MS brain blocks (n = 24) were cryosectioned, fixed, and immunostained with goat anti‐JAML (R&D systems, 1/50) and with mouse anti‐CD68 (DAKO, 1/100), mouse anti‐CD11c (BD Biosciences, 1/200), rabbit anti‐CD3 (DAKO, 1/200) and mouse anti‐MHC‐II (DAKO, 1/100) followed by corresponding secondary antibodies (Jackson ImmunoResearch ‐ West Grove, PA). Imaging quantification was performed as previously described.6
Adhesion and transmigration assays
Monocytes and CD8 T cells were isolated from blood of healthy donors as previously described8 and were allowed to adhere 1 h to monolayers of human BBB‐ECs. Cells were then washed, fixed, and immunostained for JAML. intercellular adhesion molecule‐1 (ICAM‐1) (mouse anti‐ICAM1, Biolegend, San Diego ‐ CA) and p120 (mouse anti‐p120, BD Biosciences 1/100) as described before.8 To enable investigation of leukocyte migration across the BBB, we used a transwell model in which BBB‐ECs were grown on the upper chamber for 72 h.6, 7, 8 Before migration, CD8 T cells were activated using plate‐bound anti‐CD3 (eBioscience, 2.5 μg/mL) (Santa Clara, CA) and soluble anti‐CD28 (BD Pharmingen, 2 μg/mL) in X‐vivo 15 medium (Lonza) for 4 days. Monocytes were isolated by positive selection according to the manufacturer's protocol (Miltenyi). Activated CD8 T cells and monocytes (106 cells per well) were added to the transwell chamber containing anti‐JAML (R&D systems, 20 μg/mL) or control immunoglobulins and migrated for 18 h. Migrated cells were imaged using DIC microscopy (Leica MZ10F; Leica Microsystems, Wetzlar ‐ Germany), and were collected and counted as previously described.6, 7, 8
Statistical analyses
Statistical analyses were performed using Graphpad PRISM 4.0 (La Jolla, CA). Data are expressed as the mean ± standard error of the mean (SEM). Data were analyzed using Students' t‐test and only P‐values <0.05 were considered statistically significant.
Results
To determine JAML expression on the CNS vasculature, primary cultures of human BBB‐ECs were grown under resting and inflammatory (IFN‐γ/TNF) conditions. Expression of JAML was low on BBB‐ECs grown under resting conditions, but was significantly upregulated upon inflammation (Fig. 1A–C and Fig. S1A). In MS brains JAML was moderately expressed by normal‐appearing white matter blood vessels and by perivascular macrophages, while significant upregulation was found in vessels within MS lesions (Fig. 1D and Fig. S1B). Macrophages/microglia (CD68+), dendritic cells (CD11c+) and few CD3+ T cells also stained positive for JAML in MS lesions (Fig. 1D). We thus decided to characterize JAML expression in peripheral and CNS leukocytes from RRMS patients and healthy controls. Roughly 52% of CD14+ monocytes derived from the blood of healthy donors expressed JAML, but a significant increase in percentage (80%) and level of expression (twofold upregulation) was observed in monocytes of RRMS patients (Fig. 1E and F). In contrast, only 2.1% of CD8 T cells isolated from healthy controls (n = 9) expressed JAML versus 5.5% in MS patients (n = 15) (Fig. 1G and H). However, the frequency of JAML‐expressing CD8 T cells significantly increased (up to 30%) in the CSF of RRMS patients (n = 4) (Fig. 1G and H). The low frequency of monocytes in the CSF of MS patients did not permit proper analysis. JAML expression in CD4 T cells and B lymphocytes was marginal (below 1%) and minimal differences were found between healthy subjects and RRMS patients (data not shown). These findings suggest that the inflammatory milieu in the periphery and in the CNS of MS patients regulates JAML expression in myeloid cells and CD8 T lymphocytes.
Figure 1.
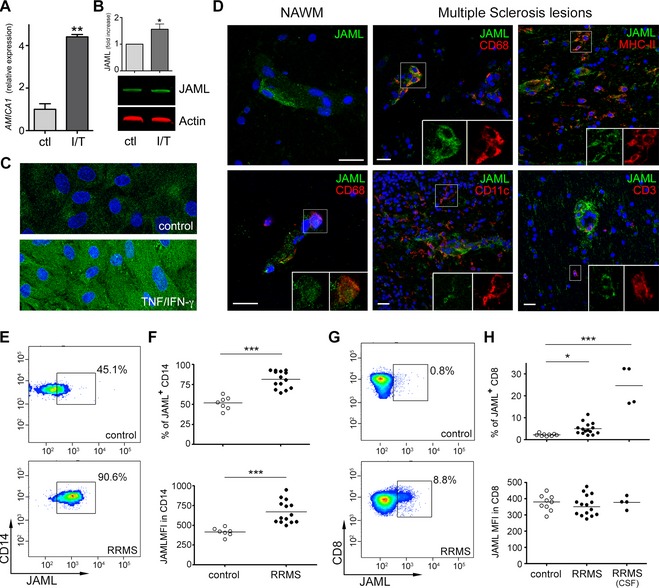
JAML expression on the BBB and immune cells during MS. qPCR (A) western blot (B) and immunocytofluorescent analysis (C) expression of JAML in primary cultures of resting and inflamed (TNF/IFN‐γ) human BBB‐ECs. Gene nomenclature for JAML is AMICA1. Data shown are representative of two to three independent experiments (n = 2–3 EC preparations), Scale bar: 10 μm (D) JAML expression was evaluated in MS tissues (both NAWM and lesions) and costained with the immune markers CD68 (macrophages/microglia), CD11c (dendritic cells), MHC‐II (antigen‐presenting cells) and CD3 (T cells), scale bars: 20 μm. (E and F) Representative flow cytometry dot plot showing the expression of JAML on human ex vivo CD14 monocytes and CD8 T cells in the peripheral blood of RRMS patients and controls. (G and H) MFI and percentage of JAML‐expressing CD14 monocytes and CD8 T cells in the peripheral blood of controls and RRMS patients and in the CSF of patients with active disease. (*P < 0.05, **P < 0.01, ***P < 0.001). JAML, junctional adhesion molecule‐like; BBB‐ECs, blood–brain barrier endothelial cells; MS, multiple sclerosis; NAWM, normal appearing white matter; CSF, cerebrospinal fluid; MFI, mean fluorescent intensity.
As part of its active role supporting leukocyte transmigration, the BBB projects transmigratory cups rich in actin and CAMs that anchor and embrace adherent leukocytes.9 Monocyte adhesion to BBB‐ECs was accompanied by the formation of transmigratory cup‐like structures rich in JAML (Fig. 2A). Analysis of junctional molecules supporting BBB function such as p120 demonstrated localization at inter‐endothelial junctions and in docking structures supporting adherent monocytes (Fig. 2A). Transmigratory cup‐like structures expressing JAML were also detected in areas of contact between CD8 T cells and the BBB (Fig. 2B). We next investigated whether JAML is required for efficient transmigration of leukocytes in vitro. Pretreatment of BBB‐ECs with a JAML blocking antibody significantly compromised the transmigration of CD14+ monocytes (Fig. 2C – 57% reduction) and of activated CD8 T cells (Fig. 2D – 45% reduction). These findings are strongly suggestive of a role of JAML in mediating the migration of monocytes and CD8 T cells across the BBB.
Figure 2.
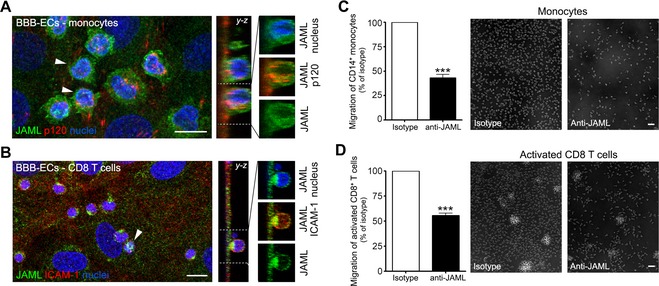
JAML support leukocyte migration across the BBB. (A and B) Expression of JAML (green) on CD14+ monocytes and CD8 T cells adhering to primary cultures of human BBB‐ECs. Costaining for p120 – ICAM‐1 (red) and nuclei (blue) are included. Y‐Z projections show JAML enhancement surrounding the leukocytes in transmigratory cups. Data shown are representative of four independent experiments. Scale bar = 10 μm. (C and D) Human ex vivo CD14 monocytes and activated CD8 T cells were allowed to migrate for 18 h across a monolayer of primary human BBB‐ECs pretreated with isotype control or anti‐JAML antibody. JAML blockade significantly restricted the migration of CD14 monocytes and activated CD8 T cells across BBB‐ECs. Data shown are representative of three independent experiments (n = 3 and 6 blood donors) performed in triplicate using two distinct BBB‐EC preparations (***P < 0.001). JAML, junctional adhesion molecule‐like; BBB‐ECs, blood–brain barrier endothelial cells.
Discussion
Here, we demonstrate the expression of JAML at the BBB level and its upregulation during neuroinflammatory conditions. Monocytes/macrophages and CD8 T cells also expressed JAML and during active MS is significantly upregulated. The fact that JAML localizes in ring‐like structures reminiscent of transmigratory cups at sites where leukocytes adhere to BBB‐ECs and that its blockade reduces monocyte and CD8 T cell transmigration across the BBB suggest an important function for JAML in the recruitment of specific leukocyte subsets into the CNS.
The upregulated expression of JAML on inflamed BBB‐ECs and immune cells highlight its potential role in leukocyte transmigration across the BBB. We support this notion by showing for the first time JAML expression in structures reminiscent of transmigratory cups/tetraspanin‐enriched microdomains, which serve as docking structures thought to facilitate leukocyte migration.9, 10 Interestingly, monocyte adhesion to the endothelium also induced the formation of transmigratory cups enriched in p120, providing further evidence for the expression of junctional proteins by leukocytes11 and emphasizing the role of p120 as an intracellular mediator of monocyte migration.12 In addition, JAML expression on myeloid and CD8 T cells populations within the CNS is an important aspect that warrants further investigation as JAML is also a costimulatory molecule able to support cell activation and effector functions.13 The migration of neutrophils and monocytes across epithelium and EC lines was shown to be decreased when JAML is blocked.4, 14 Here, we show JAML expression by primary cultures of human BBB‐ECs and demonstrate that JAML blockade compromises the migration of monocytes and activated CD8 T cells. CD8 T cell migration across the BBB is partially dependent on α4 integrin,15 a process in which JAML might play an active role as its dimerization is controlled by α4 and leads to strong leukocyte‐EC binding.16
In addition, JAML also serves as a ligand for CAR, a molecule known to localize at epithelial tight junctions,17 but is expressed diffusely in choroid plexus.18 Thus, during RRMS JAML might support monocyte and CD8 T cell migration into the CNS through homophilic interactions with the BBB endothelium and heterophilically by binding to CAR on the choroidal epithelium forming the blood–CSF barrier. JAML emerges as a CAM involved in monocyte and CD8 T cell migration during neuroinflammatory conditions and holds significant promise as a therapeutic target, given its role in controlling the trafficking of specific populations of pathogenic leukocytes into the CNS.
Authorship Contribution
J. I. A. designed the study, performed the experiments, analyzed the data, and wrote the manuscript. H. K. isolated protein/RNA, help with flow cytometry analysis and manuscript preparation. L. C. performed qPCR and WB experiments. M. C. collected and analyzed patient data. C. L. participated in carrying flow cytometry experiments and activation of CD8 T cells. A. P. supervised data interpretation, provided the funds and input in manuscript revision/preparation.
Conflict of Interest
Dr. Alexandre reports grants from CIHR, MS Society, during the conduct of the study.
Supporting information
Figure S1. JAML expresion on BBB‐ECs and MS tissues. Relative levels of JAML expression were measured by pixel intensity analysis of control and TNF/IFN‐g treated BBB‐endothelial cells (A). The pixel intensity of JAML was also measured on blood vessels within NAWM and MS lesions (B). In vitro analysis of JAML expression was performed in five different regions of a BBB‐EC monolayer (controls vs. inflamed) (n = 3 experiments), whereas in situ analysis represents five patients and a total of 25 vessels analyzed per region (NAWM vs. MS lesions). P‐values obtained using an unpaired t‐test are indicated as ***P < 0.001.
Acknowledgments
We thank Igal Ifergan for assisting in flow cytometry analysis, Julia Pollman for assistance on immunostainings, Lyne Bourbonniere for maintaining human BBB‐EC cultures and Camille Pittet for technical assistance. We also want to thank the laboratories of Tracy Bale and Ellen Pure (PennVet) for their technical support. This study was funded by the Multiple Sclerosis Society of Canada (MSSC), The EndMS network and the Canadian Institutes of Health Research (CIHR). J. I. A holds the David L. Torrey EndMS transitional career development award from the MSSC. C. L. holds a scholarship and fellowship from the MSSC. A. P. holds a Senior Scholar Award of the Fonds de Recherche du Québec‐Santé and a senior Canada Research Chair in Multiple Sclerosis.
References
- 1. Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta 2011;1812:252–264. [DOI] [PubMed] [Google Scholar]
- 2. Abbott NJ, Ronnback L, Hansson E. Astrocyte‐endothelial interactions at the blood‐brain barrier. Nat Rev Neurosci 2006;7:41–53. [DOI] [PubMed] [Google Scholar]
- 3. Arcangeli ML, Frontera V, Aurrand‐Lions M. Function of junctional adhesion molecules (JAMs) in leukocyte migration and homeostasis. Arch Immunol Ther Exp 2013;61:15–23. [DOI] [PubMed] [Google Scholar]
- 4. Zen K, Liu Y, McCall IC, et al. Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule‐like protein on neutrophils. Mol Biol Cell 2005;16:2694–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moog‐Lutz C, Cave‐Riant F, Guibal FC, et al. JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood 2003;102:3371–3378. [DOI] [PubMed] [Google Scholar]
- 6. Alvarez JI, Dodelet‐Devillers A, Kebir H, et al. The Hedgehog pathway promotes blood‐brain barrier integrity and CNS immune quiescence. Science 2011;334:1727–1731. [DOI] [PubMed] [Google Scholar]
- 7. Ifergan I, Kebir H, Bernard M, et al. The blood‐brain barrier induces differentiation of migrating monocytes into Th17‐polarizing dendritic cells. Brain 2008;131(Pt 3):785–799. [DOI] [PubMed] [Google Scholar]
- 8. Larochelle C, Cayrol R, Kebir H, et al. Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain 2012;135(Pt 10):2906–2924. [DOI] [PubMed] [Google Scholar]
- 9. Carman CV, Springer TA. Trans‐cellular migration: cell‐cell contacts get intimate. Curr Opin Cell Biol 2008;20:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barreiro O, Zamai M, Yanez‐Mo M, et al. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol 2008;183:527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mandel I, Paperna T, Glass‐Marmor L, et al. Tight junction proteins expression and modulation in immune cells and multiple sclerosis. J Cell Mol Med 2012;16:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alcaide P, Newton G, Auerbach S, et al. p120‐Catenin regulates leukocyte transmigration through an effect on VE‐cadherin phosphorylation. Blood 2008;112:2770–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Witherden DA, Verdino P, Rieder SE, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science 2010;329:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo YL, Bai R, Chen CX, et al. Role of junctional adhesion molecule‐like protein in mediating monocyte transendothelial migration. Arterioscler Thromb Vasc Biol 2009;29:75–83. [DOI] [PubMed] [Google Scholar]
- 15. Ifergan I, Kebir H, Alvarez JI, et al. Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on alpha4 integrin. Brain 2011;134(Pt 12):3560–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luissint AC, Lutz PG, Calderwood DA, et al. JAM‐L‐mediated leukocyte adhesion to endothelial cells is regulated in cis by alpha4beta1 integrin activation. J Cell Biol 2008;183:1159–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen CJ, Shieh JT, Pickles RJ, et al. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA 2001;98:15191–15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Persson A, Fan X, Widegren B, Englund E. Cell type‐ and region‐dependent coxsackie adenovirus receptor expression in the central nervous system. J Neurooncol 2006;78:1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. JAML expresion on BBB‐ECs and MS tissues. Relative levels of JAML expression were measured by pixel intensity analysis of control and TNF/IFN‐g treated BBB‐endothelial cells (A). The pixel intensity of JAML was also measured on blood vessels within NAWM and MS lesions (B). In vitro analysis of JAML expression was performed in five different regions of a BBB‐EC monolayer (controls vs. inflamed) (n = 3 experiments), whereas in situ analysis represents five patients and a total of 25 vessels analyzed per region (NAWM vs. MS lesions). P‐values obtained using an unpaired t‐test are indicated as ***P < 0.001.


